Abstract
Flexibility in task performance is essential for a robust system of division of labour. We investigated what factors determine which social insect workers respond to colony-level changes in task demand. We used radio-frequency identification technology to compare the roles of corpulence, age, spatial location and previous activity (intra-nest/extra-nest) in determining whether worker ants (Temnothorax albipennis) respond to an increase in demand for foraging or brood care. The less corpulent ants took on the extra foraging, irrespective of their age, previous activity or location in the nest, supporting a physiological threshold model. We found no relationship between ants that tended the extra brood and corpulence, age, spatial location or previous activity, but ants that transported the extra brood to the main brood pile were less corpulent and had high previous intra-nest activity. This supports spatial task-encounter and physiological threshold models for brood transport. Our data suggest a flexible task-allocation system allowing the colony to respond rapidly to changing needs, using a simple task-encounter system for generalized tasks, combined with physiologically based response thresholds for more specialized tasks. This could provide a social insect colony with a robust division of labour, flexibly allocating the workforce in response to current needs.
Keywords: division of labour, task allocation, foraging, brood care, social insects, radio-frequency identification
1. Introduction
Division of labour is a fundamental property of social organization, seen in many group-living organisms (Smith 1776; Oster & Wilson 1978; Robinson 1992). Division of labour allows task specialization and parallel performance of tasks, and is thought to be key to the success of social insects such as bees, wasps, termites and ants (Oster & Wilson 1978). In a perfectly stable environment, each task could have a group of highly specialized workers dedicated solely to that task. However, in dynamic and unpredictable environments, it is necessary to have flexibility in task performance. Social insect colonies are able to respond to changes in demand for a particular task by re-allocating parts of the workforce (Calabi & Traniello 1989; Johnson 2002; Couvillon et al. 2008). This flexibility and the resulting robustness are key benefits of decentralized control of division of labour (Bourke & Franks 1995).
Division of labour can be modelled as a threshold-based process, with individuals taking on a task when the stimulus for performing that task reaches their threshold (Robinson 1987a; Bonabeau & Théraulaz 1999). If demand for a particular task increases, then the stimulus for performing that task will rise. Under this model, individuals that respond to the change in demand will be either those with low thresholds that are reached by the stimulus or, if thresholds are similar, those experiencing the stimulus most strongly. An individual's task threshold could be affected by its age, physiology or previous experience, while the level of stimulus experienced could be affected by spatial location. We monitored all these factors to establish which individuals responded to changes in demand for two tasks vital in all social insect colonies: brood care and foraging.
Social insects that forage outside the nest tend to be older, while brood carers are usually younger. One hypothesis to explain this is that thresholds for particular tasks change with age (Robinson 1987b). The prediction of the age-related threshold hypothesis is that older ants will have lower thresholds for foraging, so in our experiment they should be more likely to respond to the change in demand for foragers, while younger ants will respond to the change in demand for brood care. However, physiological changes occur during an insect's life, and physiological state may be more important than age in determining task thresholds (Robinson et al. 1994; Robinson 2009). Amount of fat stored is a physiological state that often correlates with task, with leaner foragers or soldiers and with more corpulent brood carers (Porter & Jorgensen 1981; Lachaud et al. 1992; Blanchard et al. 2000; Toth & Robinson 2005). The physiological threshold hypothesis predicts that in our experiment the leaner ants will have lower thresholds for foraging and be more likely to respond to the change in demand for foragers, irrespective of age, while more corpulent ants will have lower thresholds for brood care.
One variation on threshold models includes self-reinforcement. In this model, performing a task reduces an individual's threshold for that task, so experience will affect future performance (Théraulaz et al. 1998; Ravary et al. 2007). The experience hypothesis predicts that ants responding to the increased demand for brood care would be ants that had carried out this intra-nest task previously (i.e. in our experiment those with few previous extra-nest trips), while those responding to the increased demand for foragers would be those with many previous extra-nest trips.
In spatial task-encounter models, workers perform a task until the stimulus drops below their threshold and they then move away to search for a new task (Tofts & Franks 1992; Tofts 1993). This spatial hypothesis predicts that ants nearer the entrance should respond to the change in the demand for foraging as they experience the stimulus provided by returning ants (Robinson et al. 2009a) and are likely to encounter the nest entrance as they move around. Ants patrolling the inside of the nest should also be likely to respond to the change in demand, as they will be exposed to stimulus from a hungry brood (McDonald & Topoff 1985). When the demand for brood care is increased, the spatial hypothesis predicts that ants nearer the inside of the nest where the new brood is added, or those tending to patrol the inside of the nest, would be more likely to discover the new brood and respond to this stimulus by providing brood care.
These individual attributes—age, physiology (corpulence), experience and spatial location—are interrelated. In Temnothorax albipennis, younger ants tend to be more central in the nest, and extra-nest workers tend to be leaner and older (Blanchard et al. 2000). We compare the contributions of these multiple factors in determining which individual ants respond to the demand for more work in a particular task. We used colonies of ants individually tagged using radio-frequency identification (RFID) to identify which ants responded to the changing task demand, and to match this information with their previous intra- and extra-nest activity, spatial fidelity to particular nest regions, and their age cohort and corpulence.
2. Material and methods
(a). Experimental colonies
Sixteen complete queenright T. albipennis colonies were collected from Dorset, UK, in March 2008. At this time of year pre-pupae and pupae are present, but new workers have not yet eclosed, so all workers have overwintered from the previous year (Partridge 1993). Colonies were maintained in the laboratory according to established protocol (Franks et al. 2006). Six experimental colonies were constructed, each to consist of a queen, brood and two age cohorts of workers: callow and mature. The queen, 128 brood items and 55 mature workers (each marked on the head with a dot of white Pactra paint) were taken from one source colony. These mature ants had overwintered from the previous year. To these we added 50 late-stage pupae, taken as evenly as possible from five to nine other colonies. This artificial colony was allowed to emigrate into a new nest. No rejection of the pupae was observed. Forming mixed origin colonies in this way does not have any observable effect on brood rearing or foraging behaviour (see electronic supplementary material). Final colonies included 50 callows and 50 older workers—the initial 55 allowed for a small number of mature ants dying during the pre-trial week, presumably owing to old age. If there were still more than 50 prior to the trial commencing, we randomly removed excess ants. The brood items used in colony construction were larvae (of a range of sizes), because eggs are too small to count accurately, and pupae could eclose during the experiment, altering colony composition. This ratio of workers to brood (1 : 1.28) is the average ratio in wild colonies (Franks et al. 2006). Pupae eclosed within 2 days of colony construction. Callows are inactive for the first 1–2 days after eclosion, but by 3 days they are mobile and take part in all tasks in the colony (Elva J. H. Robinson, personal observation). Eight days after colony construction, we tagged every worker with an RFID microtransponder (500 × 500 × 120 µm, weight 89 µg; PharmaSeq, Inc., New Jersey, USA) glued to the thorax using established protocol (Robinson et al. 2009a,b). The microtransponders were scanned before being attached to the ants, so that the IDs assigned to callows (pale cuticle) and matures (dark cuticle, paint mark on head) were known. During tagging, we placed the callows under CO2 anaesthetization a second time, to match the treatment of the mature ants, which had previously been anaesthetized for paint marking.
(b). Experimental protocol
The tagged colony was allowed to move into a two-chamber nest (figure 1) placed in an arena 23 × 23 cm. In all trials the colony moved the brood only into the first chamber. A trial commenced 9 days after colony construction, so the callows were 6–7 days old while the mature workers were at least 8 months old. Prior to the start of a trial, colonies were fed with protein (Drosophila melanogaster) and honey solution. Water was available ad libitum throughout the trials. We performed six trials, each on a different colony.
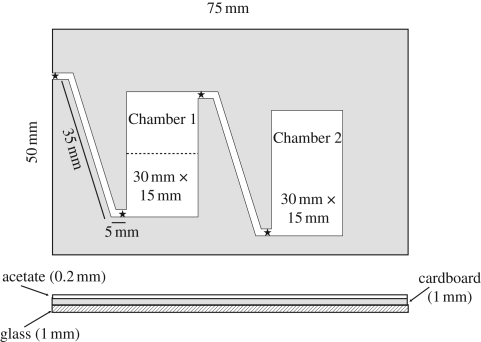
Figure 1.
Two-chamber nest. Chamber 1 is separated for scanning purposes into two regions by a dividing line drawn on the acetate, perpendicular to the long chamber walls and positioned through the centroid of the brood area. This does not correspond to a physical barrier. Chamber 2 has a trapdoor in the acetate cover, allowing access for adding brood. Corridors 1.5 mm wide. Stars denote reader locations.
During the trial, we collected data in two ways. During ‘activity recording’, four RFID readers (comprising a Hitachi HL6738MG laser that provided 35 mW of energy to the passive tags and an antenna to detect the radio identification signal) were placed above the entrances and exits to the corridor between the two chambers of the nest, and the corridor between chamber 1 and the arena (figure 1). This had the advantage that directional data could be collected on the movements of the ants, including overnight. This method does not record ants that stay in one chamber; hence, to provide information about the locations of these ants, ‘spatial scanning’ was performed. For this, we used a hand-held RFID reader to identify the ants in each chamber and outside. Chamber 1 was separated into inner and outer regions (figure 1). Not every ant was read in each spatial scan, because some ants were upside down or under other ants, but over repeated scans during a trial, 88 to 99 per cent of tagged ants were detected. Temnothorax spp. show a spatial separation of tasks (Sendova-Franks & Franks 1994; Backen et al. 2000), so this method allowed us to link spatial movements with movements between tasks.
Once the colony moved into the new nest, we turned on the readers for activity recording and the ants were left undisturbed for 65 h, to settle into the new nest, sort the brood and explore the arena. From day 4 onwards, we carried out spatial scans twice a day (at 1000 and 1600 h). Two manipulations were carried out. On day 5, immediately after the first spatial scan, we added 32 large brood items to chamber 2 through a trapdoor in the acetate ceiling. This manipulated worker-to-brood ratio (1:1.6) is lower than the natural average, but still within the range seen in wild T. albipennis (Franks et al. 2006). In subsequent spatial scans, we counted the brood items in chamber 2, and recorded workers scanned in chamber 2 as either in contact with the brood or not. On day 7, immediately after the first spatial scan, we supplied honey solution in the foraging arena. The vast majority of extra-nest trips in T. albipennis are made by only a few ants (e.g. the four most active ants made almost half of all foraging trips in Robinson et al. 2009a). To avoid these very active ants responding to the added food without any switching occurring, when adding the food we also removed any external ants and any ants that left the nest in the first 2 h (up to a maximum of eight tagged ants). This ensured that task switching was required for ants in the nest to respond to the demand for food. Demand for food would be expected to be high in a colony that has been unfed for 5 days and has a high brood-to-worker ratio. Removed ants were immediately frozen, and at the end of day 8 the whole colony was killed by freezing. Three days after a trial ended, we defrosted the colony and recorded the tag ID, age cohort, corpulence and final location of all workers in the nest. We determined age cohort from tag ID. We were also able to determine the age of ants that had lost their RFID tags because the mature ants had been paint-marked on the head and had darker cuticles. Corpulence was approximated from gaster dry weight (Blanchard et al. 2000), which was measured according to the protocol in Robinson et al. (2009a).
(c). Data analysis
We used generalized linear mixed models (GLMMs) to analyse what factors predict switching behaviour, with whether ants switched task or not as the dependent factor, age cohort, spatial fidelity, previous intra-nest activity, previous extra-nest activity and corpulence as fixed factors, colony as a random factor, and a binomial error structure. Previous intra-nest activity was defined as the number of trips an ant made between the two chambers of the nest prior to the manipulation (adding brood or food); previous extra-nest activity equated to the number of trips an ant made outside the nest. These metrics assess activity at particular key points; spatial fidelity was also measured to give a general assessment of how much the ant moved around chamber 1. Spatial fidelity was calculated from the spatial scan data and measured the proportion of time an ant spent in a particular region of chamber 1 (inner region for brood care; outer region for foraging; see figure 1). Positive values indicate high spatial fidelity to one region of chamber 1; negative values indicate high fidelity to the opposite region; 0 would indicate no spatial fidelity. Spatial fidelity values are weighted by the amount of data available for each ant and compared to the actual distribution of ants between the two regions in each scan (see appendix).
Ants responded to the addition of extra brood in two ways: some moved to chamber 2 and started tending the new brood by feeding and grooming it; some transported brood to the main brood pile in chamber 1. In all colonies, at least some of the brood was transported to the main brood pile, but in five of the six colonies some of the added brood was still in chamber 2 at the end of the trial. We defined ants as switching to transporting brood if their rate of visiting chamber 2 in the 6 h immediately after the brood was added was higher than their rate of visiting chamber 2 over the previous 89 h; ants whose rate of visiting chamber 2 stayed the same or decreased were defined as not switching to brood transport. We defined ants as switching to tending the new brood if they were in contact with the brood in chamber 2 during the next spatial scan after brood was added; ants that did not fulfil these criteria were defined as not switching to care of the new brood, even if they were present in chamber 2. The first spatial scan was used because there was the largest quantity of brood still present in chamber 2 at this time and also the most brood carers. In two colonies (A and E), much of the brood was transported to chamber 1 straight away, and no ants fulfilled the above criteria; hence these colonies were not included in the model analysing switching to tending the new brood. Switching to tending the new brood is not significantly associated with switching to transporting brood (χ21 = 0.61, p = 0.43); hence we treated these two behaviours separately in the analysis.
We defined ants as switching to extra-nest tasks if their rate of leaving the nest was higher during the 30 h after food was added than in the previous 137 h. Ants that had an equal or lower rate of leaving the nest were defined as not-switching, except those that made more than 0.1 extra-nest trips per hour both before and after the manipulation. These ants (mean two per trial) were excluded, as they were continuing to perform extra-nest tasks at a similar rate, and therefore could not be classed as either switching or not switching to extra-nest tasks. Apart from this exception, we included in the models all the ants that kept their tags to the end of the trial (mean 86% of ants). GLMMs were performed in R 2.4.1 and all other statistics in Minitab 15.1.20.0.
3. Results
(a). Age cohort, corpulence and spatial location
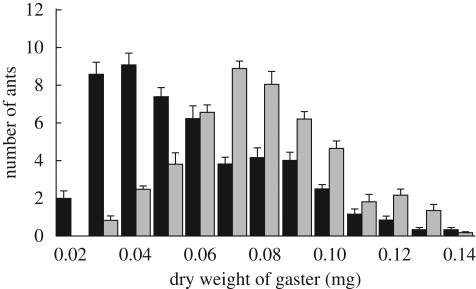
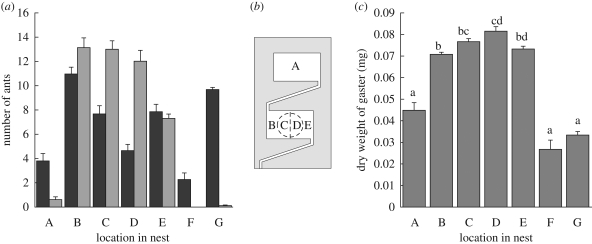
The different traits measured are interrelated. Callows have a higher mean corpulence than mature workers, which show a left-skewed distribution of gaster weights (figure 2). However, there is considerable overlap in the corpulence range of the two cohorts, with the dry gaster weight of mature ants ranging from 0.014 to 0.138 mg and of callow ants ranging from 0.027 to 0.139 mg (figure 2). In addition, callows tend to be more centrally located in the nest than mature ants (figure 3a) and, within both age groups, ants in chamber 1 are more corpulent than ants outside or in chamber 2 (figure 3c).
Figure 2.
Corpulence distribution, measured as gaster dry weight of worker ants in the two age cohorts, mean + s.e., n = 6 colonies. Callow gaster dry weight (grey bar) (mean ± s.d. = 0.078 ± 0.023 mg) is higher than mature gaster dry weight (black bar; 0.0583 ± 0.0260 mg; two-way ANOVA: F1,5 = 99.9, p < 0.001). There was also significant inter-colony variation (F1,5 = 5.7, p < 0.001).
Figure 3.
Locations of ants at the end of the trial. (a) Age cohort of ants at different spatial locations: number, mean + s.e.; n = 6 colonies (black bar, mature; grey bar, callow). (b) Key to locations within nest and arena (F, outside; G, extra-nest workers removed during food manipulation). (c) Corpulence of ants at different spatial locations, mean + s.e.; n = 6 colonies. Corpulence varied across locations (two-way ANOVA: F5,8 = 24.5, p < 0.001). There was also significant inter-colony variation (F5,8 = 6.3, p < 0.001). Groups sharing the same letter are not significantly different under Tukey's multiple comparisons. Ants located in corridors are not shown: they totalled mean ± s.d. = 2.8 ± 2.5 ants in the corridor leading outside, 0.8 ± 0.75 in the corridor between chambers.
(b). Increased demand for foragers
Corpulence was the best predictor for which ants left the nest when the demand for foragers increased, with leaner ants being more likely to switch to extra-nest tasks (table 1). Age cohort was not a significant predictor: although more mature ants leave the nest than callows, callows still make up 20 per cent of the new extra-nest workers and, at a given corpulence, mature ants are not significantly more likely to leave than callow ants (figure 4a; table 1). There is also no effect of spatial fidelity to the external end of chamber 1, nor previous activity inside or outside the nest.
Table 1.
Results of GLMMs on response to increased task demands. Non-significant interactions were eliminated from the models. Non-significant terms are shown for information; their exclusion does not qualitatively change the results. Signs on t-values indicate direction of relationship (e.g. switching to extra-nest activity was less likely with increasing corpulence).
| model | predictor | t-value | d.f. | p |
|---|---|---|---|---|
| switch to extra-nest activity | corpulence | −3.23 | 236 | <0.01 |
| age cohort | 0.93 | 236 | 0.35 | |
| spatial fidelity | 0.96 | 236 | 0.34 | |
| previous intra-nest activity | −0.58 | 236 | 0.57 | |
| previous extra-nest activity | 0.64 | 236 | 0.52 | |
| switch to tending extra brood | corpulence | −0.48 | 119 | 0.63 |
| age cohort | 0.61 | 119 | 0.54 | |
| spatial fidelity | 1.30 | 119 | 0.19 | |
| previous intra-nest activity | 1.55 | 119 | 0.13 | |
| previous extra-nest activity | −0.08 | 119 | 0.93 | |
| switch to transporting extra brood | corpulence | −4.43 | 165 | <0.001 |
| age cohort | 1.27 | 165 | 0.21 | |
| spatial fidelity | −0.52 | 165 | 0.60 | |
| previous intra-nest activity | 4.19 | 165 | <0.001 | |
| previous extra-nest activity | −1.59 | 165 | 0.11 |
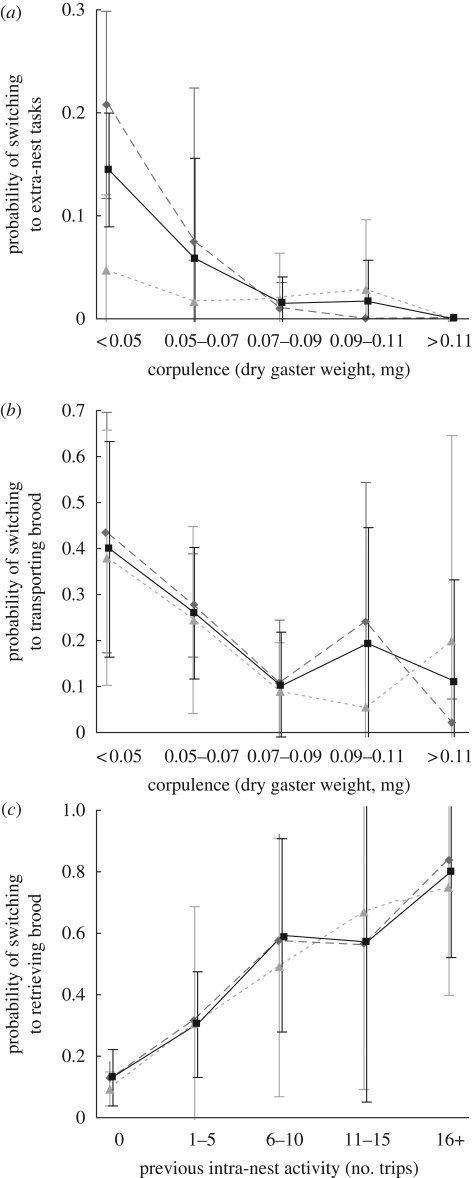
Figure 4.
The probabilities of switching to (a) external tasks and (b) transporting brood decrease with increasing corpulence. (c) The probability of switching to transporting brood increases with previous intra-nest activity. Differences between age cohorts (mature and callow) are not statistically significant. Probabilities, mean ± s.d.; n = 6 colonies. See table 1 for statistical details. Diamond, mature; triangle, callow; square, total.
(c). Increased demand for brood care
The ants that switch to tending the new brood are not predicted by age cohort, corpulence, activity or spatial fidelity to the internal end of chamber 1 (table 1). This behaviour seems to be random within the population, at least with respect to our measurable factors.
The ants that transport the extra brood to chamber 1 are best predicted by high previous intra-nest activity and low corpulence (figure 4b,c; table 1). Extra-nest activity, age and spatial fidelity are not significant predictors.
(d). Specialist switchers
Both switching to extra-nest tasks and switching to transporting brood are well predicted by low corpulence (table 1). Are the same lean ants responding to the increase in demand for both tasks? Overall, there is a significant association between switching to extra-nest tasks and switching to transporting brood (χ21 = 11.4, p < 0.001), with 10.8 ± 7.9 per cent (mean ± s.d.) of switchers switching to both new tasks. This pattern is quite variable across colonies: in colonies B, E and F all the extra foragers went on to become extra brood transporters, whereas in colony A no ants responded to both of the increases in demand. There is no relationship between switching to extra-nest tasks and switching to tending the new brood (χ21 = 0.134, p = 0.72).
4. Discussion
(a). Increased demand for foragers
Our results clearly show that corpulence is the best predictor for whether an ant responds to the increased demand for foragers by making trips outside the nest. This supports the physiological threshold hypothesis (table 2). Leaner ants are more likely to go outside, even when other factors (age, experience and spatial location) are taken into account. This demonstrates that the previously observed correlation between low corpulence and likelihood of leaving the nest (Robinson et al. 2009a) is not a side-effect of age-based thresholds, with leaner ants also being older. In our results, both mature and callow ants made extra-nest trips and there was considerable overlap in their corpulence distributions.
Table 2.
Summary of hypotheses predicting which ants will respond to increased demand for each task and empirical results.
| hypothesis | foraging |
brood care |
hypothesis supported? | ||
|---|---|---|---|---|---|
| prediction | result | prediction | result | ||
| age-related thresholds | older ants | no effect | younger ants | no effect | no |
| physiological thresholds | leaner ants | leaner ants | more corpulent ants | no effect on tending new brood; leaner ants retrieve brood | yes (foraging) |
| experience hypothesis | weak effect of more previous extra-nest trips | no effect | ants making fewer previous extra-nest trips | no effect | no |
| spatial hypothesis | ants near entrance or exploring nest | no effect | ants near the inside of nest or exploring nest | no effect on tending new brood; ants exploring nest retrieve brood | yes (brood retrieval) |
Previous work has demonstrated that recent extra-nest experience is not as important as corpulence in predicting which ants leave the nest (Robinson et al. 2009a). Our current results support this pattern, although any effect seen would have to be weak because, by definition, if ants had very high extra-nest activity before the manipulation and simply continued with their previous behaviour, they were not considered to have responded to the change in demand. More experienced ants might be expected to be more competent foragers; however, the propensity of a T. albipennis worker to forage is not a predictor of its foraging efficiency (Dornhaus 2008), so response to increased demand for foraging may be unrelated to experience-related competence.
Finally, the role of corpulence in predicting which ants leave the nest does not seem to be simply a side-effect of location in the nest, although the least corpulent ants were more peripheral (figure 3c). At least at the scale of spatial fidelity we studied, corpulence was more important than whether an ant was usually near the entrance to the nest. However, further study at a finer spatial scale would provide more detail on this, as the scale of stimulus perception is likely to be smaller than our spatial fidelity zone measures. The spatial task-encounter hypothesis also predicts that high levels of intra-nest activity (nest patrolling) could make it more likely that the stimulus for foraging is perceived, for example if the stimulus is brood hunger. We did not find any support for this hypothesis.
(b). Increased demand for brood care
Ants responded to the new brood in two ways. Certain ants moved into the second chamber, where the brood was added, and began to tend the brood, while other ants retrieved some of the extra brood to the main brood pile. The ants that took on the task of tending the new brood appear to have been a random subset of the colony, not predicted by age cohort, corpulence, previous activity or spatial fidelity. This contrasts with the ideas of age-correlated task thresholds, which predict that the youngest ants should be most likely to tend the brood (table 2). Instead our data suggest a high degree of flexibility over who performs brood care. This fits with data from Camponotus floridanus and Pheidole dentata in which ants taking on extra brood care are randomly distributed with respect to age (Tripet & Nonacs 2004; Muscedere et al. 2009). Muscedere et al. (2009) found that older P. dentata workers were more effective than younger workers at raising larvae, suggesting that there was no brood care specialization among younger workers.
Physiological brood care specializations are seen in many social insects. Honeybee and stingless bee nurses have specialized glands producing secretions for feeding larvae (Fluri et al. 1982; Huang et al. 1989; Gracioli-Vitti et al. 2004). In some ants and wasps, nurses feed larvae on trophic eggs, and hence have larger or more numerous ovarioles than other workers (Fresneau 1984; Fénéron et al. 1996). In other ant species, however, the brood carers may not require physiological specializations (Muscedere et al. 2009). We found no evidence that more corpulent ants are more likely to take on extra brood care tasks. This suggests that the usual central location of corpulent ants may be a side-effect of their low activity (Blanchard et al. 2000) rather than a task-based location near the brood pile. We also found no evidence that being previously located near to where the brood was added affects the likelihood of tending the new brood.
We do not know whether ants that tended the extra brood were previously actively engaged in a task, or what that task would have been. It is possible that they were brood care specialists already. However, it is a reasonable assumption that brood care specialists would tend to stay inside the nest, and so their number of extra-nest trips would be low. We did not find that extra-nest trips were low among ants that tended the new brood; hence, this does not support the idea that previous tasks affected which ants responded to the demand for extra brood care.
Ants that took on the task of transporting the extra brood back into chamber 1 were lean, with high levels of previous intra-nest activity. High intra-nest activity indicates that these ants were moving between the chambers of the nest, and supports the spatial task-encounter hypothesis, because these ants are most likely to come across the new brood and perceive the stimulus for brood transport (table 2). We found no age effect, suggesting that younger ants do not have lower thresholds for brood transport than older ants. In P. dentata, brood retrieval is preferentially performed by older workers (Muscedere et al. 2009); however, corpulence correlates have not been measured in this species, and hence it is possible that leanness plays a role here too. Although this is a brood-related task, we found that the leaner ants, not the most corpulent, took it on. This suggests that the leanest ants may be ‘elites’ with generally low thresholds to engage in work (Plowright & Plowright 1988; Gautrais et al. 2002). This is supported to some extent by the finding that in some colonies the same lean individuals responded to the increase in demand both for foragers and for brood retrieval, but it does not explain why they did not also tend the new brood. Another possibility is that these lean individuals represent a group of ‘patroller’ ants, which search the nest for things out of place, breaches in the walls, etc. Moving unattended brood to brood carers could be part of this task. Further work identifying the tasks performed by these ants prior to transport would be required to determine this.
(c). Flexible division of labour
Our data support a flexible task-allocation system that allows the colony to respond rapidly to changes in demand for particular tasks. An individual's physiological state may make the individual more or less suited to certain tasks. Using individual corpulence as a physiological threshold for foraging task performance could be adaptive, because leaner individuals are less costly to lose during the risky task of foraging, may attract fewer predators and will also be more efficient, as they are more mobile and have a greater capacity for feeding in the field (Porter & Jorgensen 1981; Blanchard et al. 2000). As demand for foragers increases, the foraging thresholds of successively more corpulent ants would be reached. Corpulence level could also act as a summary cue for the individual's hunger state, the general hunger state of the colony and possibly the individual's experience, making it a simple yet powerful organizational mechanism (Robinson et al. 2009a; Richardson et al. in preparation).
For tasks that do not require physiological specialization, any members of the colony can potentially respond to increased demand. In our study, ants responded to the increased need for brood transport as predicted by a spatial task-encounter model (Tofts & Franks 1992; Tofts 1993), suggesting that any ants that perceive the stimulus can respond adequately. Johnson (2003) proposed that selective pressure for division of labour conflicts with opposing selection for flexible task allocation, leading to a mixed-strategy compromise within social insect colonies. Our data support this view, suggesting that combining a ‘foraging for work’ system for generalized tasks with physiological thresholds for more specialized tasks could provide a social insect colony with a robust system of work organization, flexibly allocating the workforce in response to current needs.
Acknowledgements
We thank A. E. Walsby for advice on weighing gasters, E. A. Langridge for experimental assistance and E. L. Franklin, E. A. Langridge, S. Perez-Espona, T. O. Richardson, A. B. Sendova-Franks and N. Stroeymeyt for useful discussions. N.R.F. and E.J.H.R. acknowledge EPSRC grant EP/D076226/1. O.F. is supported by a Burroughs-Wellcome Career Award at the Scientific Interface.
Appendix
The spatial fidelity (SF) of ant n to region r of chamber 1 (figure 1) was calculated as
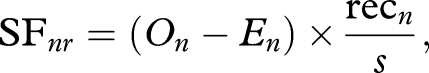 |
where
On = recnr/recn;
recnr = total number of scans in which ant n was recorded in region r;
recn = total number of scans in which ant n was recorded in chamber 1;
p = number of ants in region r in scan i/total number of ants in chamber 1 in scan i; and
s = total number of spatial scans.
 |
For brood care switching, region r = inner end of chamber 1 and s = 3, all scans prior to the addition of the new brood. For foraging switching, region r = outer end of chamber 1 and s = 7, all scans prior to the addition of food.
References
- Backen S. J., Sendova-Franks A. B., Franks N. R.2000Testing the limits of social resilience in ant colonies. Behav. Ecol. Sociobiol. 48, 125–131 (doi:10.1007/s002650000219) [Google Scholar]
- Blanchard G. B., Orledge G. M., Reynolds S. E., Franks N. R.2000Division of labour and seasonality in the ant Leptothorax albipennis: worker corpulence and its influence on behaviour. Anim. Behav. 59, 723–738 (doi:10.1006/anbe.1999.1374) [DOI] [PubMed] [Google Scholar]
- Bonabeau E., Théraulaz G.1999Role and variability of response thresholds in the regulation of division of labor in insect societies. In Information processing in social insects (eds Detrain C., Deneubourg J. L., Pasteels J. M.), pp. 141–163 Basel, Switzerland: Birkhäuser [Google Scholar]
- Bourke A. F. G., Franks N. R.1995Social evolution in ants Monographs in behaviour and ecology Princeton, NJ: Princeton University Press [Google Scholar]
- Calabi P., Traniello J. F. A.1989Behavioral flexibility in age castes of the ant Pheidole dentata. J. Insect Behav. 2, 663–677 (doi:10.1007/BF01065785) [Google Scholar]
- Couvillon M. J., Robinson E. J. H., Atkinson B., Child L., Dent K. R., Ratnieks F. L. W.2008Onslaught of conspecific intruders triggers rapid shifts in guarding behaviour in honey bees (Apis mellifera). Anim. Behav. 76, 1653–1658 (doi:10.1016/j.anbehav.2008.08.002) [Google Scholar]
- Dornhaus A.2008Specialization does not predict individual efficiency in an ant. PLOS Biol. 6, 1–8 (doi:10.1371/journal.pbio.0060285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fénéron R., Durand J. L., Jaisson P.1996Relation between behaviour and physiological maturation in a ponerine ant. Behaviour 133, 791–806 (doi:10.1163/156853996X00477) [Google Scholar]
- Fluri P., Lüscher M., Wille H., Gerig L.1982Changes in weight of the pharyngeal gland and the haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 28, 61–68 (doi:10.1016/0022-1910(82)90023-3) [Google Scholar]
- Franks N. R., Dornhaus A., Best C. S., Jones E. L.2006Decision making by small and large house-hunting ant colonies: one size fits all. Anim. Behav. 72, 611–616 (doi:10.1016/j.anbehav.2005.11.019) [Google Scholar]
- Fresneau D.1984Développement ovarien et statut social chez une fourmi primitive Neoponera obscuricornis Emery (Hym. Formicidae, Ponerinae). Insect. Soc. 31, 387–402 (doi:10.1007/BF02223655) [Google Scholar]
- Gautrais J., Théraulaz G., Deneubourg J. L., Anderson C.2002Emergent polyethism as a consequence of increased colony size in insect societies. J. Theor. Biol. 215, 363–373 (doi:10.1006/jtbi.2001.2506) [DOI] [PubMed] [Google Scholar]
- Gracioli-Vitti L. F., Abdalla F. C., da Cruz-Landim C.2004Characterization of the mandibular glands in different adult types of Scaptotrigona postica Latreille (Hymenoptera: Apidae). Neotrop. Entomol. 33, 703–708 (doi:10.1590/S1519-566X2004000600007) [Google Scholar]
- Huang Z. Y., Otis G. W., Teal P. E. A.1989Nature of brood signal activating the protein-synthesis of hypopharyngeal gland in honey bees, Apis mellifera (Apidae, Hymenoptera). Apidologie 20, 455–464 (doi:10.1051/apido:19890601) [Google Scholar]
- Johnson B. R.2002Reallocation of labor in honeybee colonies during heat stress: the relative roles of task switching and the activation of reserve labor. Behav. Ecol. Sociobiol. 51, 188–196 (doi:10.1007/s00265-001-0419-1) [Google Scholar]
- Johnson B. R.2003Organization of work in the honeybee: a compromise between division of labour and behavioural flexibility. Proc. R. Soc. Lond. B 270, 147–152 (doi:10.1098/rspb.2002.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaud J. P., Passera L., Grimal A., Detrain C., Beugnon G.1992Lipid storage by major workers and starvation resistance in the ant Pheidole pallidula (Hymenoptera, Formicidae). In Biology and evolution of social insects (ed. Billen J.), pp. 153–160 Leuven, Belgium: Leuven University Press [Google Scholar]
- McDonald P., Topoff H.1985Social regulation of behavioral development in the ant, Novomessor albisetosus (Mayr). J. Comp. Psychol. 99, 3–14 (doi:10.1037/0735-7036.99.1.3) [DOI] [PubMed] [Google Scholar]
- Muscedere M. L., Willey T. A., Traniello J. F. A.2009Age and task efficiency in the ant Pheidole dentata: young minor workers are not specialist nurses. Anim. Behav. 77, 911–918 (doi:10.1016/j.anbehav.2008.12.018) [Google Scholar]
- Oster G. F., Wilson E. O.1978Caste and ecology in the social insects Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- Partridge L. W.1993Facets of the ecology, behaviour and evolution of ants. PhD thesis, University of Bath, Bath, UK, pp. 156–178 [Google Scholar]
- Plowright R. C., Plowright C. M. S.1988Elitism in social insects: a positive feedback model. In Interindividual behavioral variability in social insects (ed. Jeanne R. L.), pp. 419–431 Boulder, CO: Westview [Google Scholar]
- Porter S. D., Jorgensen C. D.1981Foragers of the harvester ant, Pogonomyrmex owyheei—a disposable caste? Behav. Ecol. Sociobiol. 9, 247–256 (doi:10.1007/BF00299879) [Google Scholar]
- Ravary F., Lecoutey E., Kaminski G., Châline N., Jaisson P.2007Individual experience alone can generate lasting division of labor in ants. Curr. Biol. 17, 1308–1312 (doi:10.1016/j.cub.2007.06.047) [DOI] [PubMed] [Google Scholar]
- Richardson T. O., Robinson E. J. H., Christensen K., Jensen H. J., Franks N. R., Sendova-Franks A. B.In preparation Record dynamics in ants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. J. H.2009Physiology as a caste-defining feature. Insect. Soc. 56, 1–6 (doi:10.1007/s00040-008-0994-5) [Google Scholar]
- Robinson E. J. H., Richardson T. O., Sendova-Franks A. B., Feinerman O., Franks N. R.2009aRadio-tagging reveals the roles of corpulence, experience and social information in ant decision making. Behav. Ecol. Sociobiol. 63, 627–636 (doi:10.1007/s00265-008-0696-z) [Google Scholar]
- Robinson E. J. H., Smith F. D., Sullivan K. M. E., Franks N. R.2009bDo ants make direct comparisons? Proc. R. Soc. B 276, 2635–2641 (doi:10.1098/rspb.2009.0350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G. E.1987aModulation of alarm pheromone perception in the honey bee—evidence for division of labor based on hormonally regulated response thresholds. J. Comp. Physiol. A 160, 613–619 (doi:10.1007/BF00611934) [Google Scholar]
- Robinson G. E.1987bRegulation of honey-bee age polyethism by juvenile hormone. Behav. Ecol. Sociobiol. 20, 329–338 (doi:10.1007/BF00300679) [Google Scholar]
- Robinson G. E.1992Regulation of division of labor in insect societies. Ann. Rev. Entomol. 37, 637–665 (doi:10.1146/annurev.en.37.010192.003225) [DOI] [PubMed] [Google Scholar]
- Robinson G. E., Page R. E., Huang Z. Y.1994Temporal polyethism in social insects is a developmental process. Anim. Behav. 48, 467–469 (doi:10.1006/anbe.1994.1260) [Google Scholar]
- Sendova-Franks A. B., Franks N. R.1994Social resilience in individual worker ants and its role in division-of-labor. Proc. R. Soc. Lond. B 256, 305–309 (doi:10.1098/rspb.1994.0085) [Google Scholar]
- Smith A.1776An inquiry into the nature and causes of the wealth of nations London, UK: W. Strahan and T. Cadell [Google Scholar]
- Théraulaz G., Bonabeau E., Deneubourg J. L.1998Response threshold reinforcement and division of labour in insect societies. Proc. R. Soc. Lond. B 265, 327–332 (doi:10.1098/rspb.1998.0299) [Google Scholar]
- Tofts C.1993Algorithms for task allocation in ants (a study of temporal polyethism theory). Bull. Math. Biol. 55, 891–918 [Google Scholar]
- Tofts C., Franks N. R.1992Doing the right thing: ants, honeybees, and naked mole-rats. Trends Ecol. Evol. 7, 346–349 (doi:10.1016/0169-5347(92)90128-X) [DOI] [PubMed] [Google Scholar]
- Toth A. L., Robinson G. E.2005Worker nutrition and division of labour in honeybees. Anim. Behav. 69, 427–435 (doi:10.1016/j.anbehav.2004.03.017) [Google Scholar]
- Tripet F., Nonacs P.2004Foraging for work and age-based polyethism: the roles of age and experience on task choice in ants. Ethology 110, 863–877 (doi:10.1111/j.1439-0310.2004.01023.x) [Google Scholar]