Abstract
Weaponry is ubiquitous in male ungulates and is driven by intrasexual selection, but the mystery surrounding its sporadic presence in females remains unsolved. Female horns are often smaller and shaped differently to male horns, suggesting a different function; indeed, hypotheses explaining the presence of female horns include competition for food, male mollification and defence against predators. Here we use comparative phylogenetic analyses to show that females are significantly more likely to bear horns in bovids that are conspicuous due to large body size and living in open habitats than inconspicuous species living in closed habitats or that are small. An inability to rely on crypsis or take refuge in deep vegetation has apparently driven the evolution of horns for defence against predators in female bovids, a finding supported by many field observations. Typically, exceptions are small species where females are territorial (e.g. duikers) and use horns in intrasexual contests. Furthermore, we suggest that conspicuousness and territoriality hypotheses may explain other instances of female cranial weaponry (i.e. antlers and ossicones) in other horned ruminants. Our phylogenetic reconstruction indicates that the primary function of horns in females is linked to antipredator defence in most clades, but occasionally to intrasexual competition in others.
Keywords: horns, bovidae, antlers, weaponry, territoriality, antipredator
1. Introduction
In most artiodactyl families, males of all species have horns or antlers that are widely thought to have evolved for intrasexual combat over territories or mates (Geist 1966; Clutton-Brock 1982; Janis 1982). In contrast, females have antlers in only one species of cervid, and the presence of horns is highly variable across female bovid species, a problem that vexed even Darwin (1874). There have been several attempts to explain the evolutionary causes of horn production in female bovids. Roberts (1996) suggested that horns in females may have evolved due to competition over food resources, providing evidence that presence in females is correlated with increased group size. Estes (1991) proposed that female horns are a form of mimicry of males to mollify male aggression targeted at sons, citing a general trend where females of species that form mixed-sex groups are more likely to bear horns than females of species where the sexes remain separate. Packer (1983) suggested that female horns serve in defence against predators, showing that females of heavier species are more likely to bear horns. Finally, Kiltie (1985) proposed that in most species, female horns are non-adaptive and persist due to pleiotropy, and a genetic shift to a hornless condition may lead to sterility.
At present, it is known that horns in female bovids are correlated with body weight (Packer 1983; Kitchener 1985), that strength might be important in antipredator defence, and that female horns are straight and backward-facing with tips facing the midline of the skull (Lundrigan 1996; Caro et al. 2003), appearing specialized for stabbing predators rather than parrying blows from an opponent's horn (Packer 1983). Moreover, there are numerous accounts of female bovids defending themselves or offspring against predatory attack using their horns as weapons (Kruuk 1972; Sinclair 1977; Mech & Nelson 1990; Gese 1999). Yet, despite these observations, attempts to explain varied presence of female horns between taxa have continued to flounder owing to lack of clear predictions, failure to pit alternative hypotheses against each other, and absence of phylogenetic controls.
Antipredator defences in animals fall into two exclusive categories: (i) crypsis or (ii) defence against predatory attack, which can be active or passive as in the case of aposematism (Poulton 1890). In birds and mammals, including bovids (Jarman 1974), crypsis is enhanced in small species that can hide even in low grass (Caro 2005), and in species living in forested habitat where vegetation makes hiding effective (Stoner et al. 2003; Bro-Jørgensen 2008). As such, we propose that it is not body size per se that primarily influences the need for defensive weaponry (Packer 1983), but rather that conspicuous species that are visible to predators from long distances will benefit more from weaponry because they are more likely to be noticed and hunted than short or forest-living species. After all, heavy species living in dense forests (e.g. tragelaphines) are sufficiently concealed and can probably still rely on crypsis to avoid predation. Therefore, a ‘conspicuousness’ hypothesis for the presence of horns in females predicts that if horns function for antipredator defence, then conspicuous species (large species living in open habitats where they cannot hide from predators) would benefit from using morphological structures in active defence, whereas inconspicuous species (small species or species living in exclusively closed habitats) should not invest in developing energetically costly weaponry. In addition, we propose that in some species, female horns might be favoured due to female intrasexual combat over territories (Clutton-Brock 2009). Although female territoriality is unusual in bovids (26/117 spp.), an alternative ‘territoriality’ hypothesis would predict that selection would favour horns in species where females mark and defend territories against conspecifics. Roberts (1996) stressed competition for food in large groups in his ‘female competition hypothesis’ but did discuss the role of female territoriality per se. He did not test female territoriality explicitly, and his reliance on the positive correlation between group size and horn presence actually opposes the prediction that species showing female territory defence should be more likely to bear horns because these species do not form large groups.
2. Material and methods
We categorized the presence or absence of horns in females in 117 species of bovid (Caro et al. 2003). Categorizations did not take into account intraspecific variation in the presence of horns in females (i.e. if any female members of the species possessed horns, they were scored as present). For example, females in certain but not all populations of Oreotragus oreotragus express horns, so we scored horns as present in this species.
In order to test the ability of different hypotheses to predict the evolution of horns in female bovids, we conducted different phylogenetically corrected multiple regressions using forward stepwise model selection procedures (see below). These models allow different predictor variables that relate to a specific hypothesis regarding the evolution of horns to compete against each other for inclusion in the final model. We will outline each model selection exercise in order as they build upon each other.
(a). Body size
Packer (1983) predicted that larger species are better able to defend themselves against predators and would benefit more from having defensive weaponry than smaller species that could rely on crypsis and rapid flight from predators. As in previous studies (Kiltie 1985; Roberts 1996), we tested for the effect of two different measures of body size, shoulder height and body mass, on the evolution of horns. We distinguish between these two measures in our analyses because, in addition to their impacts on defensive capability, shoulder height has a greater impact on conspicuousness because taller species would probably be more conspicuous to predators from longer distances, whereas body mass has a greater impact on reducing escape speed. Following Roberts (1996), for each species, we recorded the midpoint of the range of shoulder heights and the minimum body mass reported by Nowak (1999). Both shoulder height and body weight were logarithmically transformed to achieve normality.
(b). Openness of habitat
Since species that live in more open habitats with less protective cover are likely to be more conspicuous to predators, we created an openness score for each species by editing previously scored (Caro et al. 2004) habitat categories using Nowak (1999) to include only the primary habitat types (limiting these to only one to two habitat types per species) to give greater weight to those habitats in which the species spends the most time. We created openness scores for each habitat type (dense forest = swamp = 0.001, light forest = 0.1, scrubland = 0.2, grassland = 0.75, rocky = 0.9 and tundra = desert = 1), which were formulated based on how much relative cover each habitat provided and from how far away an animal could be seen in each environment. Animals are probably only detectable from very short distances in dense forest and heavy swamps, therefore they received very low openness scores (0.001); animals are likely much easier to detect at long distances in desert and tundra habitats (both 1).
(c). Territoriality
Part of Roberts' (1996) female competition hypothesis suggested that females that compete with same sex conspecifics for access to territories would benefit more from horns than non-territorial females; however, he did not test this aspect of the hypothesis. Roberts' tests were limited to the food competition aspect of female competition (see below). To test for the effect of female territoriality on horn evolution, we compiled published (Nowak 1999; Caro et al. 2003) and online (IUCN 2009; UMMZ 2009) data in the presence or absence of female territoriality in bovid species. Females were scored as ‘territorial’ if they actively participated in territorial marking and defence against conspecifics; male territoriality was disregarded.
(d). Group size
The second part of Roberts' (1996) female competition hypothesis suggested that females living in larger groups would be subjected to a greater degree of intraspecific agonism and competition over access to food resources, and he found a significant correlation between group size and presence of horns in females. To pit this hypothesis against the others, we categorized group size for each species into a 1–4 scale: 1 = ‘solitary only’, 2 = ‘solitary and intermediate-sized groups’, 3 = ‘intermediate-sized groups only’, and 4 = ‘intermediate-sized and large groups’ based on published data (Caro et al. 2004).
(i). Analysis I
We used a published Cetartiodactyla supertree (Price et al. 2005) as a base tree, resolved most polytomies using Marcot (2007), and resolved all other taxon-specific polytomies using other sources (Kuznetsova & Kholodova 2003; Pitra et al. 2004; Pidancier et al. 2006; Mona et al. 2007; Huffman 2009). We determined that the tracing of female horn evolution on this composite tree has strong phylogenetic signal (Blomberg et al. 2003; p < 0.001) using PDAP 6.0 software (Garland et al. 1993); therefore, related species tend to resemble each other in their tendency for females to have horns. We pitted hypotheses based on body size, habitat openness and intraspecific competition against each other. We calculated standardized independent contrasts (FIC: Felsenstein 1985) for each of the above factors (territoriality, shoulder height, body mass, habitat openness and group size) using the PDTREE module (Midford et al. 2005) in Mesquite 2.5 (Maddison & Maddison 2008) to account for shared ancestry. In order to test the effect of each factor on horn presence simultaneously, we tested these contrast variables using multiple linear regression models (forced through the origin: Garland et al. 1992) in SPSS 17.0. Preliminary analyses revealed significant bivariate correlations between all predictor variables (all p < 0.02), except between body mass and territoriality (p = 0.115). To avoid collinearity issues and to determine which of these predictors best explained the variance in the presence of horns in females, we used forward stepwise model selection procedures: all five factors were simultaneously tested against each other with the most significant factor entering the model first. The cutoff for entry into the model was set at p = 0.1, and factors were entered until only those with probabilities less than this value were in the model. Statistical results given for non-significant factors excluded from the model are the results for that factor if it were to be included in the model at the next step.
(e). Conspicuousness
We hypothesized that species that are taller and live in open habitats are more exposed, visible from longer distances, and would be more likely to benefit from the presence of horns for defence against predators. While analysis I tested shoulder height and habitat openness separately, we sought out a composite measure of conspicuousness that simultaneously accounted for continuity in shoulder height and weighted openness of primary habitats more heavily. Therefore, we compiled an ‘exposure’ metric by multiplying a species’ log10(shoulder height) by the mean openness (0–1) of its primary habitat(s). Shoulder height was used instead of body mass because shoulder height likely has a greater impact on visibility than mass. The multiplication of openness by height allows tall species living in dense forests (e.g. bongos, Tragelaphus eurycerus) to score very low on the exposure scale, moderately sized species living in deserts to retain intermediate scores (e.g. gazelles), and tall species living in completely open habitats (e.g. muskoxen: Ovibos moschatus) to score very high.
(i). Analysis II
We ran a second forward stepwise multiple regression identical to that described in analysis I except that a variable containing FIC values from the exposure variable was included in the list of candidate predictor variables.
(ii). Analysis III
While the raw continuous form of ‘exposure’ was used in the formal phylogenetically corrected analyses I and II, we divided species into ‘exposed’ and ‘not exposed’ categories to summarize the effects of exposure at the species level. There was a clear breakpoint in the distribution of ‘exposure’ at a score of 1.0, so species with scores less than 1.0 were classified as ‘not exposed’, and those with scores greater than 1.0 were classified as ‘exposed’. The effects of these uncorrected categorical exposure and territoriality variables on horn presence in females were tested with simple χ2 tests using SPSS 17.0. We tested exposure and territoriality against horns separately. We then combined the two predictors into a single variable (‘exposed OR territorial’ versus ‘not exposed and non-territorial’) and tested it against horns.
3. Results
(a). Analysis I
First, we pitted shoulder height, body mass, openness of habitat, female territoriality, and group size against each other to test their relative ability to explain horn presence in females. We found that openness of habitat had the greatest effect on the presence of horns (t = 4.592, n = 116, p < 0.001, r = 0.397), where females of species living in more open environments were more likely to bear horns. We also found that territoriality had a significant effect on the presence of horns in females (t = 2.930, n = 116, p = 0.004, r = 0.266) in which females that actively mark or defend territories have horns. Females of heavier species were also more likely to have horns (t = 2.218, n = 116, p = 0.029, r = 0.204). With body mass included in the final model, shoulder height did not have a significant effect on female horn presence (t = − 0.164, n = 116, p = 0.870, r = − 0.015). Similarly, group size had no effect on the presence of horns in females (t = 1.255, n = 116, p = 0.212, r = 0.118) and was left out of the final regression model (F3,113 = 11.103, p < 0.001, adjusted R2 = 0.207).
(b). Analysis II
When the exposure metric was allowed to compete with shoulder height, body mass, and openness in the model to further test the conspicuousness hypothesis, we found that exposure outcompeted openness as a predictor of horns in females: females of highly exposed species were much more likely to have horns than less-exposed species (t = 4.604, n = 116, p < 0.001, r = 0.397). Because exposure was included in the model, habitat openness was not included in the final model (t = −0.037, n = 116, p = 0.970, r = − 0.004). Similar to the first test, species in which females defend territories and large species were also more likely to bear horns (territoriality: t = 2.958, n = 116, p = 0.004, r = 0.268; body mass: t = 2.108, n = 116, p = 0.037, r = 0.194). Similar to the first analysis, the final model (F3,113 = 11.144, p < 0.001, adjusted R2 = 0.208) excluded shoulder height and group size (shoulder height: t = −0.184, n = 116, p = 0.854, r = −0.017; group size: t = 1.282, n = 116, p = 0.203, r = 0.120).
(c). Analysis III
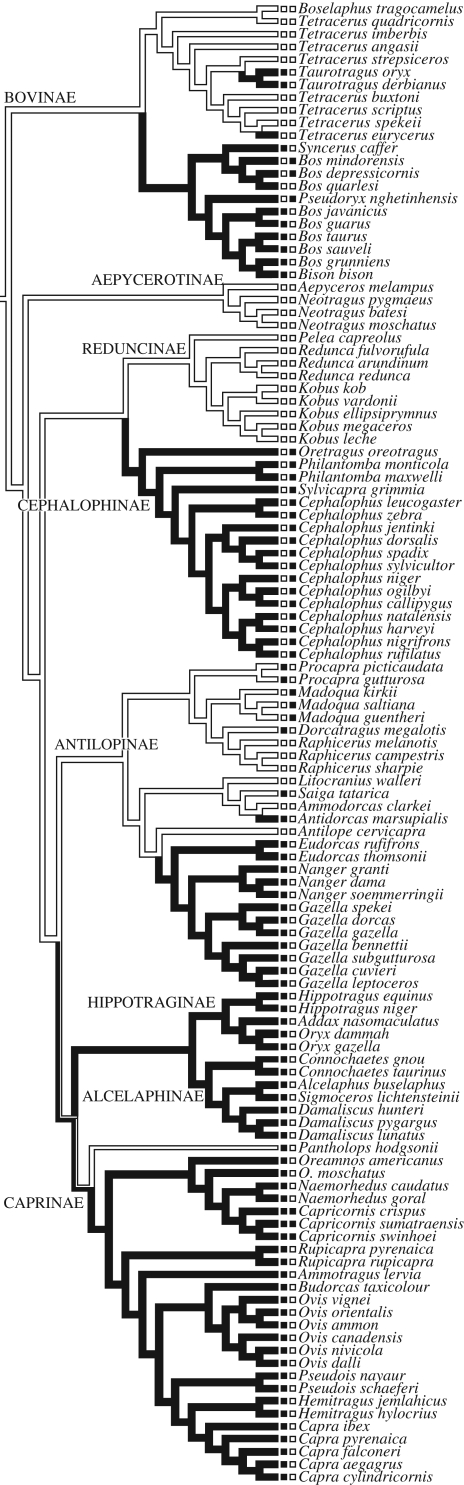
Using the categorical version of the exposure metric, we found that 92.3 per cent (60/65) of exposed species had horns (figure 1; χ2 = 32.919, d.f. = 1, p < 0.001). Similarly, 88.4 per cent (23/26) of species where females are territorial also exhibit horns in females (figure 1; χ2 = 5.384, d.f. = 1, p = 0.020). Females of the remaining three territorial hornless species (Madoqua spp.) mark territories but do not defend them. Importantly, when the territoriality and conspicuousness hypotheses were considered simultaneously, 80 out of the 82 species where female carry horns could be accounted for (figure 1; χ2 = 73.430, d.f. = 1, p < 0.001, table 1).
Figure 1.
(Opposite.) Phylogenetic tree of the Bovidae. Branch coloration signifies the presence (black) or absence (white) of horns in females after reconstruction based on maximum parsimony. Partial black coloration indicating presence or absence is parsimoniously equivocal. The row of boxes on the left nearest the tree tips denotes whether the species is ‘exposed’ (black) or ‘not exposed’ (white) using the exposure measure as indicated in analysis III. The row of boxes on the right nearest the species names denotes whether females of the species are territorial (black), or non-territorial (white). Subfamilies are labelled on the branches (n.b., Oreotragus and Neotragus are also traditionally included in Subfamily Antilopinae, but current phylogenetic evidence suggests that they occupy taxonomic positions outside this clade; Huffman 2009).
Table 1.
Number of exposed and territorial species where female bovids bear horns (n = 82).
| territoriality |
||
|---|---|---|
| exposure | territorial | non-territorial |
| exposed | 3 | 57 |
| not exposed | 20 | 2a |
aT. eurycerus, B. quarlesi (see text for explanations). Note that species where females lack horns are not included in this table; statistical analyses included all 117 species.
4. Discussion
Conspicuousness and territoriality hypotheses explained nearly every instance of horns in female bovids (80 of 82 species; table 1). In all models, measures of conspicuousness (openness and exposure) explained the greatest amount of variance in the presence of horns, followed by female territoriality. That body mass was also a significant factor in the evolution of horns suggests that heavier animals are less able to rely on swift flight to escape predators and, as Packer (1983) suggested, may be better able to defend themselves against predators. Group size was less effective at explaining the variance in female horn presence and its significant role in past studies may be a byproduct of its significant correlation with the other predictors in the model. Figure 1 shows that horns probably evolved several times in female bovids for defence (bovines, antilopines, caprines, hippotragines and alcelaphines) and once for intrasexual combat (cephalophines). Interestingly, in female territorial species where only certain populations display female horns, those populations that do display horns tend to have higher levels of female territoriality (e.g. klipspringers), suggesting that differences among populations in species ecology can have significant impact on natural selection favouring the evolution of horns in females; although we did not test this explicitly.
Only two bovid species (see above) fail to conform to our predictions that female horns evolved in conspicuous species as weapons against predators, or in territorial species for use in conspecific aggression (table 1). Female bongos (T. eurycerus) are non-territorial, large, and live in dense forest, but use their horns in aggressive interactions in female groups to establish a rank order (Estes 1991) with smaller-horned cows tending to defer to individuals with longer horns (Kingdon 1982). Little is known about female mountain anoas (Bubalus quarlesi), which bear horns, but the species appears to be monogamous (Massicot 2005), and females may indeed defend territories against conspecifics similar to the other Bubalus spp.
The most parsimonious reconstruction of the evolution of female horns based on our hypothesized phylogenetic tree (figure 1) indicates one potential case of horn loss. Female Tibetan antelope or ‘chiru’ (Pantholops hodgsonii) lack horns, have large bodies, live in open deserts and grasslands, and would appear to be highly exposed to predators in this landscape. However, chiru are unique in that they dig shallow (approx. 30 cm) depressions in the dirt to lie down in and hide from predators, probably reducing the degree to which they are exposed to predators from afar. This novel means of reducing exposure may have tipped the balance in favour of crypsis and relaxed the need to evolve or maintain weaponry in females of this species.
We believe that our arguments are also relevant to other female ruminants; although, they cannot be easily tested systematically due to small sample sizes. Three admittedly speculative points are relevant and may provide ideas for others to investigate. (i) While 11 species of cervid would be classified as ‘exposed’ based on the bovid criterion (exposure > 1.0), the necessity of growing antlers each year may increase the cost of weaponry and shift the threshold of conspicuousness needed for the costs to outweigh the protective benefits of antlers in females. Indeed, females of only one cervid species, reindeer (Rangifer tarandus) have antlers, and females keep their antlers longer after the rut than males, allowing them to achieve dominance over males and gain access to valuable feeding sites for themselves, their unborn offspring, and their yearling calves (Espmark 1964). (ii) Among the giraffids, where females of both species have horn-like ossicones, giraffes (Giraffa camelopardalis) are conspicuous on the plains (T. Caro 2008, personal observation), use strong kicks to fend-off predators, but it is not known if ossicones are used as well. Okapi (Okapia johnstoni) are inconspicuous (large body, exclusively closed habitat), but females live alone in exclusive home ranges (Hart 1992) and may therefore, defend these territories against other females using weaponry. (iii) Antilocaprids (pronghorn: Antilocapra americana) currently lack any natural non-human predators, are highly conspicuous, live exclusively in open environments, are large and both sexes have pronghorns; although, female ornaments are very short and stubby. Many aspects of pronghorn antipredator behaviour are geared to predators that have recently gone extinct on the North American continent (Byers 1999), and this may apply to female horns too. Note that the other ruminant groups, moschids (musk deer) and tragulids (chevrotains), are all inconspicuous (all live in closed habitats and are small), and both sexes lack antlers entirely in both groups.
If horns are an antipredator defence mechanism, there should be less need for their bearers to possess cryptic pelage. While the sample is too small to run meaningful formal analyses, of the nine bovids exhibiting dappled or striped coloration (T. imberbis, T. angasii, T. strepsiceros, T. scriptus, T. spekeii, T. eurycerus, Taurotragus derbanius, Cephalophus zebra, C. sylvicultor), the first five are indeed hornless. Females of the last four are horned although the function of horns in three of them is probably for intrasexual female combat, leaving Taurotragus derbianus as the bovid anomaly. This suggests that female horns and cryptic body pelage might be evolutionarily dissociated in bovids. In light of these speculations, the relationship between weaponry and crypsis deserves further attention.
We maintain that simple understanding of basic antipredator and social strategies can explain the presence and absence of defensive weaponry in this taxon. Conspicuous, open country species that are tall or large in size cannot hide, may have slower escape speeds, and are driven to carry horns to defend themselves and offspring; in addition, their large size must be a benefit in self-defence. Conversely, inconspicuous, small or closed habitat species are cryptic and relieved of the burden of evolving antipredator weaponry. Nearly all other cases of horns are found in territorial taxa where females use horns to defend their territories against conspecific females. Our results suggest that competition between females over food in large groups has had little effect on the evolution of horns in females. Weaponry in female bovids is thus principally driven by natural selection, whereas weaponry in males in all taxa and in females in certain taxa is driven by intrasexual selection, both processes driving evolution of the same morphological structure in one taxonomic group.
Acknowledgements
We thank J. Andersen, P. Bergmann, J. M. Gaillard, D. Collar, C. Stoner and B. Taft for statistical input; the Biology Computer Resource Center at the University of Massachusetts Amherst for computing resources; and members of the Behaviour and Morphology (BAM) group at the University of Massachusetts Amherst and anonymous reviewers for comments on previous versions of this manuscript.
References
- Blomberg S. P., Garland T., Jr, Ives A. R.2003Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745 [DOI] [PubMed] [Google Scholar]
- Bro-Jørgensen J.2008Dense habitats selecting for small body size: a comparative study on bovids. Oikos 117, 729–737 (doi:10.1111/j.0030-1299.2008.16069.x) [Google Scholar]
- Byers J. A.1999American pronghorn: social adaptations and the ghosts of predators past Chicago, IL: University of Chicago Press [Google Scholar]
- Caro T. M.2005Antipredator defenses in birds and mammals. Interspecific interactions Chicago, IL: University of Chicago Press [Google Scholar]
- Caro T. M., Graham C. M., Stoner C. J., Flores M. M.2003Correlates of horn and antler shape in bovids and cervids. Behav. Ecol. Sociobiol. 55, 32–41 (doi:10.1007/s00265-003-0672-6) [Google Scholar]
- Caro T. M., Graham C. M., Stoner C. J., Vargas J. K.2004Adaptive significance of antipredator behaviour in artiodactyls. Anim. Behav. 67, 205–228 (doi:10.1016/j.anbehav.2002.12.007) [Google Scholar]
- Clutton-Brock T. H.1982The functions of antlers. Behaviour 79, 108–125 (doi:10.1163/156853982X00201) [Google Scholar]
- Clutton-Brock T.2009Sexual selection in females. Anim. Behav. 77, 3–11 [Google Scholar]
- Darwin C.1874The descent of man, and selection in relation to sex London, UK: John Murray [Google Scholar]
- Espmark Y.1964Studies in dominance–subordination relationship in a group of semi-domestic reindeer (Rangifer tarandus L.). Anim. Behav. 12, 420–426 (doi:10.1016/0003-3472(64)90061-2) [Google Scholar]
- Estes R. D.1991The behavior guide to African mammals Berkeley, CA: University of California Press [Google Scholar]
- Felsenstein J.1985Phylogenies and the comparative method. Am. Nat. 125, 1 (doi:10.1086/284325) [DOI] [PubMed] [Google Scholar]
- Garland T., Jr, Harvey P. H., Ives A. R.1992Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32 [Google Scholar]
- Garland T., Jr, Dickerman A. W., Janis C. M., Jason A. J.1993Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 42, 265–292 [Google Scholar]
- Geist V.1966The evolution of horn-like organs. Behaviour 27, 175–214 [Google Scholar]
- Gese E. M.1999Threat of predation: do ungulates behave aggressively towards different members of a coyote pack? Can. J. Zool.-Rev. Can. Zool. 77, 499–503 (doi:10.1139/cjz-77-3-499) [Google Scholar]
- Hart J. A.1992Forage selection, forage availability and use of space by okapi (Okapia johnstoni) a rainforest giraffe in Zaire. Ongulés/Ungulates 91, 217–221 [Google Scholar]
- Huffman B. 2009. See www.ultimateungulate.com . Accessed April 2009.
- IUCN. The IUCN red list of threatened species. 2009. See www.iucnredlist.org .
- Janis C.1982Evolution of horns in ungulates: ecology and paleoecology. Biol. Rev. Cambr. Phil. Soc. 57, 261–317 (doi:10.1111/j.1469-185X.1982.tb00370.x) [Google Scholar]
- Jarman P. J.1974The social organization of antelope in relation to their ecology. Behaviour 48, 215–267 (doi:10.1163/156853974X00345) [Google Scholar]
- Kiltie R. A.1985Evolution and function of horns and hornlike organs in female ungulates. Biol. J. Linn. Soc. 24, 299–320 (doi:10.1111/j.1095-8312.1985.tb00377.x) [Google Scholar]
- Kingdon J.1982East African mammals, vol. 3C, D London, UK: Academic Press [Google Scholar]
- Kitchener A.1985The effect of behavior and body weight on the mechanical design of horns. J. Zool. 205, 191–203 [Google Scholar]
- Kruuk H.1972The spotted hyena Chicago, IL: University of Chicago Press [Google Scholar]
- Kuznetsova M. V., Kholodova M. V.2003Revision of phylogenetic relationships in the Antilopinae subfamily on the basis of the mitochondrial rRNA and β-spectrin nuclear gene sequences. Doklady Biol. Sci. 391, 333–336 (doi:10.1023/A:1025102617714) [DOI] [PubMed] [Google Scholar]
- Lundrigan B.1996Morphology of horns and fighting behavior in the family bovidae. J. Mammal. 77, 462–475 (doi:10.2307/1382822) [Google Scholar]
- Maddison W. P., Maddison D. R.2008Mesquite: a modular system for evolutionary analysis. See www.mesquiteproject.org [Google Scholar]
- Marcot J. D.2007Molecular phylogeny of terrestrial artiodactyls. In The evolution of artiodactyls (eds Prothero D. R., Foss S. E.), pp. 4–18 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Massicot P. Animal info—mountain anoa. 2005. See www.animalinfo.org/species/artiperi/anoaquar.htm . Accessed 14 January 2009.
- Mech L. D., Nelson M. E.1990Evidence of prey-caused mortality in three wolves. Am. Midl. Nat. 123, 207–208 (doi:10.2307/2425775) [Google Scholar]
- Midford P. E., Garland T., Jr, Maddison W. P.2005PDAP package of Mesquite. See www.mesquiteproject.org/pdap_mesquite/ [Google Scholar]
- Mona S., Randi E., Tommaseo-Ponzetta M.2007Evolutionary history of the genus Sus inferred from cytochrome b sequences. Mol. Phylogenet. Evol. 45, 757–762 (doi:10.1016/j.ympev.2007.05.025) [DOI] [PubMed] [Google Scholar]
- Nowak R. M.1999Walker's mammals of the world Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Packer C.1983Sexual dimorphism: the horns of African antelopes. Science 221, 1191–1193 (doi:10.1126/science.221.4616.1191) [DOI] [PubMed] [Google Scholar]
- Pidancier N., Jordan S., Luikart G., Taberlet P.2006Evolutionary history of the genus Capra (Mammalia, Artiodactyla): discordance between mitochondrial DNA and Y-chromosome phylogenies. Mol. Phylogenet. Evol. 40, 739–749 (doi:10.1016/j.ympev.2006.04.002) [DOI] [PubMed] [Google Scholar]
- Pitra C., Fickel J., Meijaard E., Groves P. C.2004Evolution and phylogeny of old world deer. Mol. Phylogenet. Evol. 33, 880–895 (doi:10.1016/j.ympev.2004.07.013) [DOI] [PubMed] [Google Scholar]
- Poulton E. B.1890The colours of animals: their meaning and use especially considered in the case of insects The International Scientific Series London, UK: Kegan Paul, Trench Trübner, & Co. Ltd [Google Scholar]
- Price S. A., Bininda-Emonds O. R. P., Gittleman A. L.2005A complete phylogeny of the whales, dolphins and even-toed hoofed mammals (Cetartiodactyla). Biol. Rev. 80, 445–473 (doi:10.1017/S1464793105006743) [DOI] [PubMed] [Google Scholar]
- Roberts S. C.1996The evolution of hornedness in female ruminants. Behaviour 133, 399–442 (doi:10.1163/156853996X00521) [Google Scholar]
- Sinclair A. R. E.1977The African buffalo Chicago, IL: University of Chicago Press [Google Scholar]
- Stoner C. J., Caro T. M., Graham C. M.2003Ecological and behavioral correlates of coloration in artiodactyls: systematic analyses of conventional hypotheses. Behav. Ecol. 14, 823–840 (doi:10.1093/beheco/arg072) [Google Scholar]
- UMMZ (University of Michigan Museum of Zoology) Animal diversity web. 2009. See www.animaldiversity.ummz.umich.edu/site/index.html .