Abstract
Social learning, defined as learning from other individuals, has had dramatic effects on some species, including humans, in whom it has generated a rich culture. As a first step in examining the evolution of and mechanisms underlying social learning in insects, we tested for social learning in fruitflies (Drosophila melanogaster). Focal females (observers) that experienced novel food together with mated females (models), who had laid eggs on that food, subsequently exhibited a stronger preference for laying eggs on that food over another novel food compared with focal females that experienced the food alone. We observed no social learning, however, when observers experienced food with potentially more ambiguous social information provided by the presence of either virgin models or aggregation pheromone. This first documentation of social learning about egg-laying substrates in fruitflies builds on recent data indicating intricate use of social information by fruitflies and opens up exciting avenues for research on the evolution and neurogenetics of social learning using biology's major model system.
Keywords: social learning, fruitflies, Drosophila melanogaster, social behaviour
1. Introduction
Social learning, defined as the acquisition of novel information from other individuals, has had important effects on a variety of species including humans (Heyes & Galef 1996). Social learning has been well studied in social Hymenoptera (Dukas in press b; von Frisch 1967; Franks & Richardson 2006; Leadbeater & Chittka 2007) and can enhance colony fitness in honeybees (Apis mellifera) (Sherman & Visscher 2002). Although social learning may have played an important role in the ecology and evolution of insects, neither the evolution of social learning nor its neurogenetic mechanisms have been closely examined.
Many animals exhibit a variety of socially influenced behaviours that could affect their learning (Galef 1976; Galef & Giraldeau 2001). Here, we restrict our discussion of social learning to cases where an individual (observer) acquires new information through interaction with either another individual (model) or cues left by that individual. The new information learned may involve individuals other than the model, other biotic entities (e.g. food, predators or competitors), or physical factors (e.g. shelter or nutrients). Hence, our critical test for social learning involves examining whether focal individuals show higher learning scores when novel information is presented with a model than alone.
Well-replicated data indicating social learning exist only for vertebrates and social insects (but see suggestive data for octopus (Octopus vulgaris) and crickets (Nemobius sylvestris) (Fiorito & Scotto 1992; Coolen et al. 2005)). A variety of non-social insects, however, have life histories that could promote the evolution of social learning (Dukas in press b; Dukas & Simpson 2009). Many non-social insects live in aggregations and some taxa even rely on pheromones for recruitment to such aggregations (Prokopy & Roitberg 2001; Wertheim et al. 2005; Costa 2006). The co-occurrence in aggregations of animals with distinct experiences could allow for inexperienced individuals to gain important information from experienced conspecifics via social learning. Under such circumstances, learning from other individuals could enhance fitness because it is often faster than individual learning, it circumvents costly errors associated with inexperience and it could enable learning of otherwise inaccessible information (Galef 1976; Dukas & Simpson 2009).
The most widely known insect with an aggregation pheromone is the fruitfly, Drosophila melanogaster, in which both sexes exhibit long-distance attraction to a male-derived pheromone that is transferred to females during copulation and emitted by recently mated females (Bartelt et al. 1985; Ejima et al. 2007). Fruitflies exhibit robust individual learning, which has been subjected to detailed neurogenetic analyses (Quinn et al. 1974; Tully 1996; Keene & Waddell 2007). Fruitflies also respond to social experience by altering their circadian clock and pheromonal expression (Levine et al. 2002; Kent et al. 2008; Krupp et al. 2008). There are also recent conflicting reports about mate choice copying in fruitflies (Auld et al. 2009; Mery et al. 2009).
Because fruitflies have been employed as a model system for research on the neurogenetics of both learning and social behaviour, they seem a highly attractive species for examining the evolution and neurogenetics of social learning. We commenced a research programme addressing this issue by testing whether inexperienced female fruitflies (focals) exhibit a stronger preference for a food type previously encountered with experienced, mated conspecifics (models) than food encountered alone. Further experiments examined the influence of the aggregation pheromone and models' mating status on focals' social learning.
2. Material and methods
(a). General
We obtained Canton-S flies from J. Levine's laboratory (University of Toronto Mississauga Campus, Ontario, Canada) and kept them in 20 × 20 × 35 cm population cages containing a total of a few thousand individuals inside an environmental chamber at 25°C and 70 per cent relative humidity, on a 12 : 12 h light : dark cycle, with lights on at 10.00. Each population cage had two standard 240 ml food bottles each containing 50 ml of standard fly medium made of corn meal, glucose, yeast, sucrose, agar and methyl paraben. The flies used in the experiment were developed at a low density in food bottles containing about 200 larvae. We collected and sexed flies within 8 h of eclosion and placed them in groups of 20 in single-sex vials with food made of sucrose (20 g l−1), agar (10 g l−1) and water with a sprinkle of live yeast added on top. All focal females were trained and tested when they were 4 days post-eclosion.
The flavoured food used in the experiments consisted of water, agar (10 g l−1), sucrose (20 g l−1) and either amyl acetate (AA, 0.9 ml l−1) or benzaldehyde (BA, 0.09 ml l−1). These chemical concentrations yielded equal food preference in previous experiments (A. Dunlap, unpublished data). To increase egg visibility, we added six drops of green and blue commercial food colouring to the AA and BA foods, respectively. Finally, to increase egg laying, we also added 0.1 ml of a live yeast suspension (30 g of yeast and six drops of red food colouring per 1 l of warm water) on top of each food dish.
We evaluated the effect of aggregation pheromone on social learning using cis-vaccenyl acetate (cVA) (99% pure, PheroBank, Wageningen, The Netherlands), which is the major active ingredient in the aggregation pheromone of D. melanogaster. Previous studies have indicated that cVA is as attractive to flies as the natural aggregation pheromone (Bartelt et al. 1985). We diluted the cVA in hexane and applied it to food dishes in a standard dose of 4.5 µg in 15 µl hexane, which is approximately equivalent to the deposition by 15 recently mated females (Bartelt et al. 1985; Wertheim et al. 2006).
(b). Experience phase
Each experiment consisted of an experience phase followed immediately with a test. For the experience phase, we introduced focal females individually into standard vials and provided them with distinct experiences as detailed below for each experiment. The vials were then placed inside the environmental chamber at full light. The experience phase commenced at 19.00 and lasted for 1 h.
(c). Test phase
After the experience phase, we transferred focal females individually into 23 × 13 × 18 cm (l × w × h) transparent plastic cages. The cages were placed inside a humidified room kept at 25°C and in dim light from 20.00 until 22.00 and in darkness from 22.00 until the following morning at 8.00. Each cage contained two 35 mm Petri dishes, one with AA- and the other with BA-flavoured foods at the opposite sides of the cage close to the wall. We randomized the positions of the flavoured foods such that half the cages had AA on the right and half on the left side. In all experiments, an observer blind to the experimental treatments counted the eggs in each dish and we then examined the effect of experience on females' oviposition preference with ANOVAs conducted on the arcsine square-root-transformed proportions of eggs. Analyses using non-parametric statistics revealed similar results. Only females that laid eggs during the testing phase were included in the analyses. In all three experiments, females belonging to distinct treatments laid similar average numbers of eggs.
(d). Preliminary experiments
We briefly describe here two preliminary experiments designed to verify, first, that focal females prefer food they have experienced for a long period over a novel food and, second, that focal females prefer food emitting an aggregation pheromone over food alone.
In the preliminary learning experiment, we mated 4-day-old females on the morning of the experiment and kept them in groups of 10 in vials containing sucrose and agar food until 20.00. At 20.00, we transferred the females in the same groups of 10 into vials containing either AA- or BA-flavoured food for a 24 h experience phase. Then, we immediately transferred the females individually into the test cages, where they remained for 12 h. We tested 120 females of which 75 (36 AA trained and 39 BA trained) laid eggs.
The females laid a significantly greater proportion of their eggs on the food experienced during the experience phase (70.0 ± 4.6% (mean ± 1 s.e.), F1,72 = 7.3, p < 0.001) and there was no significant effect of food type (F1,72 = 0.053, p = 0.819) or side (F1,72 = 1.47, p = 0.228).
In the other preliminary experiment, which did not involve training, we mated females on the morning of the experiment and kept them in individual vials with sucrose and agar food until testing. In the test phase, we introduced each female into a cage containing two identically flavoured foods (AA or BA) where one dish contained 15 µl of synthetic cVA and the other dish contained 15 µl of hexane. To control for possible side bias, we counterbalanced the Petri dish side such that the cVA-treated food was placed on the left-hand side of the cages for half of the trials. Of the 136 females tested, 85 laid eggs (47 AA tested and 38 BA tested).
The flies laid a significantly higher proportion of their eggs on the medium treated with cVA than hexane (78.9 ± 4.6%, one sample t-test, t84 = 6.58, p < 0.001), and there was no effect of either food type (F1,82 = 0.065, p = 0.80) or side (F1,82 = 0.016, p = 0.90).
3. Experiment 1: model versus alone
Here we tested whether inexperienced focal females would exhibit a stronger preference for a food type previously encountered with experienced, mated conspecifics (models) than food encountered alone. On day 1, we mated and placed 4-day-old model females two per vial in vials containing either AA- or BA-flavoured foods for 24 h. We confirmed that models laid at least 10 eggs per vial during that period. In order to differentiate between observers and models, we marked models by clipping the ends of their right wings. On the morning of day 2, we mated and placed observer females in individual vials containing sucrose water and agar until the experience phase.
In the experience phase, each focal female experienced one of the following: (i) AA-flavoured food in the presence of two models and the eggs they had laid on that food in the previous 24 h, (ii) BA-flavoured food in the presence of two models and the eggs they had laid on that food in the previous 24 h, (iii) AA-flavoured food alone or (iv) BA-flavoured food alone. Direct observations on a subset of vials indicated that, during the experience phase, the flies typically first explored and then rested at the top of the vials. On average, focal females with models (n = 32) approached models to within antenna distance 1.97 ± 0.37 times during training. We observed no egg laying during that period as it typically takes longer than 1 h for flies to commence egg laying after disturbance. Immediately after the experience phase, we tested the oviposition preference of each female. Of the 544 females tested, 356 laid eggs (89 AA trained alone, 91 AA trained with models, 87 BA trained alone and 89 BA trained with models). The average number of eggs laid by focals with models versus alone was 14.7 ± 1.1 versus 14.6 ± 1.1 eggs, respectively (F1,354 = 0.075, p = 0.78).
(a). Results
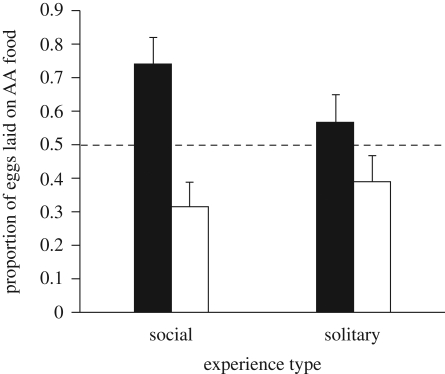
Focal females that had experienced food with models and their eggs laid a greater proportion of their eggs on that food compared with focal females that experienced food alone (F1,350 = 6.0, p = 0.015, figure 1). The effects of food type (F1,350 = 0.34, p = 0.85) and side (F1,350 = 1.4, p = 0.24) were not significant. Separate analyses for each of the two treatments revealed that both focal females with models (F1,178 = 41.6, p < 0.001) and focal females alone (F1,174 = 6.45, p = 0.012) laid a greater proportion of their eggs on the food they had experienced. That is, all focal females preferred the food they had previously experienced, but the experience together with models had a significantly larger effect than the experience alone.
Figure 1.
The mean (+1 s.e.) proportion of eggs laid on AA-flavoured food out of the total numbers of eggs laid by females (n = 356) trained either alone or with two models on either AA- or BA-flavoured foods. Black bars, AA (amyl acetate); white bars, BA (benzaldehyde).
4. Experiment 2: aggregation pheromone
Here we tested whether cVA was the primary signal mediating social learning. Because the general protocol was similar to that of experiment 1, we focus here on the distinct features of this experiment. During the experience phase, each focal female experienced one of the following four treatments: (i) cVA and AA food, (ii) cVA and BA food, (iii) hexane and AA food, or (iv) hexane and BA food. Immediately after the experience phase, we tested the oviposition preference of the focal females. Of the 544 females tested, 335 laid eggs (86 AA with hexane added, 91 AA with cVA added, 73 BA with hexane added and 85 BA with cVA added). The average number of eggs laid by focals that experienced cVA versus hexane was 28 ± 2.2 versus 28.8 ± 2.2 eggs, respectively (F1,333 = 0.3, p = 0.6).
(a). Results
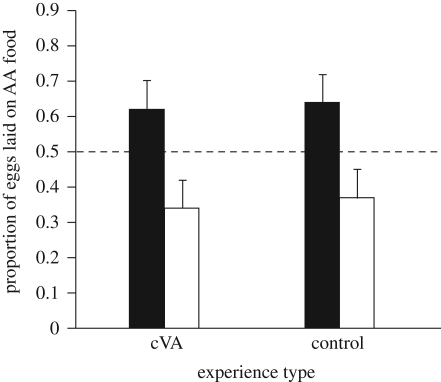
Focal females that experienced food with cVA did not lay a larger proportion of eggs on that food than focal females that experienced food with hexane (F1,331 = 0.003, p = 0.96, figure 2). The effects of food type (F1,331 = 0.034, p = 0.81) and side (F1,331 = 0.27, p = 0.49) were not significant. Separate analyses for each treatment revealed that focal females laid a greater proportion of their eggs on the food they had experienced whether it contained cVA (F1,174 = 13.4, p < 0.001) or hexane (F1,157 = 12.8, p < 0.001).
Figure 2.
The mean (+1 s.e.) proportion of eggs laid on AA-flavoured food out of the total numbers of eggs laid by females (n = 335) trained with either cVA or hexane added to either AA- or BA-flavoured foods. Black bars, AA (amyl acetate); white bars, BA (benzaldehyde)
5. Experiment 3: models' mating status
Here we examined whether social learning was affected by the type of models used, either mated or virgin females. Focal females can probably distinguish between mated and virgin females based on a variety of features including the presence of cVA and fertilized eggs only in the former.
In the experience phase, we randomly assigned focal females to one of six treatments: (i) AA food and two mated models and the eggs they had laid on that food in the previous 24 h, (ii) BA food and two mated models and the eggs they had laid on that food in the previous 24 h, (iii) AA food and two virgin models and the few unfertilized eggs they had laid on that food in the previous 24 h, (iv) BA food and two virgin models and the few unfertilized eggs they had laid on that food in the previous 24 h, (v) AA food alone or (vi) BA food alone. Immediately after the experience phase, we tested the oviposition preference of each focal female. Of the 660 females tested, 420 laid eggs (79 AA trained with mated models, 62 AA trained with virgin models, 68 AA trained alone, 82 BA trained with mated models, 50 BA trained with virgin models and 79 BA trained alone). Focal females laid 21.0 ± 1.7, 18.9 ± 1.8, and 20.8 ± 1.7 eggs, after they had experienced a food with mated models, virgin models or alone, respectively (F2,417 = 2.1, p = 0.12). We tested two a priori predictions: first, that there would be stronger social learning with mated than virgin models, and, second, that there would be stronger learning with virgin than no models, using ANOVA with two planned contrasts.
(a). Results
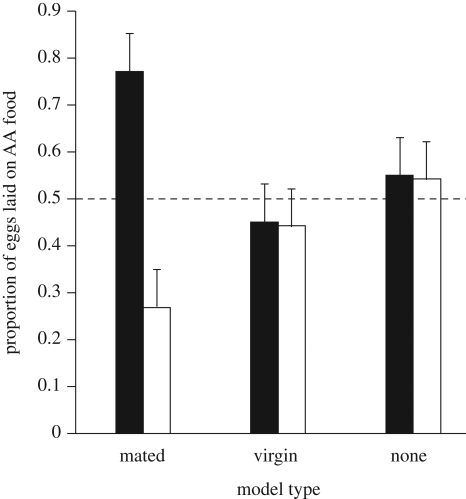
The models' mating status significantly affected focals' subsequent egg-laying behaviour (ANOVA, F2,417 = 12.89, p < 0.001). Focals that experienced food with mated models laid a greater proportion of their eggs on that food compared with focals that experienced food with virgin models (t417 = 4.18, p < 0.001), but there was no difference between focals that experienced food with virgin models and focals that experienced food alone (t417 = 0.01, p = 0.99, figure 3). Separate analyses for each treatment revealed that only focals that experienced mated models laid a significantly greater proportion of their eggs on the experienced food (F1,161 = 54.12, p < 0.001 for focal with mated models; F1,110 = 0.081, p = 0.78 for focals with virgins models; F1,145 = 0.069, p = 0.79 for focals alone).
Figure 3.
The mean (+1 s.e.) proportion of eggs laid on AA-flavoured food out of the total numbers of eggs laid by females (n = 420) trained with mated models, virgin models or alone on either AA- or BA-flavoured foods. Black bars, AA (amyl acetate); white bars, BA (benzaldehyde).
6. Discussion
Fruitflies exhibited a stronger, more robust preference for a novel food when they had a brief experience with it together with experienced, mated females and their eggs than food encountered alone (figures 1 and 3). In contrast to the strong effect of mated models and their eggs, neither virgin models nor the aggregation pheromone (cVA) alone generated socially influenced learning (figures 2 and 3). That is, the focal flies biased their food preference only after observing models who had decided that a given food was suitable for egg laying (Yang et al. 2008). The focal flies, however, apparently relied on their own sampling after experiencing the somewhat ambiguous cues provided either by virgin females who had not laid fertilized eggs or by an aggregation pheromone with neither flies nor eggs. Further experiments are necessary for elucidating the social cues focal females rely on and the relative importance of mated females versus their fertilized eggs. Our results are in agreement with theoretical predictions that animals should be selective in their reliance on social information (Laland 2004; Kendal et al. in press). Note that, to avoid ceiling effects, focal females were allowed only a short, 1 h experience prior to the test. While this generated little to no learning under the solitary condition (rightmost bars in all figures), either 24 h experience with food (§2d) or 1 h experience together with mated models and their eggs consistently generated robust learning (figures 1 and 3, leftmost bars).
We can readily understand what fruitflies gain from social learning by examining their natural history. Females that either eclose at the food substrate or smell residual odour of the food on the pupal case prefer that food over alternatives of similar quality (Jaenike 1982; Barron & Corbet 1999; Stamps & Blozis 2006; Stamps et al. 2009). By the time the females eclose, however, the food substrate may no longer be either available or suitable for egg laying. Although the females can then merely rely on their preference for the odours of yeast and decaying fruit as well as their own assessment of the food they find (Yang et al. 2008), they exhibit strong attraction to the aggregation pheromone emitted by conspecific, mated females. By joining conspecifics, fruitflies, as well as many other species, may locate suitable food faster than alone (Galef 1976; Galef & Giraldeau 2001). Furthermore, larger adult aggregations on fruit substrates increase larval fitness probably owing to increased inoculation rate of wild yeast species by the joining adults and decreased detrimental fungal growth (Wertheim et al. 2002, 2005). Whereas we replicated previous studies indicating that fruitflies prefer food with aggregation pheromone over food lacking the pheromone (§2d), our experiments went beyond documenting socially influenced behaviour to critically test for social learning.
In our experiments, the fruitflies preferred food that they had experienced with mated conspecifics more than food they had experienced alone during a choice test in which the food types were presented in the absence of conspecifics and fly-derived cues (figures 1 and 3). What is the adaptive significance of such socially influenced learning? The focal flies that had to choose between two foods of similar quality after experiencing one food alone in the experience phase had no relevant information indicating that one food was a better egg-laying substrate than the other. In contrast, the focal flies that had observed mated conspecifics and their eggs on one food during the experience phase could infer that this food was suitable while the quality of the other food was unknown. Hence, the observers could gain from laying eggs on the certain food rather than the uncertain alternative even when neither food contained conspecifics during the test.
While we have a growing understanding of the evolution and neurogenetics of individual learning (Davis 2005; Keene & Waddell 2007; Wu & Chiang 2008; Dukas in press a) and the ecological settings thought to favour social over individual learning in vertebrates (Kendal et al. in press), we know little about the evolution and neurogenetics of social learning. Further work on fruitflies can help us elucidate these biologically important issues. Moreover, information exchange among cooperating group members has been identified as an important feature of insect sociality (Wilson 1971; Fitzgerald & Peterson 1988; Costa 2006). Our finding that even aggregating solitary insects exhibit social learning raises the possibility that social learning has promoted the evolution of sociality in insects by increasing the benefits of living in groups. This possibility, along with its ecological, behavioural and neurogenetic foundations, can also be closely examined in fruitfly species.
Acknowledgements
We thank Z. Gong, and A. Patel for dedicated assistance, L. Dukas, J. Galef and two anonymous referees for their perceptive comments on the manuscript, and A. Dunlap for sharing with us unpublished data. This study was supported by the Natural Sciences and Engineering Research Council of Canada, Canada Foundation for Innovation, Ontario Innovation Trust (R.D.) and a McMaster University Graduate Fellowship (S.S.).
References
- Auld H. L., Punzalan D., Godin J.-G. J., Rundle H. D.2009Do female fruit flies (Drosophila serrata) copy the mate choice of others? Behav Process 182, 78–80 [DOI] [PubMed] [Google Scholar]
- Barron A. B., Corbet S. H.1999Preimaginal conditioning in Drosophila revisited. Anim. Behav. 58, 621–628 (doi:10.1006/anbe.1999.1169) [DOI] [PubMed] [Google Scholar]
- Bartelt R. J., Schaner A. M., Jackson L. L.1985Cis-vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J. Chem. Ecol. 11, 1747–1756 (doi:10.1007/BF01012124) [DOI] [PubMed] [Google Scholar]
- Coolen I., Dangles O., Casas J.2005Social learning in noncolonial insects? Curr. Biol. 15, 1931–1935 (doi:10.1016/j.cub.2005.09.015) [DOI] [PubMed] [Google Scholar]
- Costa J. T.2006The other insect societies Cambridge, MA: Harvard University Press [Google Scholar]
- Davis R. L.2005Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Ann. Rev. Neurosci. 28, 275–302 (doi:10.1146/annurev.neuro.28.061604.135651) [DOI] [PubMed] [Google Scholar]
- Dukas R.In press a Learning: mechanisms, ecology and evolution. In Cognitive ecology II (eds Dukas R., Ratcliffe J.). Chicago, IL: University of Chicago Press [Google Scholar]
- Dukas R.In press b Social learning in insects. In Encyclopedia of animal behavior (eds Breed M., Moore J.). Amsterdam, The Netherlands: Elsevier [Google Scholar]
- Dukas R., Simpson S. J.2009Locust show rapid individual learning but no social learning about food. Anim. Behav 78, 307–311 [Google Scholar]
- Ejima A., Smith B. P. C., Lucas C., van der Goes van Naters W., Miller C. J., Carlson J. R., Levine J. D., Griffith L. C.2007Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr. Biol. 17, 599–605 (doi:10.1016/j.cub.2007.01.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito G., Scotto P.1992Observational learning in Octopus vulgaris. Science 256, 545–547 (doi:10.1126/science.256.5056.545) [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. D., Peterson S. C.1988Cooperative foraging and communication in caterpillars. BioScience 38, 20–25 (doi:10.2307/1310642) [Google Scholar]
- Franks N. R., Richardson T.2006Teaching in tandem-running ants. Nature 439, 153–153 (doi:10.1038/439153a) [DOI] [PubMed] [Google Scholar]
- Galef B. G.1976Social transmission of acquired behavior: a discussion of tradition and social learning in vertebrates. Adv. Study Behav. 6, 77–100 [Google Scholar]
- Galef B. G., Giraldeau L. A.2001Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 (doi:10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
- Heyes C. M., Galef B. G. (eds) 1996Social learning in animals San Diego, CA: Academic Press [Google Scholar]
- Jaenike J.1982Environmental modification of oviposition behavior in Drosophila. Am. Nat. 119, 784–802 (doi:10.1086/283955) [Google Scholar]
- Keene A. C., Waddell S.2007Drosophila olfactory memory: single genes to complex neural circuits. Nat. Rev. Neurosci. 8, 341–354 (doi:10.1038/nrn2098) [DOI] [PubMed] [Google Scholar]
- Kendal R. L., Coolen I., Laland K. N.In press Adaptive trade-offs in the use of social and personal information. In Cognitive ecology II (ed. Ratcliffe D. R. J.). Chicago, IL: University of Chicago Press [Google Scholar]
- Kent C., Azanchi R., Smith B., Formosa A., Levine J. D.2008Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18, 1384–1389 (doi:10.1016/j.cub.2008.07.088) [DOI] [PubMed] [Google Scholar]
- Krupp J. J., Kent C., Billeter J. C., Azanchi R., So A. K. C., Schonfeld J. A., Smith B. P., Lucas C., Levine J. D.2008Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18, 1373–1383 (doi:10.1016/j.cub.2008.07.089) [DOI] [PubMed] [Google Scholar]
- Laland K. N.2004Social learning strategies. Learn Behav. 32, 4–14 [DOI] [PubMed] [Google Scholar]
- Leadbeater E., Chittka L.2007Social learning in insects—from miniature brains to consensus building. Curr. Biol. 17, R703–R713 (doi:10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- Levine J. D., Funes P., Dowse H. B., Hall J. C.2002Social experience influences circadian timing in Drosophila melanogaster. Behav. Genet. 32, 475–476 [Google Scholar]
- Mery F., Varela S. A. M., Danchin É., Blanchet S., Parejo D., Coolen I., Wagner R. H.2009Public versus personal information for mate copying in an invertebrate. Curr. Biol. 19, 730–734 (doi:10.1016/j.cub.2009.02.064) [DOI] [PubMed] [Google Scholar]
- Prokopy R. J., Roitberg B. D.2001Joining and avoidance behavior in nonsocial insects. Ann. Rev. Entomol. 46, 631–665 (doi:10.1146/annurev.ento.46.1.631) [DOI] [PubMed] [Google Scholar]
- Quinn W. G., Harris W. A., Benzer S.1974Conditioned behavior in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 71, 708–712 (doi:10.1073/pnas.71.3.708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman G., Visscher P. K.2002Honeybee colonies achieve fitness through dancing. Nature 419, 920–922 (doi:10.1038/nature01127) [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Blozis S. A.2006Effects of natal experience on habitat selection when individuals make choices in groups: a multilevel analysis. Anim. Behav. 71, 663–672 (doi:10.1016/j.anbehav.2005.07.015) [Google Scholar]
- Stamps J., Luttbeg B., Krishnan V. V.2009Effects of survival on the attractiveness of cues to natal dispersers. Am. Nat. 173, 41–46 (doi:10.1086/593306) [DOI] [PubMed] [Google Scholar]
- Tully T.1996Discovery of genes involved with learning and memory: an experimental synthesis of Hirschian and Benzerian perspectives. Proc. Natl Acad. Sci. USA 93, 13 460–13 467 (doi:10.1073/pnas.93.24.13460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Frisch K.1967The dance language and orientation of bees Cambridge, MA: Harvard University Press [Google Scholar]
- Wertheim B., Marchais J., Vet L. E. M., Dicke M.2002Allee effect in larval resource exploitation in Drosophila: an interaction among density of adults, larvae, and micro-organisms. Ecol. Entomol. 27, 608–617 (doi:10.1046/j.1365-2311.2002.00449.x) [Google Scholar]
- Wertheim B., van Baalen E.-J. A., Dicke M., Vet L. E. M.2005Pheromone-mediated aggregation in nonsocial arthropods: an evolutionary ecological perspective. Ann. Rev. Entomol. 50, 321–346 (doi:10.1146/annurev.ento.49.061802.123329) [DOI] [PubMed] [Google Scholar]
- Wertheim B., Allemand R., Vet L. E. M., Dicke M.2006Effects of aggregation pheromone on individual behaviour and food web interactions: a field study on Drosophila. Ecol. Entomol. 31, 216–226 (doi:10.1111/j.1365-2311.2006.00757.x) [Google Scholar]
- Wilson E. O.1971The insect societies Cambridge, MA: Harvard University Press [Google Scholar]
- Wu C.-L., Chiang S.2008Genes and circuits for olfactory-associated long-term memory in Drosophila. J. Neurogen. 22, 257–284 (doi:10.1080/01677060802307755) [DOI] [PubMed] [Google Scholar]
- Yang C.-H., Belawat P., Hafen E., Jan L. Y., Jan N.2008Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319, 1679–1683 (doi:10.1126/science.1151842) [DOI] [PMC free article] [PubMed] [Google Scholar]