Abstract
Laboratory and field studies have documented better cognitive performance associated with marked hemispheric specialization in organisms as diverse as chimpanzees, domestic chicks and topminnows. While providing an evolutionary explanation for the emergence of cerebral lateralization, this evidence represents a paradox because a large proportion of non-lateralized (NL) individuals is commonly observed in animal populations. Hemispheric specialization often determines large left–right differences in perceiving and responding to stimuli. Using topminnows selected for a high or low degree of lateralization, we tested the hypothesis that individuals with greater functional asymmetry pay a higher performance cost in situations requiring matching information from the two eyes. When trained to use the middle door in a row of a nine, NL fish correctly chose the central door in most cases, while lateralized fish showed systematic leftward or rightward biases. When choosing between two shoals, each seen with a different eye, NL fish chose the high-quality shoal significantly more often than the lateralized fish, whose performance was affected by eye preference for analysing social stimuli. These findings suggest the existence of a trade-off between computational advantages of hemispheric specialization and the ecological cost of making suboptimal decisions whenever relevant information is located on both sides of the body.
Keywords: lateralization, asymmetry, fish
1. Introduction
In most vertebrates, the eyes are laterally placed and each eye largely sees a different portion of the visual field. As lateral positioning of the eyes is often accompanied by an almost complete crossing of fibres at the optic chiasm, the contralateral hemisphere primarily processes visual input of each eye. In these species, the presence of left–right functional asymmetries often leads the organism to analyse and respond to a stimulus in a different way depending on its placement on the left or the right side of the observer (Vallortigara & Andrew 1991; Deckel 1995; Rogers et al. 2004; Wiltschko et al. 2007).
The occurrence of functional brain asymmetries is now well documented for both bony fish and land vertebrates (reviewed in Andrew & Rogers 2002; Vallortigara & Bisazza 2002). Having two specialized hemispheres can be very advantageous. Lateralized chicks that had to learn to discriminate between food and non-food while a model of avian predator was moved overhead learned faster than the non-lateralized (NL) chicks, and were also more responsive to the model predator (Rogers et al. 2004). In the teleost Girardinus falcatus, fish artificially selected for a high degree of lateralization were twice as fast as NL fish at catching live prey when fish had to share attention with a concurrent task, predator vigilance (Dadda & Bisazza 2006). Lateralized fish could attain this result by attending the feeding task primarily with one eye while using the other eye to monitor the predator. These examples suggest that hemispheric specialization might increase the capacity to carry out simultaneous processing, by channelling different types of information into the two separate halves of the brain and by enabling separate and parallel processing to take place in the two hemispheres. Indeed, it has been suggested that this might have been the major selective force driving evolution of lateralization of cognitive functions in early vertebrates (Rogers 2000, 2002). Strongly lateralized individuals have been found to outperform less lateralized individuals in many other contexts that do not explicitly involve the sharing of attentional resources among concurrent tasks, such as termite fishing in chimpanzees (McGrew & Marchant 1999), visual discrimination in pigeons (Gunturkun et al. 2000), and schooling and spatial orientation in fish (Bisazza & Dadda 2005; Sovrano et al. 2005), implying that other, unidentified advantages of cerebral lateralization may exist.
Some heritability of lateralization has been demonstrated in fish (Barth et al. 2005; Bisazza et al. 2007), rodents (Collins 1990) and primates (Hopkins et al. 2001; Anneken et al. 2004), and one would expect natural selection to favour individuals with specialized hemispheres. Yet the literature rarely reports a highly skewed or an antisymmetric distribution of laterality (but see Zucca & Sovrano 2008; Giljov et al. 2009). Animal populations normally show a great variation in the degree of laterality and, not infrequently, NL (or weakly lateralized) individuals outnumber strongly lateralized ones (Bisazza et al. 1997, 2000; Gunturkun et al. 2000; Brown et al. 2007; Takeuchi & Hori 2008).
As noted by Rogers (2002), there are potential costs associated with cerebral asymmetries, and in particular with transferring and integrating the information that reaches the two hemispheres. Toads, for example, are more likely to strike at a prey moving in their right lateral field of vision while agonistic responses are delivered preferentially to a conspecific seen on their left side (Vallortigara et al. 1998). Similar differences have been found in birds and reptiles (Deckel 1995; Dharmaretnam & Rogers 2005). Even in species with frontally placed eyes, such as humans, hemispheric dominance can sometimes hinder performance when strict cooperation between the two halves of the brain is required. For example, the human right hemisphere is usually dominant for spatial processing, and this determines left–right perceptual and attentional biases, a phenomenon known as ‘pseudoneglect’. Simple tests show that more attention is paid to the left side of a happy–sad chimeric face (David 1989), that a systematic leftward error is made in the manual bisection of a line (Jewell & McCourt 2000) or that objects appear to have significantly different size when seen by the right and the left eye (McManus & Tomlinson 2004).
Normally, biologically relevant stimuli such as a predator, a prey or a rival, are equally likely to appear on the left or the right side, and it is not difficult to see the potential disadvantages arising from having side biases in the promptness or effectiveness of a response to a particular class of objects (see discussion in Rogers 2000, 2002; Vallortigara & Rogers 2005). As a consequence, a trade-off is expected between these disadvantages and the cognitive advantages of lateralization such as the possibility of parallel processing (Rogers 2002; Corballis 2006). However, to date, the possibility that a left–right difference in the way an animal analyses and responds to environmental stimuli translates into a disadvantage for more lateralized individuals has not been empirically tested.
Here, we tested the hypothesis that individuals with marked cerebral lateralization pay a higher cost in terms of reduced efficiency in tasks relying on hemispheric communication and cooperation. Lateralization in fish is partly under genetic control, and this allows one to obtain fish that differ in the degree or direction of cerebral lateralization (Barth et al. 2005; Bisazza et al. 2007). To pursue our goal, we compared fish from lines artificially selected for a high or low degree of lateralization in two conditions requiring the integration of information from left and right visual hemifields. In the first experiment, fish were asked to find the middle of a row of small doors that was presented frontally, an adaptation for fish of the ‘line bisection test’, a standard method of neuropsychology to measure visuo-spatial biases. In the second experiment, we measured how efficiently a subject chose between two stimuli (two groups of social companions) differing in quality that were presented at the opposite sides of the body, thus with the critical information split up between the two lateral hemifields.
2. Material and methods
(a). Subjects
The goldbelly topminnow, G. falcatus (Cyprinodontiformes, Poeciliidae), is a small viviparous fish originally from Cuba.
For this experiment, we used subjects from three stocks of fish that differed in laterality and that were obtained through selective breeding (Bisazza et al. 2007). From 1997 to 2001, topminnows were artificially selected for eye preference to monitor a potential predator using the detour test, which scores the direction taken by a fish when facing a barrier behind which a model predator is visible (Facchin et al. 1999; see details in the electronic supplementary material).
One hundred and ten females were used in this study. Subjects (approx. six to seven months old) were subdivided into three experimental groups: fishes that turned 80 per cent or more to the left (LD), fishes that turned 80 per cent or more to the right (RD) and fishes that turned 50 per cent of times in each direction (NL). Groups were maintained in 80 l glass aquaria with abundant vegetation (Ceratophillum sp.) and a 14D : 10L photoperiod; water temperature was 25 ± 2°C and all fish were fed dry fish food and Artemia salina nauplii twice a day.
(b). Experiment 1: finding the centre of a figure (bisection test)
In this experiment, 16 lateralized (eight LD and eight RD, collectively called LAT) and 10 NL adult females were trained to use the central door in a row of nine, using the possibility to rejoin the social group as reinforcement. Only females were used in this experiment because they are several times heavier than males and can more easily open the experimental doors.
(i). Apparatus
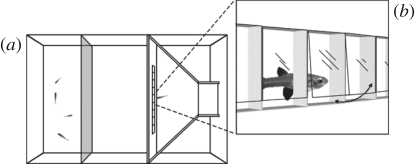
The apparatus (figure 1) consisted of a rectangular glass tank (80 × 40 × 40 cm) divided into three sectors. A small ‘start box’ (9.5 × 5.5 × 9 cm), which contained the focal female at the beginning of the test, was provided with a trapdoor (8 × 12 cm) leading into the choice area. Once in this area, the fish faced a white partition (40 × 40 cm) provided with a succession of nine identical doors (4 × 2 cm each) placed at 2 cm from the bottom and spaced 5 mm one from another, constituting a ‘line’ of 22 cm. All the doors were similar, but only the fifth, central door could be opened by pressing on the flexible plastic material with the snout. Through this door, the subject could gain access to a sector (35 × 40 × 40 cm) in which a group of four stimulus fish were visible and acted as reward. The apparatus was placed in a dark room and lit by two neon lamps (15 W). A video camera was suspended 1 m above the experimental tank and used to record the behaviour of the focal fish during the tests.
Figure 1.
(a) Apparatus used in the bisectioning task. (b) Subjects were required to use the middle door in a row of nine.
Prior to the experiment, subjects were placed in a pre-training tank for 10 days. This procedure had the twofold aim of accustoming the fish to use of the movable doors and training them to use the middle door in a short row (three doors). A partition divided the tank into two compartments, one provided with vegetation acting as cover and the other containing food. Three doors identical to those of the experimental apparatus were positioned at the centre of the partition and only the central one allowed the fish to move between the compartments.
(ii). Procedure
At the beginning of the test, the focal female was dip-netted and released into the sector of the experimental apparatus facing the four stimuli fish for a 15 min period of acclimatization. The female was then gently dip-netted and inserted into the start box for a 2 min period, where the plastic door was raised and the focal female released into the choice area. The female was allowed to try the different doors until the correct door was found. The intertrial interval was 5 min, during which the fish was allowed to remain in the sector with visible social group and reinforced with food (A. salina nauplii). The fish were then gently captured and reinserted into the start box for another trial and the procedure was repeated until six trials were completed. From the video recordings, we scored the frequencies of attempts for each of the nine doors.
(c). Experiment 2: efficiency in bilateral information processing
In this experiment, 44 LAT (21 LD and 23 RD) and 40 NL adult females were allowed to choose between two social groups that differed in quality (see below) in a situation in which each stimulus was seen by a different eye. Only females were used in this experiment because males of this species demonstrate a reduced tendency to shoal, behave aggressively towards same sex stimuli and mate with opposite sex stimuli. The experiment was carried out in two variants. Twenty-six LAT (13 LD and 13 RD) and 22 NL females were tested with variant A, in which stimulus shoals differed by number of fish (four versus two females). Eighteen LAT (eight LD and 10 RD) and 18 NL females were tested with variant B, in which stimulus shoals differed by the size of the fish (same size as the subject versus smaller size).
(i). Apparatus
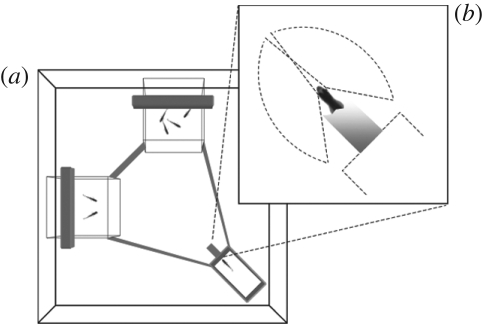
The experimental apparatus consisted of an aquarium (60 × 60 × 35 cm; figure 2) subdivided into four compartments. One, the start box, consisted of a small rectangular area (25 × 8 × 22 cm) made by green opaque plastic walls. Fish could exit only through a small corridor (4 × 3 × 2 cm) that led to the choice compartment. The corridor was built so that the focal female, upon exiting, saw two simultaneous stimulus shoals, each visible in a different visual hemifield. A transparent door (5 × 11.5 cm) was placed at the beginning of the corridor and was connected to a monofilament line on a pulley, which made it possible for an observer to raise it from a remote location. Two transparent glass cubes (20 × 20 × 20 cm) were placed at the opposite sides 25 cm apart and hosted stimulus fish. Two 8 W fluorescent lamps were suspended on both cubes while the start box was kept in dark. The floor was covered with gravel (with the exception of the start box) and the tank was filled with 15 cm of water (temperature 25 ± 2°C). A video camera was suspended 1 m above the experimental tank and used to record the choice of the focal fish.
Figure 2.
(a) Apparatus used in the bilateral information processing task. (b) Once outside the small corridor, the focal female simultaneously saw two stimulus shoals, each visible in a different visual hemifield.
(ii). Procedure
As in the previous experiment, during the week preceding the experiment, subjects were placed in a pre-training tank in order to familiarize them with the corridor that allows movement between the compartments.
Ten minutes before the test, the two stimulus shoals were inserted in the two cubes. A single experimental female was inserted in the start box and allowed to acclimatize for 1 min. The door was then raised and the female was allowed to enter the experimental area. A preliminary test had shown that in this circumstance fish joined one shoal immediately without stopping or turning around. We recorded the first choice made by the subject as marked by reaching one body length (4 cm) from the front glass of the cube containing the shoal. Each subject was tested twice daily at about a 120 min interval and the left–right position of the two stimuli was inverted between the two trials.
(iii). Determination of social preference
Shoal preferences have been shown in a number of teleosts, and virtually all studied species show consensus in preferring shoals containing more individuals and shoals containing fish of the same size as the subject (Hager & Helfman 1991; Krause & Godin 1994).
A pilot experiment was performed to determine the preference of female topminnows for these two features. Thirty-two females from an unselected laboratory stock (therefore containing females with a variable degree of lateralization) were tested in an apparatus similar to that used for the experiment except for the corridor, which measured 16 × 20 cm and was provided with a glass door allowing the subjects to see the two stimulus shoals with both eyes for 2 min before being released in the choice compartment. Sixteen females were allowed to choose between a large (four females) and a small (two females) shoal, and 16 females were allowed choose between three similar-sized females and three smaller females (70% of the length of the subject). When tested for their preference between a shoal of two and a shoal of four females, 15 out of 16 fish tested preferred the latter (χ2 = 12.3, p < 0.001). When tested for their preference between a shoal containing smaller females and one containing same-size females, 14 out of 16 fish tested preferred the latter (χ2 = 9.0, p = 0.004).
Both these social preferences were used in the main experiment. In one variant, subjects were presented with four females (the preferred stimulus in pilot experiment) and two females. In the other variant, the subject could choose between three similar-sized females (preferred stimulus) and three smaller females (70% of the length of the subject). Half of the subjects did the first trial with the preferred shoal on the left and the second trial with the preferred shoal on the right; the other half of the subjects did the reverse. The performance of each subject was scored on three levels: both choices of the preferred stimulus; one choice of the preferred stimulus and one for the non-preferred one; and both choices of the non-preferred stimulus.
3. Results
(a). Finding the centre of a figure (bisection test)
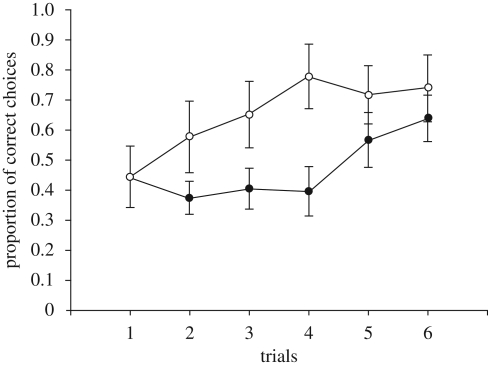
Topminnows rapidly generalized line bisection from the three-door row pre-training phase to the nine-door row in the testing phase. As shown in figure 3, from the second trial onwards, the proportion of choices of the normally preferred shoal was higher in NL than in LAT fish and both progressively reduced the number of incorrect choices in successive trials. On the whole, we found a significant difference between NL and LAT in the number of choices for preferred stimuli and a significant reduction in the proportion of choice of non-preferred stimuli in successive trials (repeated measure ANOVA, degree of lateralization F1,24 = 9.655, p = 0.005; difference among trials F5,120 = 2.111, p = 0.069; linear trend F1,24 = 10.65, p < 0.003; interaction F5,120 = 0.975, p = 0.436). We found no difference in accuracy between RD and LD fish (F1,14 = 0.461, p = 0.508; difference among trials F5,70 = 1.796, p = 0.125; interaction F5,70 = 0.446, p = 0.815).
Figure 3.
Proportion of correct choices (mean ± s.e.) of central door during the six trials of the bisectioning task in lateralized and NL topminnows. Filled circles, lateralized; open circles, non-lateralized.
To obtain detailed information about the type of errors made by the three groups of subjects, we scored the results assigning a value from 1 for the leftmost door to 9 for the rightmost. Correct choice of the central door corresponded to a value of 5. The average score of NL fish is close to this value (mean ± s.d., 4.73 ± 0.50) with no significant left–right bias in bisection (t(9) = 1.698, p = 0.124). LD and RD fish showed significant leftward (4.21 ± 0.66; t(7) = 3.315, p = 0.013) and rightward biases (5.56 ± 0.65; t(7) = 2.435, p = 0.045), respectively. The difference between RD and LD is highly significant (t(14) = 4.071, p < 0.001).
(b). Efficiency in bilateral information processing
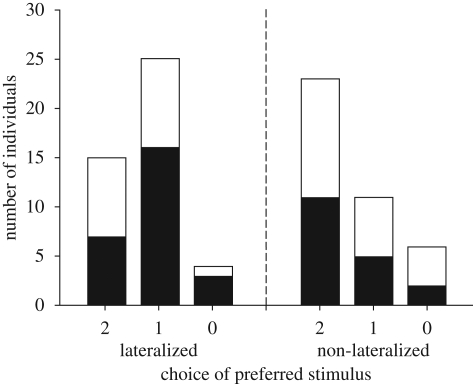
The frequency of choices of the preferred stimulus was not significantly different between variant A and B trials (χ2 = 1.468, p = 0.428, figure 4), and the results of the two variants were considered together for subsequent analyses. The majority of NL fish chose the preferred stimulus in both trials while most LAT fish chose the preferred stimulus in one trial and the non-preferred stimulus in the other. The difference between these two groups in the proportion of subjects choosing the preferred shoal in both trials, in one trial or in none is significantly different (χ2 = 7.355, p = 0.025). The difference between LD and RD is not significant (Fisher's exact test, p = 0.752); however, owing to reduced sample size, the chance to detect a difference between the two subgroups of LAT fish is lower.
Figure 4.
Bilateral information processing task. Lateralized and NL topminnows were given the choice between two different social groups each seen by a different eye. Bars represent the number of individuals choosing the normally preferred shoal in both trials, in one trial or in none. In variant A, the choice was between four (preferred stimulus) and two females. In variant B, the choice was between similar-sized (preferred stimulus) and smaller females. Black bars, variant A; white bars, variant B.
RD and LD differ significantly in left–right preference (χ2 = 4.482, p = 0.034), with both groups choosing more often the stimulus presented on the eye dominant for analysing social stimuli (the right eye in RD, the left eye in LD; Dadda et al. 2007). In NL fish, no difference was found in the proportion of choices of the preferred stimulus between the right and left presentation (χ2 = 0.313 p = 0.584).
4. Discussion
The results of our experiments support the hypothesis that a marked cerebral lateralization may hinder efficiency when tasks require hemispheric communication and cooperation. To our knowledge, this is the first study documenting an advantage of less lateralized individuals and indicating possible ecological costs of brain asymmetries that may be responsible for the maintenance of NL phenotypes in animal populations.
Previous studies comparing the cognitive performance of poorly and strongly lateralized individuals have found a greater efficiency of the latter in all organisms examined (fish: Bisazza & Dadda 2005; birds: Dharmaretnam & Rogers 2005; primates: McGrew & Marchant 1999; insects: Pascual et al. 2004). Many of the observed differences involved functions that significantly affect survival, such as finding food or escaping predators. Yet at the behavioural level lateralization often results in side biases in perception, information processing and motor output that could potentially give rise to disadvantages (Rogers 2002; Vallortigara & Rogers 2005). In particular, vertebrates with laterally positioned eyes encounter difficulties integrating information from left and right visual fields (Ingle 1968; Prior & Wilzeck 2008; Xiao & Gunturkun 2009), and one can envisage circumstances in which individuals with reduced left–right functional differences might outperform the more strongly lateralized ones.
We have compared poorly and strongly lateralized topminnows in two situations that might be expected to hinder individuals with pronounced left–right differences in analysing the sensory input. In both experiments, subjects were required to integrate information from the left and the right visual hemifield in order to take the appropriate decision. Experiment 1 consisted of an adaptation for the fish of the line bisection test, a major diagnostic tool for the identification of visuo-spatial deficits in patients with brain lesions and population-level visuo-spatial biases in normal human subjects (reviewed in Jewell & McCourt 2000). Fish familiar with using the middle door of three were trained to find the middle of a nine-door row. NL topminnows rapidly learned the new task, making only a few errors that were equally distributed on either side. By contrast, the performances of the two groups of LAT fish were impaired by systematic errors on the left or right of the centre. In particular, the efforts of RD subjects were centred nearly one door to the right of the correct door, while LD subjects made similar systematic errors on the left of the correct door.
Comparable data are available only for humans. Studies on neurologically normal subjects have found substantial individual variation in both the magnitude and the direction of the bisection bias. Individual performance can vary from approximately 10 per cent to the left to 10 per cent to the right, but is usually much less (Schenkenberg et al. 1980; Manning et al. 1990; McCourt & Olafson 1997), a range that appears similar to that observed in our study. Unlike most other vertebrates, humans show strong population biases in many lateralized functions. For example, more than 90 per cent of the population is right-handed, and a similar percentage shows left hemisphere dominance for language. The strong leftward population bias in line bisection that is normally observed in human studies is traditionally ascribed to a bias in the allocation of attention resources towards the left visual field deriving from right hemisphere dominance in spatial tasks in the majority of the population (Heilman & Van Den Abell 1980; Jewell & McCourt 2000).
It is not easy to estimate the extent to which such deficits can affect an individual's fitness in a fish. It is possible, for example, that a topminnow in need of rapidly gaining a refuge and using visible landmarks fails to follow the most favourable trajectory as a consequence of a strong lateralization of spatial attention.
The second situation considered in this paper is one in which an animal must select between two options, each seen by a different eye. Shoaling is one of the major antipredatory strategies of fish. However, not all shoals are equally safe. Fish shoaling with individuals different from themselves are more easily spotted by predators and those shoaling in large shoals benefit from better vigilance and greater dilution of risk (reviewed in Krause & Ruxton 2002). Not surprisingly, fish consistently avoid associating with individuals of a different size and prefer large shoals to small ones (Hager & Helfman 1991; Krause & Godin 1994). Our pilot tests showed that goldenbelly topminnows have a strong preference for both these qualities, with around 90 per cent of fish choosing the larger shoal and that containing similar-sized stimuli. In our experiment, the subject entered an unfamiliar area where it could choose between two shoals differing in quality but in a condition in which each hemisphere had direct access only to one-half of the information necessary to accomplish the task. In this condition, NL fish chose the normally preferred shoal significantly more often than the LAT fish, which in most cases chose the option seen with the eye that in their stock was dominant for analysing social stimuli (the right eye in RD, the left eye in LD; Dadda et al. 2007), irrespective of its relative quality. The most plausible interpretation of these data is that in LAT fish information relative to two different properties of the stimulus, shoal size and fish size, is confined, at least for the short lapse of time necessary to take a decision, to the hemisphere that initially receives the visual input, and therefore the hemisphere dominant for analysing social stimuli in this phase can only (or predominantly) access the information it receives from the contralateral eye.
The lack of integration of information reaching the two eyes that was observed especially in LAT fish might surprise a reader not familiar with lateralization literature. Our findings are consistent, however, with current knowledge of the way the teleost visual system integrates the two lateral inputs. The left and right eye systems can operate quite independently, as suggested by the observation that opposite discriminations can be simultaneously established in the two eye systems (Ingle 1968). Experiments involving subjects trained monocularly to discriminate patterns have shown that interocular information transfer is slow and incomplete (McCleary 1960; Mark 1966; Ingle 1968). Ingle found some interocular transfer of simple discrimination, but loss of information for more difficult discrimination (Ingle 1965). As yet, nothing is known about the neural bases of visual lateralization in fish and what differentiates lateralized from NL fish. This topic has been extensively investigated in birds. Neuroanatomical asymmetries have been described in detail in the two major ascending visual projections, the tectofugal and the thalamofugal pathway, and several studies have shown that stronger behavioural lateralization is associated with greater degree of asymmetry in these pathways (Deng & Rogers 2002b; Gunturkun 2002). In pigeons and domestic chicks, it was found that these neuroanatomical asymmetries are accompanied by significant left–right asymmetries of interocular transfer (Sandi et al. 1993; Skiba et al. 2000), a feature that could also be present in fish and explain the results of our experiments.
The relevance of the differences found in the second experiment for a fish's everyday life is perhaps easier to envisage. Most fish have a visual field covering almost 360° and the frontal overlap of the opposite visual fields is usually around 10° (Collin & Shand 2003). Therefore, the probability that two stimuli fall into two different visual hemifields is relatively high. Because many other aspects of behaviour—such as mating, prey capture and intraspecific aggression—are lateralized in topminnows (Bisazza et al. 2001, 2005; Dadda & Bisazza 2006), they may make suboptimal decisions in other contexts whenever a quick assessment is needed and the alternatives are placed at the opposite sides of the body.
These disadvantages are expected to decrease with increasing time allowed for decision-making as this provides a greater opportunity for integration of information from the two eyes and for looking at the different options with the same eye. In food-storing birds, for example, each hemisphere processes qualitatively different information about the location of food caches, but the different types of information are integrated when food items are retrieved some time later (Clayton & Krebs 1994). In our pilot experiment, almost all subjects chose same-size companions or the larger shoal after they were allowed 2 min of free observation of stimuli.
Problems arising from the integration of bilateral input are probably reduced also when stimuli fall into the binocular portion of the visual field as they are seen by the two eyes simultaneously. In addition, the left and right frontal binocular fields may work in a more coordinated fashion compared with the two lateral monocular fields. In pigeons, reliable interocular transfer of visual discrimination was observed when the stimuli were presented in the frontal visual field but not when they were presented in the lateral visual field (Mallin & Delius 1983). In chicks, comparison of binocular–monocular testing has shown that the left and the right frontal field are equally efficient in complex discrimination tasks, although the birds' performance was superior when both eyes were involved (Prior & Wilzeck 2008). Anatomical evidence for the integration of the two frontal binocular fields has been provided also for some teleosts (Northmore & Gallagher 2003).
A tight integration of information from left and right eyes has become the prevalent condition in the primate visual system. In human and non-human primates, there is in fact a large overlap of the two eye fields. Information from one portion of the visual field reaches both eyes and, owing to the partial decussation of the optic nerves, input from the two eyes is sent to the same hemisphere (contralateral to stimulus position). In addition, the corpus callosum enables fast and efficient information transfer between the hemispheres. However, the price to pay is that humans are no longer able to use one eye to monitor a potential danger while simultaneously and independently using the other eye to coordinate another activity, such as food gathering, in the way fish and birds are able to do (Bisazza & Dadda 2005; Dharmaretnam & Rogers 2005). Interestingly, the condition observed in topminnows more closely resembles that observed in split-brain patients. To some extent, these patients have the ability to run independent tasks with the two disconnected hemispheres. Unlike normal observers, they are capable of directing their attention to left and right field locations simultaneously and have been found to outperform normal controls in dual-task experiments (Gazzaniga & Sperry 1966; Luck et al. 1989). Yet, as expected, performance is frequently impaired in split-brain patients relative to controls in tasks relying more upon the collaboration of the hemispheres (reviewed in Gazzaniga 2000).
In all, the picture emerging from this study indicates that advantages of hemispheric specialization, such as the possibility of processing multiple information flows in parallel (Rogers et al. 2004; Dadda & Bisazza 2006), may be counterbalanced by some ecological disadvantages associated with left–right differences in the response to stimuli. The relative weights of these costs and benefits are likely to vary with ecological conditions (structure of habitat, predation risk, social density, food abundance, etc.) and the degree of lateralization is therefore expected to vary among species and populations in relation to the importance of the different factors. So far, two studies provide some support to this hypothesis. A study of 16 species of fish found that all shoaling species showed population-level lateralization of predator evasion behaviour, whereas non-shoaling species tended to have individual but not population lateralization (Bisazza et al. 2000). A field study reported in the poeciliid fish Brachyraphis episcopi that individuals from high-predation populations were more lateralized than their low-predation counterparts (Brown et al. 2004). The authors suggested that in a population with a high predation pressure, selection has favoured lateralized fish because they are better able to cope with two simultaneous tasks, such as foraging and predator vigilance.
Heritability of direction and strength of cerebral asymmetries have been reported in several vertebrates and may provide a basis for population and species differentiation. However, hereditary influences seem to account for only a fraction of the interindividual variation in laterality (Hopkins et al. 2001; Barth et al. 2005; Bisazza et al. 2007).
There is now considerable evidence that the development and expression of cerebral asymmetries can be modulated by environmental factors such as stress (Fride & Weinstock 1988), androgen exposure (Zappia & Rogers 1987) and asymmetry in physical (Collins 1975) or social (Vallortigara et al. 1999) environment. Some of these effects may represent adaptive mechanisms, allowing parents to adjust the developmental trajectories of their offspring to the environmental conditions in which they will subsequently live (Deng & Rogers 2002a; Andrew 2009). For example, maternal glucocorticoids deposited in the egg or crossing the placenta profoundly affect the development of lateralization (Diaz et al. 1995; Rogers & Deng 2005), an effect that may enable the mother experiencing stress situations (such as predator attack) at the time of embryo formation to adaptively influence the laterality pattern of their offspring (Deng & Rogers 2002a; Halpern et al. 2005).
Development of lateralization is also influenced by the amount of light reaching the embryo. In zebrafish, differential exposure to light produces wide differences in lateralization that have effects on multiple aspects of behaviour (Andrew et al. 2009a,b). In domestic chicks, the amount of light that enters through the eggshell in the days prior to hatching greatly affects the development of lateralized visual behaviour (reviewed by Deng & Rogers 2002a) and has a dramatic effect on the capacity of chicks to perform two simultaneous tasks such as feeding and predator vigilance (Rogers et al. 2004). It has been suggested that ecological factors, such as social density or abundance of predators, by influencing the choice of laying site or the time spent on the nest, affect the lateralization of the offspring and ultimately generate phenotypes with appropriate coping strategies (Deng & Rogers 2002b; Vallortigara & Rogers 2005; Andrew et al. 2009b).
Here, we demonstrated the potential disadvantages of a marked subdivision of the function between hemispheres in two contexts. The literature contains many other examples of remarkable left–right differences in the behavioural response. For example, toads, chicks and dunnarts differ in their promptness to react to a predator depending on the visual hemifield in which it appears (Lippolis et al. 2002, 2005; Dharmaretnam & Rogers 2005), and mosquitofish make closer cooperative predator inspection when predator and shoalmates are seen with the correspondingly preferred eye (De Santi et al. 2001). Gelada baboons and Anolis lizards are more likely to attack a conspecific on one side than the other (Deckel 1995; Casperd & Dunbar 1996), and side biases are shown by toads, chicks and pigeons in food detection (Vallortigara et al. 1998; Diekamp et al. 2005). Investigating whether individuals with greater left–right differences pay larger costs even in these cases will help us to assess the generality of our findings and expand our understanding of the selective mechanisms maintaining individual differences in lateralization.
Acknowledgements
The authors would like to thank Debi Roberson, George Georgiou and Annette Sieg for their useful comments.
References
- Andrew R. J.2009Origins of asymmetry in the CNS. Semin. Cell Dev. Biol. 20, 485–490 (doi:10.1016/j.semcdb.2008.11.001) [DOI] [PubMed] [Google Scholar]
- Andrew R. J., Rogers L. J.2002The nature of lateralization in tetrapods. Comparative vertebrate lateralization, pp. 94–125 Cambridge, UK: Cambridge University Press [Google Scholar]
- Andrew R. J., Dharmaretnam M., Gyori B., Miklosi A., Watkins J. A. S., Sovrano V. A.2009aPrecise endogenous control of involvement of right and left visual structures in assessment by zebrafish. Behav. Brain Res. 196, 99–105 (doi:10.1016/j.bbr.2008.07.034) [DOI] [PubMed] [Google Scholar]
- Andrew R. J., Osorio D., Budaev S.2009bLight during embryonic development modulates patterns of lateralization strongly and similarly in both zebrafish and chick. Phil. Trans. R. Soc. B 364, 983–989 (doi:10.1098/rstb.2008.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken K., Konrad C., Drager B., Breitenstein C., Kennerknecht I., Ringelstein E. B., Knecht S.2004Familial aggregation of strong hemispheric language lateralization. Neurology 63, 2433–2435 [DOI] [PubMed] [Google Scholar]
- Barth K. A., Miklosi A., Watkins J., Bianco I. H., Wilson S. W., Andrew R. J.2005fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 15, 844–850 (doi:10.1016/j.cub.2005.03.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza A., Dadda M.2005Enhanced schooling performance in lateralized fishes. Proc. R. Soc. B 272, 1677–1681 (doi:10.1098/rspb.2005.3145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza A., Pignatti R., Vallortigara G.1997Laterality in detour behaviour: interspecific variation in poeciliid fish. Anim. Behav. 54, 1273–1281 (doi:10.1006/anbe.1997.0522) [DOI] [PubMed] [Google Scholar]
- Bisazza A., Cantalupo C., Capocchiano M., Vallortigara G.2000Population lateralisation and social behaviour: a study with 16 species of fish. Laterality 5, 269–284 (doi:10.1080/135765000406111) [DOI] [PubMed] [Google Scholar]
- Bisazza A., Sovrano V. A., Vallortigara G.2001Consistency among different tasks of left–right asymmetries in lines of fish originally selected for opposite direction of lateralization in a detour task. Neuropsychologia 39, 1077–1085 (doi:10.1016/S0028-3932(01)00034-3) [DOI] [PubMed] [Google Scholar]
- Bisazza A., Dadda M., Cantalupo C.2005Further evidence for mirror-reversed laterality in lines of fish selected for leftward or rightward turning when facing a predator model. Behav. Brain Res. 156, 165–171 (doi:10.1016/j.bbr.2004.05.022) [DOI] [PubMed] [Google Scholar]
- Bisazza A., Dadda M., Facchin L., Vigo F.2007Artificial selection on laterality in the teleost fish Girardinus falcatus. Behav. Brain Res. 178, 29–38 (doi:10.1016/j.bbr.2006.11.043) [DOI] [PubMed] [Google Scholar]
- Brown C., Gardner C., Braithwaite V. A.2004Population variation in lateralized eye use in the poeciliid Brachyraphis episcopi. Proc. R. Soc. Lond. B 271, S455–S457 (doi:10.1098/rsbl.2004.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., Western J., Braithwaite V. A.2007The influence of early experience on, and inheritance of, cerebral lateralization. Anim. Behav. 74, 231–238 (doi:10.1016/j.anbehav.2006.08.014) [Google Scholar]
- Casperd J. M., Dunbar R. I. M.1996Asymmetries in the visual processing of emotional cues during agonistic interactions by gelada baboons. Behav. Process. 37, 57–65 (doi:10.1016/0376-6357(95)00075-5) [DOI] [PubMed] [Google Scholar]
- Clayton N. S., Krebs J. R.1994Memory for spatial and object-specific cues in food-storing and non-storing birds. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 174, 371–379 [Google Scholar]
- Collin S. P., Shand J.2003Retinal sampling and the visual field in fishes. Sensory processing in the aquatic environment, pp. 139–169 New York, NY: Springer [Google Scholar]
- Collins R. L.1975When left-handed mice live in right-handed worlds. Science 187, 181–184 (doi:10.1126/science.1111097) [DOI] [PubMed] [Google Scholar]
- Collins R. L.1990Asymmetry and lateralization in the mouse—a genetic perspective. Behav. Genet. 20, 711–712 [Google Scholar]
- Corballis M. C.2006Cerebral asymmetry: a question of balance. Cortex 42, 117–118 (doi:10.1016/S0010-9452(08)70335-6) [DOI] [PubMed] [Google Scholar]
- Dadda M., Bisazza A.2006Does brain asymmetry allow efficient performance of simultaneous tasks? Anim. Behav. 72, 523–529 (doi:10.1016/j.anbehav.2005.10.019) [Google Scholar]
- Dadda M., Zandonà E., Bisazza A.2007Emotional responsiveness in fish from lines artificially selected for a high or low degree of laterality. Physiol. Behav. 92, 764–772 (doi:10.1016/j.physbeh.2007.06.001) [DOI] [PubMed] [Google Scholar]
- David A. S.1989Perceptual asymmetry for happy–sad chimeric faces: effects of mood. Neuropsychologia 27, 1289–1300 (doi:10.1016/0028-3932(89)90041-9) [DOI] [PubMed] [Google Scholar]
- De Santi A., Sovrano V. A., Bisazza A., Vallortigara G.2001Mosquitofish display differential left- and right-eye use during mirror image scrutiny and predator inspection responses. Anim. Behav. 61, 305–310 (doi:10.1006/anbe.2000.1566) [Google Scholar]
- Deckel A. W.1995Laterality of aggressive responses in Anolis. J. Exp. Zool. 272, 194–200 (doi:10.1002/jez.1402720304) [Google Scholar]
- Deng C., Rogers L. J.2002aFactors affecting the development of lateralization in chicks. Comparative vertebrate lateralization, pp. 206–246 Cambridge, UK: Cambridge University Press [Google Scholar]
- Deng C., Rogers L. J.2002bPrehatching visual experience and lateralization in the visual Wulst of the chick. Behav. Brain Res. 134, 375–385 (doi:10.1016/S0166-4328(02)00050-5) [DOI] [PubMed] [Google Scholar]
- Dharmaretnam M., Rogers L. J.2005Hemispheric specialization and dual processing in strongly versus weakly lateralized chicks. Behav. Brain Res. 162, 62–70 (doi:10.1016/j.bbr.2005.03.012) [DOI] [PubMed] [Google Scholar]
- Diaz R., Ãgren S. O., Blum M., Fuxe K.1995Prenatal corticosterone increases spontaneous and d-amphetamine induced locomotor activity and brain dopamine metabolism in prepubertal male and female rats. Neuroscience 66, 467–473 (doi:10.1016/0306-4522(94)00605-5) [DOI] [PubMed] [Google Scholar]
- Diekamp B., Regolin L., Gunturkun O., Vallortigara G.2005A left-sided visuospatial bias in birds. Curr. Biol. 15, R372–R373 (doi:10.1016/j.cub.2005.05.017) [DOI] [PubMed] [Google Scholar]
- Facchin L., Bisazza A., Vallortigara G.1999What causes lateralization of detour behavior in fish? Evidence for asymmetries in eye use. Behav. Brain Res. 103, 229–234 (doi:10.1016/S0166-4328(99)00043-1) [DOI] [PubMed] [Google Scholar]
- Fride E., Weinstock M.1988Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci. 42, 1059–1065 (doi:10.1016/0024-3205(88)90561-9) [DOI] [PubMed] [Google Scholar]
- Gazzaniga M. S.2000Cerebral specialization and interhemispheric communication. Does the corpus callosum enable the human condition? Brain 123, 1293–1326 (doi:10.1093/brain/123.7.1293) [DOI] [PubMed] [Google Scholar]
- Gazzaniga M. S., Sperry R. W.1966Simultaneous double discrimination response following brain bisection. Psychon. Sci. 4, 261–262 [Google Scholar]
- Giljov A. N., Karenina K. A., Malashichev Y. B.2009An eye for a worm: lateralisation of feeding behaviour in aquatic anamniotes. Laterality 14, 273–286 (doi:10.1080/13576500802379665) [DOI] [PubMed] [Google Scholar]
- Gunturkun O.2002Ontogeny of visual asymmetry in pigeons. Comparative vertebrate lateralization, pp. 247–273 Cambridge, UK: Cambridge University Press [Google Scholar]
- Gunturkun O., Diekamp B., Manns M., Nottelmann F., Prior H., Schwarz A., Skiba M.2000Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr. Biol. 10, 1079–1081 (doi:10.1016/S0960-9822(00)00671-0) [DOI] [PubMed] [Google Scholar]
- Hager M. C., Helfman G. S.1991Safety in numbers: shoal size choice by minnows under predatory threat. Behav. Ecol. Sociobiol. 29, 271–276 (doi:10.1007/BF00163984) [Google Scholar]
- Halpern M. E., Gunturkun O., Hopkins W. D., Rogers L. J.2005Lateralization of the vertebrate brain: taking the side of model systems. J. Neurosci. 25, 10 351–10 357 (doi:10.1523/JNEUROSCI.3439-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman K. M., Van Den Abell T.1980Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology 30, 327–330 [DOI] [PubMed] [Google Scholar]
- Hopkins W. D., Dahl J. F., Pilcher D.2001Genetic influence on the expression of hand preferences in chimpanzees (Pan troglodytes): evidence in support of the right-shift theory and developmental instability. Psychol. Sci. 12, 299–303 (doi:10.1111/1467-9280.00355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle D. J.1965Interocular transfer in goldfish: color easier than pattern. Science 149, 1000–1002 (doi:10.1126/science.149.3687.1000) [DOI] [PubMed] [Google Scholar]
- Ingle D.1968Interocular integration of visual learning by goldfish. Brain Behav. Evol. 1, 58–85 (doi:10.1159/000125493) [Google Scholar]
- Jewell G., McCourt M. E.2000Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 38, 93–110 (doi:10.1016/S0028-3932(99)00045-7) [DOI] [PubMed] [Google Scholar]
- Krause J., Godin J. G.1994Shoal choice in the banded killifish (Fundulus diaphanus, Teleostei, Cyprinodontidae): effects of predation risk, fish size, species composition and size of shoals. Ethology 98, 128–136 [Google Scholar]
- Krause J., Ruxton G. D.2002Living in groups Oxford, UK: Oxford University Press [Google Scholar]
- Lippolis G., Bisazza A., Rogers L. J., Vallortigara G.2002Lateralisation of predator avoidance responses in three species of toads. Laterality 7, 163–183 (doi:10.1080/13576500143000221) [DOI] [PubMed] [Google Scholar]
- Lippolis G., Westman W., McAllan B. M., Rogers L. J.2005Lateralisation of escape responses in the stripe-faced dunnart, Sminthopsis macroura (Dasyuridae: Marsupialia). Laterality 10, 457–470 (doi:10.1080/13576500442000210) [DOI] [PubMed] [Google Scholar]
- Luck S. J., Hillyard S. A., Mangun G. R., Gazzaniga M. S.1989Independent hemispheric attentional systems mediate visual search in split-brain patients. Nature 342, 543–545 (doi:10.1038/342543a0) [DOI] [PubMed] [Google Scholar]
- Mallin H. D., Delius J. D.1983Inter-and intraocular transfer of colour discriminations with mandibulation as an operant in the head-fixed pigeon. Behav. Anal. Lett. 3, 297–309 [Google Scholar]
- Manning L., Halligan P. W., Marshall J. C.1990Individual variation in line bisection: a study of normal subjects with application to the interpretation of visual neglect. Neuropsychologia 28, 647–655 (doi:10.1016/0028-3932(90)90119-9) [DOI] [PubMed] [Google Scholar]
- Mark R. F.1966The tectal commissure and interocular transfer of pattern discrimination in cichlid fish. Exp. Neurol. 16, 215–225 (doi:10.1016/0014-4886(66)90100-2) [DOI] [PubMed] [Google Scholar]
- McCleary R. A.1960Type of response as a factor in interocular transfer in the fish. J. Comp. Physiol. Psychol. 53, 311–321 (doi:10.1037/h0040727) [Google Scholar]
- McCourt M. E., Olafson C.1997Cognitive and perceptual influences on visual line bisection: psychophysical and chronometric analyses of pseudoneglect. Neuropsychologia 35, 369–380 (doi:10.1016/S0028-3932(96)00143-1) [DOI] [PubMed] [Google Scholar]
- McGrew W. C., Marchant L. F.1999Laterality of hand use pays off in foraging success for wild chimpanzees. Primates 40, 509–513 (doi:10.1007/BF02557586) [Google Scholar]
- McManus I. C., Tomlinson J.2004Objects look different sizes in the right and left eyes. Laterality 9, 245–265 [DOI] [PubMed] [Google Scholar]
- Northmore D. P. M., Gallagher S. P.2003Functional relationship between nucleus isthmi and tectum in teleosts: synchrony but no topography. Vis. Neurosci. 20, 335–348 (doi:10.1017/S0952523803203126) [DOI] [PubMed] [Google Scholar]
- Pascual A., Huang K. L., Neveu J., Preat T.2004Brain asymmetry and long-term memory: fruitflies that have structurally similar brain hemispheres forget within a matter of hours. Nature 427, 605–606 (doi:10.1038/427605a) [DOI] [PubMed] [Google Scholar]
- Prior H., Wilzeck C.2008Selective feeding in birds depends on combined processing in the left and right brain hemisphere. Neuropsychologia 46, 233–240 (doi:10.1016/j.neuropsychologia.2007.07.014) [DOI] [PubMed] [Google Scholar]
- Rogers L. J.2000Evolution of hemispheric specialization: advantages and disadvantages. Brain Lang. 73, 236–253 (doi:10.1006/brln.2000.2305) [DOI] [PubMed] [Google Scholar]
- Rogers L. J.2002Advantages and disadvantages of lateralization. Comparative vertebrate lateralization, pp. 126–153 Cambridge, UK: Cambridge University Press [Google Scholar]
- Rogers L. J., Deng C.2005Corticosterone treatment of the chick embryo affects light-stimulated development of the thalamofugal visual pathway. Behav. Brain Res. 159, 63–71 (doi:10.1016/j.bbr.2004.10.003) [DOI] [PubMed] [Google Scholar]
- Rogers L. J., Zucca P., Vallortigara G.2004Advantages of having a lateralized brain. Proc. R. Soc. Lond. B 271, S420–S422 (doi:10.1098/rsbl.2004.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C., Patterson T. A., Rose S. P. R.1993Visual input and lateralization of brain function in learning in the chick. Neuroscience 52, 393–401 (doi:10.1016/0306-4522(93)90166-D) [DOI] [PubMed] [Google Scholar]
- Schenkenberg T., Bradford D. C., Ajax E. T.1980Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology 30, 509–517 [DOI] [PubMed] [Google Scholar]
- Skiba M., Diekamp B., Prior H., Gunturkun O.2000Lateralized interhemispheric transfer of color cues: evidence for dynamic coding principles of visual lateralization in pigeons. Brain Lang. 73, 254–273 (doi:10.1006/brln.2000.2306) [DOI] [PubMed] [Google Scholar]
- Sovrano V. A., Dadda M., Bisazza A.2005Lateralized fish perform better than nonlateralized fish in spatial reorientation tasks. Behav. Brain Res. 163, 122–127 (doi:10.1016/j.bbr.2005.04.012) [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Hori M.2008Behavioural laterality in the shrimp-eating cichlid fish Neolamprologus fasciatus in Lake Tanganyika. Anim. Behav. 75, 1359–1366 (doi:10.1016/j.anbehav.2007.09.008) [Google Scholar]
- Vallortigara G., Andrew R. J.1991Lateralization of response by chicks to change in a model partner. Anim. Behav. 41, 187–194 (doi:10.1016/S0003-3472(05)80470-1) [Google Scholar]
- Vallortigara G., Bisazza A.2002How ancient is brain lateralization? Comparative vertebrate lateralization, pp. 9–69 Cambridge, UK: Cambridge University Press [Google Scholar]
- Vallortigara G., Rogers L. J.2005Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–589 (doi:10.1017/S0140525X05000105) [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Regolin L., Pagni P.1999Detour behaviour, imprinting and visual lateralization in the domestic chick. Cogn. Brain Res. 7, 307–320 (doi:10.1016/S0926-6410(98)00033-0) [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L. J., Bisazza A., Lippolis G., Robins A.1998Complementary right and left hemifield use for predatory and agonistic behaviour in toads. NeuroReport 9, 3341–3344 (doi:10.1097/00001756-199810050-00035) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Freire R., Munro U., Ritz T., Rogers L., Thalau P., Wiltschko R.2007The magnetic compass of domestic chickens Gallus gallus. J. Exp. Biol. 210, 2300–2310 (doi:10.1242/jeb.004853) [DOI] [PubMed] [Google Scholar]
- Xiao Q., Gunturkun O.2009Natural split-brain? Lateralized memory for task contingencies in pigeons. Neurosci. Lett. 458, 75–78 (doi:10.1016/j.neulet.2009.04.030) [DOI] [PubMed] [Google Scholar]
- Zappia J. V., Rogers L. J.1987Sex differences and reversal of brain asymmetry by testosterone in chickens. Behav. Brain Res. 23, 261–267 (doi:10.1016/0166-4328(87)90026-X) [DOI] [PubMed] [Google Scholar]
- Zucca P., Sovrano V. A.2008Animal lateralization and social recognition: quails use their left visual hemifield when approaching a companion and their right visual hemifield when approaching a stranger. Cortex 44, 13–20 (doi:10.1016/j.cortex.2006.01.002) [DOI] [PubMed] [Google Scholar]