Abstract
Habituation is one of the most fundamental learning processes that allow animals to adapt to dynamic environments. It is ubiquitous and often thought of as a simple form of non-associative learning. Very little is known, though, about the rules that govern habituation and their significance under natural conditions. Questions about how animals incorporate habituation into their daily behaviour and how they can assure only to habituate to non-relevant stimuli are still unanswered. Animals under threat of predation should be particularly selective about which stimuli they habituate to, since ignoring a real threat could be fatal. In this study, we tested the response of fiddler crabs, Uca vomeris, to repeatedly approaching dummy predators to find out whether these animals habituate to potential predators and to test the selectivity of the habituation process. The crabs habituated to model predators, even though they were confronted with real predators during the same habituation process. They showed remarkable selectivity towards the stimulus: a simple change in the approach distance of the stimulus led to a recovery in their responses. The results strongly indicate that in the context of predator avoidance, habituation under natural conditions is highly selective and a stimulus is not defined just by its current sensory signature, but also its spatio-temporal history.
Keywords: habituation, fiddler crabs, predation, learning, predator avoidance, vision
1. Introduction
Habituation is a widespread form of behavioural plasticity in animals that leads to a decrease in responsiveness to repeatedly encountered, irrelevant events (Rankin et al. 2009; Thompson 2009). Despite years of research, both the underlying rules and the adaptive significance of the habituation process are still poorly understood (Rankin et al. 2009). In particular, we know little about the role of habituation in complex behavioural situations such as predator avoidance and under natural, evolution-relevant conditions. Most of our current knowledge is based on experiments performed in simplified, carefully controlled conditions. The natural environment, however, is complex and unpredictable. Similarly, natural stimuli are never precisely the same and are often extended in time rather than short and punctuated. There exists significant need, therefore, to address the phenomenon of habituation and the rules that govern this process in experiments conducted under natural conditions.
Habituation is thought to be a fundamental process serving to remove irrelevant information from the sensory input stream. This allows animals to focus on important stimuli (Rose & Rankin 2001). It is therefore crucial to understand how animals achieve an appropriate level of selectivity. If habituation is too selective, it will be ineffective and offers no advantage. If it is too general, relevant events will be missed. When dealing with predators, habituation can be effective in minimizing costs of false alarms (e.g. Dacier et al. 2006; Glaudas et al. 2006). Prey animals, with their lives at stake, have to be especially careful not to habituate to the wrong stimulus or one that is classified too broadly. In the context of predator avoidance, therefore, we expect habituation to be particularly selective.
We have previously studied predator avoidance in fiddler crabs using dummy predators that approach from a distance. Results from these experiments showed no habituation to repeated dummy approaches (Hemmi 2005a). We know very well, however, that crabs, including fiddler crabs, quickly habituate to the presence of human observers (Walker 1972; MacFarlane & King 2002; J. M. Hemmi & T. Merkle 2008, personal observation). Crabs have also been shown to habituate, under laboratory conditions, to threatening, regular stimuli (Sztarker & Tomsic 2008).
Fiddler crabs are an important food source for a variety of shore birds (e.g. Iribarne & Martinez 1999). At our study site, Uca vomeris are constantly threatened by terns that scan the mudflats for prey (Land 1999; Hemmi & Zeil 2005). Disturbance by terns can occur as frequently as every 2–3 minutes for hours at a time (J. M. Hemmi & T. Merkle 2008, personal observation). In order to feed, mate, and engage in social interactions, the crabs need to find ways to cope with this frequent threat. With their close-set eyes and poor visual acuity (Zeil & Hemmi 2006; Smolka & Hemmi in press), fiddler crabs have only incomplete information about the distance, movement direction, shape, and identity of approaching predators (Hemmi 2005b).
Previously, we proposed that fiddler crabs employ a multi-stage predator response strategy in combination with habituation to minimize the costs of false alarms inflicted by the high sensitivity and poor selectivity of their initial response (Hemmi 2005b; Hemmi & Zeil 2005). Here, we investigate whether they use habituation in predator avoidance and how they achieve the necessary selectivity despite their poor spatial acuity. We show that fiddler crabs rapidly habituate to a continuously visible ‘local’ dummy predator that approaches repeatedly. When the same dummy approaches from a distance, however, the crabs respond regardless. The results show that the crabs’ escape decision is influenced by the history of the stimulus and not just its immediate sensory signature.
2. Material and methods
(a). Experimental procedure
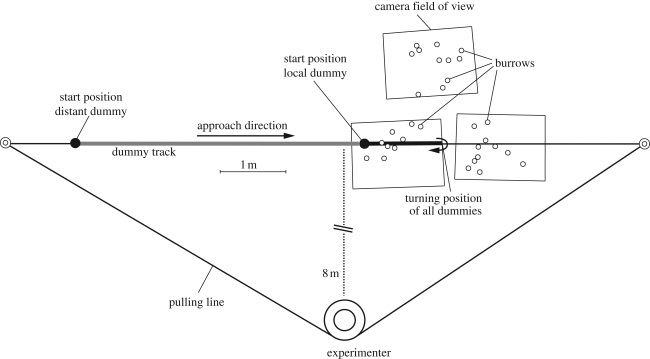
Experiments were conducted on two consecutive days using two independent groups of fiddler crabs, U. vomeris, during the early part of low tides on the mudflats of Bowling Green Bay, Townsville, Australia (19°24.3′ S, 147°6.9′ E). In each experiment, three video cameras (Panasonic NV-GS150GN) fixed to metal poles 1.6 m above the mudflat were continuously recording crab behaviour over an area of approximately 1.2 m2 each (figure 1).
Figure 1.
Experimental setup. The sketch shows the spatial arrangement of the three cameras, the dummy track, the crabs' burrows, and the experimenter for the first experimental session. The cameras recorded the crabs' responses to the dummy movements for two different approach types, a local dummy (thick black line) that moved only close to the crabs and a distant dummy (thick grey line) which moved along the same dummy track, but approached from further away.
A black styrofoam ball of 4 cm diameter was used as the dummy predator (see Hemmi 2005a). Threaded on a fine fishing line (the track), the dummy could be moved along this 20 cm high track at a speed of approximately 20 cm s−1 with a large driving wheel attached to the pulling line (figure 1). This setup enabled the experimenter to remotely control the dummy. We showed earlier (Hemmi 2005a) that this dummy, modelled on the hunting behaviour of the gull-billed tern Gelochelidon nilotica (Land 1999), successfully elicits anti-predator responses in fiddler crabs.
After setting up all equipment, crabs were given at least 10 min to resume their normal foraging behaviour before the first run started. During this time, the local dummy was already at its start position.
We used two physically identical dummy types that differed only in their starting position. The first type, the local dummy, was always close to the crabs, within one camera's field of view (figure 1). From there, it moved approximately 1 m towards the crabs before promptly returning to its starting position. These ‘runs’ were repeated every 2.1 ± 0.4 min (mean ± s.d.). Local dummies never left the area and remained visible to all crabs on the surface. After 26 runs on day 1 or 22 runs on day 2, the returning local dummy was moved about 4 m past its starting position. This second dummy type, the ‘distant’ dummy, then approached the crabs after the normal inter-stimulus interval along exactly the same track (figure 1). The distant dummy approached the crabs in runs 27, 28, 33, and 34 (day 1) and 23, 24, 29, 30, 34, and 35 (day 2).
(b). Video analysis and response measures
Video footage was digitised using DVGRAB (open-source Linux software) and the behavioural data extracted using custom-made Matlab software (by J. M. H.). All crabs were assigned to their individual burrows, which they occupy long term and return to in case of danger. Crab positions were tracked at 200 ms intervals. We calibrated camera images, removed lens distortion effects and determined the positions of the cameras relative to each other and to the ground with the help of a checker-board standard and open-source software developed by Bouguet (2005). Dummy movements were recorded by running the pulling line through three wheels, each visible to one of the video cameras. From the wheel rotations, we could calculate the dummy's position relative to known objects in the video sequences, even when the dummy itself was not visible in every image.
A home run was considered to have occurred whenever a crab moved at least 3 cm towards its burrow during a three frame period (600 ms). The response start was assigned to the first of these frames in which the crab had moved at least 1 cm during one 200 ms interval. A burrow descent was recorded when a crab entered its burrow to the point where it became invisible. Responses were only counted if they occurred during the incoming phase of the dummy movement, i.e. while it moved from its starting position (local or distant) towards the turning point (figure 1). The decision to run home or go underground was assumed to have happened one frame (200 ms) before a response could be measured. For the home run, we only considered crabs that were at least 5 cm away from their burrow at the start of the run (Hemmi 2005a). This ensured that a home run could be scored by the criterion defined above. For each valid run, we determined the distance between crab and dummy at the time of response and the proportion of the distance to the burrow covered while running home, herein referred to as response strength.
All crabs that left the cameras' field of view during the dummy movement or were involved in an interaction with another crab were excluded from the analyses.
(c). Statistics
(i). Permutation analysis
All data were analysed in the context of a simple permutation approach (Good 2005). This non-parametric approach allowed us to avoid assumptions about the underlying statistical distribution of the data, yet take into account the multiple response measures per crab. To test for statistical effects, the variable in question was randomly permuted 10 000 times across each individual crab's responses. The score of our statistical measure (see below) computed on our original, unpermuted dataset was then compared with the scores of the permuted data sets. Significance was judged by calculating the percentage of permutations that resulted in a score that was more extreme or equal to the score calculated from the unpermuted dataset. By permuting strictly within individual crabs, we eliminated crab-to-crab variability from the analysis. To test for habituation effects (e.g. decreasing response probability), our statistical measure was either the sum of presentation numbers for all responses or the sum of the product of the presentation number and the response distance or response strength. In all cases, a lower than average score indicates that crabs reduced their response probability, distance or strength over the course of the experiment.
We tested whether response probability was affected by how close the dummy was able to approach the crabs (figure 3), by resampling not within crabs but within runs. This was necessary, as owing to the fixed geometry of the setups, there was little variability in how closely the dummy was able to approach a particular crab/burrow between runs involving a particular crab.
To test whether response probability was affected by changes in bearing at which the dummy was seen, we used the same approach as we used to check for habituation. The permutation score was calculated as the product between the angular change in bearing and whether or not a response occurred. All permutation tests are in agreement with results from a generalized mixed model analysis performed in R (R Development Core Team 2008) using the lme4 package.
3. Results
(a). Habituation to local predators
In order to test whether fiddler crabs habituate to the repeated approaches of a local, continuously visible, dummy predator, we examined the home run and the burrow descent response. These represent the two ecologically most costly stages of the crabs' predator avoidance behaviour.
(i). Home run
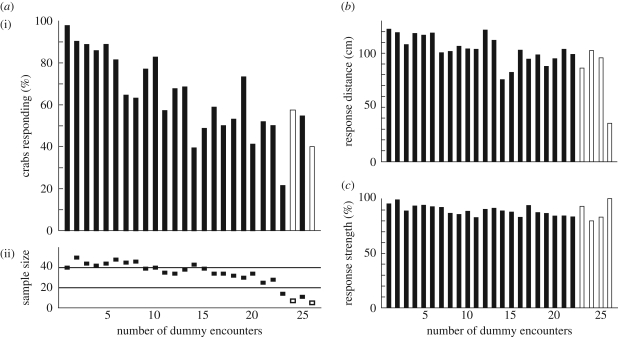
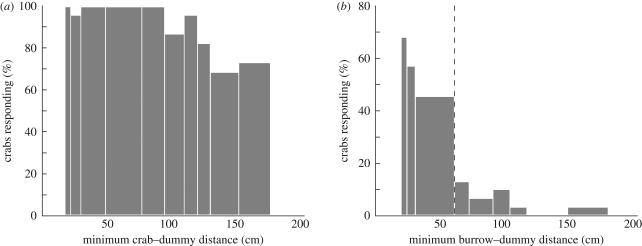
During the first confrontation with an approaching dummy predator, all but one out of 40 crabs responded by running towards their burrow (figure 2a). However, the likelihood that crabs responded dropped significantly after repeated movements of the dummy. The probability of response declined from almost 100 per cent to about 40 or 50 per cent during the first 26 dummy movements (n = 881, p < 0.001, figure 2a). The crabs also responded later, i.e. they allowed the dummy to approach them more closely before running home (n = 881, p < 0.001, figure 2b). In contrast, their response strength reduced only minimally during the course of the experiment (n = 881, p < 0.001, figure 2c). The significant but small decline visible in figure 2c is likely due to the fact that towards the end of the experiment, crabs more often ran home without actually going underground (see below). A 2 cm crab that starts 20 cm away from its burrow and runs home to just touch its burrow with its legs, would only score a strength of 90 per cent, even though it effectively ran all the way home. Response probability also depends on the crabs' distance from the dummy. Nearby crabs that were approached more closely by the dummy were more likely to respond (n = 220, p<0.01, figure 3a), but all crabs habituated.
Figure 2.
Crabs are less likely to respond to a moving dummy predator after repeated encounters. The x-axis in each panel shows the number of times a particular crab had actually encountered the local dummy move. For a crab that was underground during the first dummy movement, the second movement would be its first encounter. Open bars and boxes indicate runs where less than 10 crabs contributed. (a) (i) Percentage of crabs that ran home as the dummy approached (p < 0.001). (ii) Sample sizes. (b) Response distance, measured as the distance between dummy and crab at the time of response. Distances are only available for runs where crabs responded (p < 0.001). (c) Response strength, measured as the percentage of distance towards the burrow the crab ran home (p < 0.001).
Figure 3.
Crabs that were further away from the dummy were less likely to respond to the local dummy during the first five dummy runs. (a) Home run (p < 0.001) and (b) burrow descent (p < 0.001) probability as a function of the minimum possible distance between the dummy and crab (a) or the crab's burrow (b), respectively. The width of the distance bins has been adjusted in both panels to include approximately equal numbers of crabs (a, Nbin = 22; b, Nbin = 30/31).
(ii). Burrow descent
Not all crabs that ran home entered their burrow in a direct response to the stimulus. Only about 25 per cent of all crabs did so during the initial dummy approach. This was partly because for some crabs the dummy simply did not come close enough to trigger burrow descent. Crabs that were approached more closely were much more likely to go underground (figure 3b, n = 307, p < 0.001). We therefore restricted our analysis to those crabs whose burrows were approached to within 60 cm by the dummy (figure 3b, dashed line).
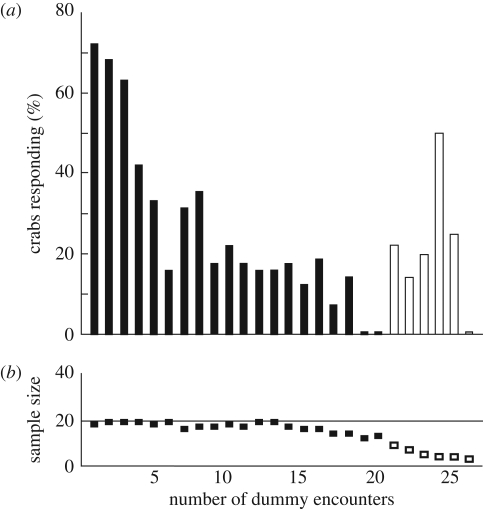
The crabs’ probability of going underground clearly decreased during successive dummy movements in a similar way as observed for the home run. Response probabilities dropped from over 70 per cent when the dummy moved for the first time to less than 20 per cent towards the end of the experiment (n = 369, p < 0.001, figure 4).
Figure 4.
The probability of burrow descent decreases with repeated movements of the local dummy predator. The x-axis shows the number of dummy runs seen by each crab (encounters). (a) Percentage of crabs that went underground during the incoming movement of the dummy (p < 0.001). (b) Sample sizes for each dummy encounter (otherwise conventions as in figure 2).
(b). Responses to distant predators
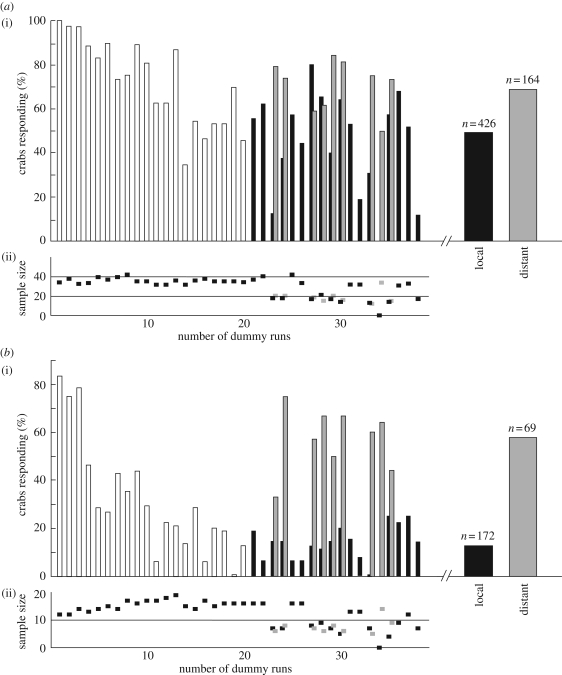
The strong decrease in the crabs’ response probability was rather unexpected, especially for the home run. Previous dummy experiments had shown no clear signs of habituation (Hemmi 2005a,b). These experiments, however, used a dummy predator that always approached from far away. We therefore investigated what would happen if the dummy moved far away for the duration of one stimulus interval, and then approached from that position along the same dummy track as before. The crabs indeed responded more strongly to this distant dummy (figure 5, grey bars) than to the local dummy (black bars), even though it was exactly the same object that approached them. This held true for both the home run (n = 590, p < 0.001, figure 5a) and the burrow descent (n = 241, p < 0.001, figure 5b). The effect was particularly strong for the burrow descent, where the response probability increased on average by more than four times for the distant dummy (figure 5b, wide bars).
Figure 5.
After an initial habituation phase to the local dummy, the crabs were more likely to respond to a dummy approaching from a distance. (a(i)) Probability of crabs running home (p < 0.001). (b(i)) Probability of crabs going underground (p < 0.001). In both panels, response probability is plotted against actual dummy runs rather than encounters as in figures 2 and 4. This was necessary to pool the data from the distant dummy movements in order to calculate meaningful percentages. Open bars, habituation phase to the continuously visible local dummy; black bars, response probability to local dummies after the habituation phase; grey bars, response probability to distant dummies. The only difference between the two dummy types was their approach distance. The distant dummy moved along the same track but started 4 m further away. Wide bars on the right show the mean response probability for the local dummy (black bar) for runs 21–38 and the distant dummy (grey bar). a(ii) and b(ii) Sample sizes for each dummy approach.
It is important to note that in most cases the crabs did not go underground until the distant dummy was well within the region where they had previously seen the local dummy. Only crabs that had their burrow close to the starting position of the local dummy responded earlier. These crabs had no other option than to respond outside this region because the dummy would have approached them too closely. However, even if we exclude all eleven runs where crabs did respond outside the region where they used to see the local dummy, our earlier conclusion that the crabs are more likely to respond to the distant dummy still holds true (n = 358, p < 0.001).
The point we stress here is that in all remaining cases, the crabs decided to respond to the distant dummy when it was already inside the region where the local dummy moved during the habituation phase (34 ± 22 cm (mean ± s.d.)). In other words, at the time of decision, the distant dummy looked exactly the same as the local dummy: it was the same dummy, seen at the same place, moving at the same speed.
(c). Is habituation retinotopic?
It is possible that the crabs in our experiments always saw the local dummy in the same part of their visual field, which may have caused habituation. The distant dummy was seen in a different part of the visual field and may, therefore, have elicited a higher response probability. Nalbach (1990) has shown that, at the neuronal level, looming sensitive neurons habituate in one part of their receptive field, but become dishabituated when the approach direction of the stimulus changes. Fiddler crabs align their longitudinal body axis with their homing direction when foraging in the vicinity of the burrow (Zeil 1998). Provided the crabs in our experiments always moved away from their burrows in the same compass direction, they would always have seen the local dummy with the same part of their retina. Habituation in this case may simply involve ignoring image motion in this particular part of the visual field. However, the body orientation of individual crabs in our experiments varied widely from one run to the next with a mean change of 65 ± 20° (mean ± s.d.) throughout the course of the experiments. Furthermore, there was no correlation between the response probability and changes in the direction in which a crab saw the dummy for successive dummy runs (n = 816, p = 0.47), indicating that habituation was not retinotopic.
4. Discussion
We have shown that fiddler crabs rapidly reduce their responsiveness to a locally moving dummy predator after repeated presentations. Both the likelihood that crabs ran home and that they descended into their burrow diminished over the course of the experiment. However, when the same dummy changed its behaviour by approaching from farther away, the habituated crabs were again more likely to respond. The decrease in response probability is therefore due to habituation and not sensory or motor fatigue, a general decrease in activity or sensory adaptation (Rankin et al. 2009). The effect was particularly strong for the underground response where the crabs were more than four times as likely to respond to the distant compared with the local dummy (figure 5b).
It is important to realize that when the crabs decided to respond to the distant dummy, they saw exactly the same object in exactly the same region of space as during the habituation phase. The only difference between the local and distant dummy was their history, i.e. their previous movement. The local dummy had started close-by and was clearly visible even before it started moving, while the distant dummy started about 4 m farther away and would have been difficult to spot. At its starting distance (4.3 m away on average) the distant dummy would be a stationary black dot of just 0.53° in size seen 2.7° above the horizon (Smolka & Hemmi in press). In this case, the dummy would only partially cover the receptive field of a single ommatidium. The same dummy at the starting distance (61 cm on average) of the local dummy, had an angular size of more than 3° and would be seen by about six ommatidia at 18° of elevation.
Equally striking is that the crabs did not dishabituate as a result of the intermittent appearance of the distant dummy. Their response probability with respect to the local dummy appears unaffected (figure 5). This strongly suggests that the crabs were able to distinguish clearly between the two events and treat them differently, indicating a high stimulus specificity. In fact, not only did the crabs distinguish between the two dummy types, but they habituated to the movements of the local dummy while still responding to real terns! During the course of each habituation experiment, we recorded an average of 25 hunting terns that elicited crab responses.
(a). Stimulus specificity
Stimulus specificity, or stimulus generalization, is thought to help animals respond to objects that are clearly novel, but habituate to objects that are similar across space and time (Rankin et al. 2009). The crabs in this study showed a remarkable specificity. A simple change in the approach distance of an otherwise identical stimulus suppressed habituation, and the crabs' response probability increased. The high stimulus specificity emphasizes how sensitive habituation is to the spatio-temporal structure of natural events. We do not yet know how this specificity is achieved. One possibility is that local interneurons integrate some approach measure over time and thereby distinguish between the two events.
Alternatively, the crabs may have associated the habituation process with the prior presence of the local dummy, treated it as a known object, and only habituated to this particular object. The local dummy was very prominent in between its movements and was constantly visible before and during the habituation phase. The approach of the distant dummy did not dishabituate the crabs: they still ignored the local dummy after exposure to the distant dummy. This implies that the dummy's identity is connected to its location in the crabs' surroundings rather than to its consistent visibility. If, in the context of predator avoidance, crabs indeed only habituate to known objects, we could explain why we did not find habituation in previous experiments that used only distant dummies (Hemmi 2005a). The association between a known object and habituation represents an associative effect on habituation that is distinct from context conditioning (e.g. Wagner 1979; Rankin 2000). During context conditioning, an association is formed between a context and a stimulus that can later modify the animal's behaviour. For instance, Tomsic et al. (1993) found that changes in context between the training and the test phase abolished the effects of habituation in the crab Chasmagnathus. Associative effects appear to be confined to long-term habituation (Wagner 1979; Maldonado et al. 1997; Pedreira et al. 1998; Rankin 2000; Rose & Rankin 2001; Dong & Clayton 2009). We do not know, at this point, whether the habituation effects presented here constitute short or long-term habituation. This could only be decided by an additional testing session hours or days later. It is clear, though, that our stimulus protocol very closely resembles the standard protocol of one stimulus every 3 min that induces long-term habituation to a predatory stimulus in Chasmagnathus (Tomsic et al. 1998).
(b). Habituation in predation
The high specificity of the fiddler crabs’ habituation process is predicted by the argument that in the context of predator avoidance animals should learn what not to fear, rather than what to fear (e.g. Deecke et al. 2002). The entire design of the fiddler crabs' anti-predator response strategy reflects this strategy. Their escape response is triggered by a sensitive, but unspecific criterion that is related to the retinal speed of objects (Hemmi 2005b). This criterion is a poor predictor of the actual risk and is always likely to lead to a high number of false alarms. Field observations emphasize this: crabs run away from terns, but also frequently from kites, flying insects, and wind-blown leaves (Smolka 2009). We have previously argued that habituation is one of the mechanisms, which might help crabs to mitigate the impact of false alarms (Hemmi & Zeil 2005). The present experiments demonstrate that crabs are indeed able to learn to ignore specific kinds of movements, even of very threatening stimuli. It is important to remember that crabs have essentially no distance information for objects above the horizon (Hemmi 2005b): a 4 cm dummy suspended 20 cm above the substrate is thus similar to a dangerous tern hovering 2 m above the mudflat. In the field, a fiddler crab that owns a burrow would never allow a real tern to approach to a distance equivalent to that of the local dummy (J. M. Hemmi & T. Merkle 2008, personal observation).
The fiddler crab strategy is, therefore, to respond to all movement, in the first instance, and then to habituate to certain, well-defined events. Habituation is an important component of the crabs’ anti-predator strategy and it is the high stimulus specificity we have shown here that makes this strategy safe.
In conclusion, we demonstrated that fiddler crabs habituate to the movements of a constantly visible, local dummy predator. The habituation process underlying this reduction in response probability is very flexible yet highly specific. On the one hand, the crabs learnt to ignore objects even though stimulation was not retinotopic, did not occur at fixed time intervals, and was interrupted by natural predator approaches. On the other hand, once habituated, crabs still responded to the same object when it approached from further away. The stimulus specificity displayed by the crabs shows that habituation to predators is sensitive to the spatio-temporal history of the stimulus and not just its current sensory signature.
Acknowledgements
We wish to thank Jochen Zeil for his advice and helpful comments on an earlier version of the manuscript, the RSB workshop team for the custom-made equipment, and Wiebke Ebeling, Nerida Harley, Shaun New, Richard Peters, Jochen Smolka, and three referees for constructive criticism on earlier drafts of the manuscript. We are grateful to Catherine Rankin for her interest and support and to Teresa Neeman from the ANU statistical consulting unit for advice and help with the statistics. We acknowledge support from the ARC Centre of Excellence program and the Centre for Visual Sciences. The work of T.M. is supported by a postdoctoral fellowship granted by the German Science Foundation (DFG).
References
- Bouguet J. Y.2005Camera calibration toolbox for Matlab MRL—Intel Corp; (See http://www.vision.caltech.edu/bouguetj/calib_doc) [Google Scholar]
- Dacier A., Maia R., Agustinho D. P., Barros M.2006Rapid habituation of scan behavior in captive marmosets following brief predator encounters. Behav. Process. 71, 66–69 (doi:10.1016/j.beproc.2005.09.006) [DOI] [PubMed] [Google Scholar]
- Deecke V. B., Slater P. J. B., Ford J. K. B.2002Selective habituation shapes acoustic predator recognition in harbour seals. Nature 420, 171–173 (doi:10.1038/nature01030) [DOI] [PubMed] [Google Scholar]
- Dong S., Clayton D. F.2009Habituation in songbirds. Neurobiol. Learn. Mem. 92, 183–188 (doi:10.1016/j.nlm.2008.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaudas X., Winne C. T., Fedewa L. A.2006Ontogeny of anti-predator behavioral habituation in cottonmouths (Agkistrodon piscivorus). Ethology 112, 608–615 (doi:10.1111/j.1439-0310.2005.01183.x) [Google Scholar]
- Good P. I.2005Permutation, parametric and bootstrap tests of hypotheses New York, NY: Springer [Google Scholar]
- Hemmi J. M.2005aPredator avoidance in fiddler crabs: 1. Escape decisions in relation to the risk of predation. Anim. Behav. 69, 603–614 (doi:10.1016/j.anbehav.2004.06.018) [Google Scholar]
- Hemmi J. M.2005bPredator avoidance in fiddler crabs: 2. The visual cues. Anim. Behav. 69, 615–625 (doi:10.1016/j.anbehav.2004.06.019) [Google Scholar]
- Hemmi J. M., Zeil J.2005Animals as prey: perceptual limitations and behavioural options. Mar. Ecol. Prog. Ser. 287, 274–278 [Google Scholar]
- Iribarne O. O., Martinez M. M.1999Predation on the southwestern Atlantic fiddler crab (Uca uruguayensis) by migratory shorebirds (Pluvialis dominica, P-squatarola, Arenaria interpres, and Numenius phaeopus). Estuaries 22, 47–54 (doi:10.2307/1352926) [Google Scholar]
- Land M. F.1999The roles of head movements in the search and capture strategy of a tern (Aves, Laridae). J. Comp. Physiol. A 184, 265–272 (doi:10.1007/s003590050324) [Google Scholar]
- MacFarlane G. R., King S. A.2002Observer presence influences behaviour of the semaphore crab, Heloecious cordiformis. Anim. Behav. 63, 1191–1194 (doi:10.1006/anbe.2002.3016) [Google Scholar]
- Maldonado H., Romano A., Tomsic D.1997Long-term habituation (LTH) in the crab Chasmagnathus: a model for behavioral and mechanistic studies of memory. Braz. J. Med. Biol. Res. 30, 813–826 [DOI] [PubMed] [Google Scholar]
- Nalbach H. O.1990Visually elicited escape in crabs. In Frontiers in crustacean neurobiology (eds Wiese K., Krent W. D., Tautz J., Reichert H., Mulloney B.), pp. 165–172 Basel, Switzerland: Birkhauser Verlag [Google Scholar]
- Pedreira M. E., Romano A., Tomsic D., Lozada M., Maldonado H.1998Massed and spaced training build up different components of long-term habituation in the crab Chasmagnathus. Anim. Learn. Behav. 26, 34–45 [Google Scholar]
- R Development Core Team 2008R: a language and environment for statistical computing. Foundation for statistical computing Vienna, Austria: See http://www.R-project.org [Google Scholar]
- Rankin C. H.2000Context conditioning in habituation in the nematode Caenorhabditis elegans. Behav. Neurosci. 114, 496–505 (doi:10.1037/0735-7044.114.3.496) [PubMed] [Google Scholar]
- Rankin C. H., et al. 2009Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 92, 135–138 (doi:10.1016/j.nlm.2008.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Rankin C. H.2001Analyses of habituation in Caenorhabditis elegans. Learn. Mem. 8, 63–69 (doi:10.1101/lm.37801) [DOI] [PubMed] [Google Scholar]
- Smolka J. Sampling visual space: topography, colour vision and visually guided predator avoidance in fiddler crabs (Uca vomeris) The Australian National University; 2009. [Google Scholar]
- Smolka J., Hemmi J. M.In press The topography of vision and behaviour. J. Exp. Biol. [DOI] [PubMed] [Google Scholar]
- Sztarker J., Tomsic D.2008Neuronal correlates of the visually elicited escape response of the crab Chasmagnathus upon seasonal variations, stimuli changes and perceptual alterations. J. Comp. Physiol. A 194, 587–596 (doi:10.1007/s00359-008-0333-3) [DOI] [PubMed] [Google Scholar]
- Thompson R. F.2009Habituation: a history. Neurobiol. Learn. Mem. 92, 127–134 (doi:10.1016/j.nlm.2008.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsic D., Massoni V., Maldonado H.1993Habituation to a danger stimulus in two semiterrestrial crabs: ontogenic, ecological and opioid modulation correlates. J. Comp. Physiol. A 173, 621–633 [Google Scholar]
- Tomsic D., Pedreira M. E., Romano A., Hermitte G., Maldonado H.1998Context-US association as a determinant of long-term habituation in the crab Chasmagnathus. Anim. Learn. Behav. 26, 196–209 [Google Scholar]
- Walker I.1972Habituation to disturbance in the fiddler crab (Uca annulipes) in its natural environment. Anim. Behav. 20, 139–146 (doi:10.1016/S0003-3472(72)80184-2) [Google Scholar]
- Wagner A. R.1979Habituation and memory. In Mechanisms of learning and motivation, (eds Dickinson A., Boakes R. A.), pp. 53–82 Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]
- Zeil J.1998Homing in fiddler crabs (Uca lactea annulipes and Uca vomeris: Ocypodidae). J. Comp. Physiol. A 183, 367–377 (doi:10.1007/s003590050263) [Google Scholar]
- Zeil J., Hemmi J. M.2006The visual ecology of fiddler crabs. J. Comp. Physiol. A 192, 1–25 (doi:10.1007/s00359-005-0048-7) [DOI] [PubMed] [Google Scholar]