Abstract
Hydrothermal vent mussels belonging to the genus Bathymodiolus are distributed worldwide and dominate communities at shallow Atlantic hydrothermal sites. While organisms inhabiting coastal ecosystems are subjected to predictable oscillations of physical and chemical variables owing to tidal cycles, the vent mussels sustain pronounced temperature changes over short periods of time, correlated to the alternation of oxic/anoxic phases. In this context, we focused on the short-term adaptive response of mussels to temperature change at a molecular level. The mRNA expression of 23 genes involved in various cell functions of the vent mussel Bathymodiolus azoricus was followed after heat shocks for either 30 or 120 min, at 25 and 30°C over a 48 h recovery period at 5°C. Mussels were genotyped at 10 enzyme loci to explore a relationship between natural genetic variation, gene expression and temperature adaptation. Results indicate that the mussel response to increasing temperature is a depression in gene expression, such a response being genotypically correlated at least for the Pgm-1 locus. This suggests that an increase in temperature could be a signal triggering anaerobiosis for B. azoricus or this latter alternatively behaves more like a ‘cold’ stenotherm species, an attribute more related to its phylogenetic history, a cold seeps/wood fall origin.
Keywords: temperature, adaptation, genotype, mRNA expression, metabolic depression, Bathymodiolus azoricus
1. Introduction
Temperature, and especially thermal gradients, is one of the major environmental factors influencing vertical and latitudinal distributions of marine organisms by constraining many biological processes from macromolecular structure and interactions to reaction fluxes (Hochachka & Somero 1984; Somero 1995). Temperature variations impact biochemical and physiological processes directly, by restricting enzyme activity efficiency or promoting protein catabolism, for example. A second effect of increasing temperature is the induction of hypoxia by limiting the oxygen dissolution in sea water. Consequently, marine organisms may shift metabolism towards anaerobiosis at both cold and warm temperature extremes (Anestis et al. 2007). This shift generally entails a metabolic depression owing to the lesser efficiency of ATP turn over and oxygen-limited thermal tolerance in organisms (Pörtner 2002; Storey & Storey 2004). In fact, it was shown that the oxygen uptake of an organism is greatly reduced near the lower and higher limits of thermal tolerance, not only because to lower oxygen availability but also through a loss of efficiency of oxygen supply mechanisms that are not able to meet the organism's temperature-dependent oxygen demand (Anestis et al. 2007). In some marine ecosystems, such as the intertidal zone (immersion/emersion cycles) or deep-sea hydrothermal vents, organisms are often exposed to steep environmental gradients (temperature and oxygen, sulphide, methane) and long periods of stressful conditions, including hypoxia owing to emersion (Chung & Zmora 2008), outranged temperatures and eutrophication (La Jeunesse & Elliott 2004) or high sulphide concentrations (Weeks et al. 2002).
The hydrothermal vent environment exhibits extreme and highly fluctuating life conditions (Chevaldonné et al. 1991; Le Bris et al. 2005) compared with the surrounding deep sea. Hydrothermal vents are active sulphide-rich springs located along mid-oceanic ridges of the deep sea. This environment is characterized by sharp and highly fluctuating temperature (tens of degrees over centimetres), oxygen and pH gradients, as well as the presence of radionuclide, high heavy metal and sulphide concentrations (Cherry et al. 1992; Johnson et al. 1994; McMullin et al. 2000). Chemical concentrations mainly depend on the nearly turbulent mixing of fluid with the surrounding abyssal sea water. Oxygen concentration is therefore negatively correlated with temperature and sulphide concentration (Johnson et al. 1986), leading to possible physiological changes during the aerobic/anaerobic alternation (Hourdez & Lallier 2007). Oxygen concentration is indeed close to zero when fluid temperature exceeds 10°C. In spite of these challenging environmental conditions (compared with the surrounding deep sea), a highly specialized fauna, often endemic (from Archaea to vertebrates), is exploiting the sulphide/methane source. Among them, hydrothermal vent mussels belonging to the genus Bathymodiolus are distributed worldwide (Von Cosel et al. 1999). In particular, they dominate communities at shallower hydrothermal sites of the Mid-Atlantic Ridge (MAR). While organisms inhabiting coastal ecosystems are subjected to predictable oscillations of physical and chemical variables controlled by tidal cycles, the vent mussels withstand large temperature changes over short periods of time (Johnson et al. 1994). For example, these authors reported that temperature can fluctuate from 5 to 20°C within a 12 h period with values over 10°C at least for 4 h. Chemical parameters variations occurred concomitantly with these temperature oscillations. Johnson et al. (1994) also established that sulphide concentrations increased from less than 200 µM to 1 mM for the highest temperature, while oxygen concentration simultaneously decreased towards zero. Numerous studies conducted on Bathymodiolus spp. were related to stress physiology and endosymbiosis (Bebianno et al. 2005; Duperron et al. 2006), but very few have investigated how temperature changes and subsequent aerobic–anaerobic shifts of the vent conditions may influence vent mussel physiology (Berger & Young 2006).
In the context of investigations on the thermotolerance of vent taxa, we focused our work on the short-term adaptive response of mussels to thermal challenge at a molecular level. We wanted to know whether vent mussels represent a ‘true’ eurytherm species, able to sustain bursts of high temperatures over long periods of time. For this purpose, we followed the mRNA expression of 23 genes involved in various cell functions (metabolic pathways, detoxifying processes, stress response, etc.) of the Atlantic vent mussel Bathymodiolus azoricus after heat shocks for 30 or 120 min, at 25 or 30°C, followed by a 48 h recovery period at 5°C. In addition, mussels were genotyped at 10 enzyme loci to explore a potential relationship between natural genetic variation, stress protein expression and temperature adaptation. This allowed us to determine whether mussels can easily recover from heat exposure and may adopt specific transcriptional strategies to cope with thermal changes.
2. Material and methods
(a). Sample collection and experimental design
Bathymodiolus azoricus individuals were collected on board the N/O L’Atalante using the ROV Victor6000 from dense mussel beds at the shallow vent field of Menez Gwen (37°51′ N, 31°30′ W, 850 m depth) and at the Rainbow vent field (36°14′ N, 33°54′ W, 2350 m) depth on the MAR during the ATOS cruise (2001).
Mussels from the Menez Gwen site were selected for heat-shock exposure experiments because they were able to rapidly recover from depressurization and live for more than a year at atmospheric pressure with sulphide/methane exposure without specialized pressure vessels (Kádár et al. 2005).
Both experiments were conducted as follows: the 72 h (or 96 h for the LabHorta set) acclimatized mussels were transferred into a sea-water tank at 25°C (experiment 1) or 30°C (experiment 2) during 30 (first series) and 120 min (second series) while a third group was maintained at 5°C as a control. The control mussels were used as a calibrator in subsequent real-time PCR analysis. The two groups of 30 heat-shocked mussels were transferred to 5°C for 48 h at the end of the heat-challenge period in two distinct tanks (series 1 and 2). Mussels were sampled at the end of the heat shock (T0) and after 2, 6, 12, 24 and 48 h during the recovery period at 5°C. Details about the experimental schedule are developed in the electronic supplementary material, S1.
In parallel to the heat-shock experiments, differential mortality experiments were conducted on board of the N/O L’Atalante using mussels from Rainbow vent field and, at LabHorta, using individuals collected from the Menez Gwen vent field (see details in the electronic supplementary material, S1).
(b). Gene selection and mRNA quantification by real-time PCR
The mRNA expression of 23 genes was followed by real-time PCR in the mussels subjected to 25°C. Among these 23 genes, 12 were subselected to follow the mRNA expression in the second experimental set of mussels (subjected to 30°C). In order to cover various metabolic pathways, genes were chosen from an EST collection generated from B. azoricus cDNA libraries (Tanguy et al. 2008) and from Bathymodiolus thermophilus SSH libraries (Boutet et al. 2009) (electronic supplementary material, S2). Total RNA extraction and real-time PCR were carried out according to the protocol described in the electronic supplementary material, S2.
(c). Individual genotyping
The correlations between gene expression and genotype were accessed by genotyping the experimented mussels for 10 enzyme systems following the protocols described in the electronic supplementary material, S2.
(d). Statistical treatment of the datasets
(i). Effect of heat-shock duration, time of recovery and genotype on mRNA expression
A canonical redundancy analysis (RDA) was performed on the data obtained from the 25°C heat-shock experiment, including mRNA expression of 23 genes (response variables), 10 explanatory variables including the experimental factors heat-shock duration and time of recovery, genotype classes at seven loci (coding described below; non-polymorphic loci Aco, Hk and Mpi were not retained for subsequent analysis), and multi-locus heterozygosity using the R-language ‘rdaTest’ function (http://www.bio.umontreal.ca/legendre/indexEn.html). RDA was used to estimate the fraction of variation in gene expression attributable to the explanatory variables. This method therefore displays correlations between the response and explanatory variables.
The explanatory variables were recoded as a set of numerical variables corresponding to experimental factors and genetic classes. Heat-shock duration and heterozygosity were considered as quantitative variables (transformation of numerical code not required). A polynomial transformation was applied to the recovery times (simple, power 1; square, power 2; cube, power 3). Genotype classes were all coded as three binary variables corresponding to the genotypes 100/100, 100/x and x/x. In order to give the same weight to all response variables in the analysis, levels of gene expression (ΔCt) were standardized to mean = 0 and variance = 1 (z-score transformation) with the R-language ‘decostand’ function of the ‘vegan’ package (Vegan: Community Ecology Package v. 1.8-3; http://cran.r-project.org/). For each gene, the very few missing values (lack of amplification) in the dataset were replaced by the mean expression across individuals; this kept unaltered the position of the centroid of the response variables (Legendre & Legendre 1998) as non-amplification of one individual for a given gene is more likely the result of polymorphism along the primer hybridization site.
A forward selection of the explanatory variables was performed using with the ‘packfor’ R-language package (Packfor: forward selection with permutation. R package v. 0.0-7; http://biomserv.univ-lyon1.fr/dray/Software.html#packfor), retaining the explanatory variables with p ≤ 0.05 after Monte Carlo permutation tests (nperm = 9999). An overall test of significance of the canonical relationship (9999 permutations) and an ordination biplot (Z-plot type) was generated from the RDA results (Legendre & Legendre 1998).
(ii). Effect of thermal stress on mRNA expression
A second RDA (using the R-language ‘rdaTest’ function) was computed on the response data combining the mRNA expression of 12 genes under the 25 and 30°C experimental conditions for the HS-mussels, and 5 explanatory variables (temperature, shock duration, time of recovery, Pgm-1 and Mdh-2: the two most contributing loci in the first RDA). Because the number of individuals differed for each experimental condition, the explanatory variables, temperature and heat-shock duration, were transformed in order to give each group the same weight in the analysis (table S1, electronic supplementary material). The other data transformations were similar to the previous RDA. Significant testing of the global analysis and the forward selection of explanatory variables were also done in the same way as in the first RDA. The results were visualized in an ordination biplot (Z-plot type).
(iii). Potential relationship between pgm-1 genotype and mRNA expression level
The differences between mean mRNA expression of each Pgm-1 genotype classes (three groups: 100/100, 100/x, x/x, where x stands for any other allelic form) were tested by using a non-parametric Wilcoxon–Mann–Whitney test with multiple test correction of Holm (1979) (R-language ‘stats’ package).
(e). Differential mortalities under a pressure of 1 atm
Batches of 50 individuals from Menez Gwen and Rainbow were acclimatized to different temperatures (10, 15, 20 and 25°C) over a period of one week and processed following protocols described in the electronic supplementary material, S1. Dead and surviving mussels (following the DL50) were compared using pairwise exact tests of genic differentiation (Genepop v. 3.4, Markov chain parameters: dememorization = 10 000, batches = 100 and iterations per batch = 5000) and a multiple test correction of Holm (1979) (R-language ‘p.adjust’ function).
3. Results
(a). mRNA expression variation in response to heat shocks
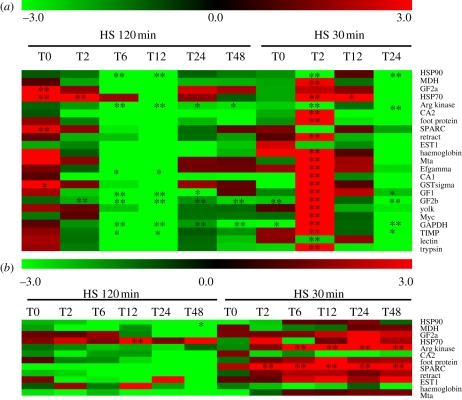
In both sets of experiments, we found a strong transcriptional depression trend affecting most of the genes during the recovery kinetics after a brief period of gene induction during the first 2 h of recovery. This global gene inhibition (often marginally significant when compared to control) was always more pronounced for the 120 min-shock (figure 1a,b).
Figure 1.
mRNA gene expression followed during a recovery period of 48 h at 5°C in mussels exposed for 30 and 120 min at 25°C (a) and 30°C (b). Relative mRNA expression was expressed as xfold control mussel expression level. T0–T48, hours following heat shock. Significant differences with control: *, p < 0.05 and **, p < 0.01.
First, different expression patterns were observed between heat-shock durations. Although a similar trend of mRNA decrease was observed after the shock, the gene induction occurring for most genes about 2 h after the beginning of the shock (i.e. at T2 = 30 min plus 2 h and T0 = 120 min) was greatly attenuated during the long-term exposure in the case of the 25°C-exposure (figure 1a) and displayed a complete change in gene expression from a constant upregulated genes situation during the short shock to a constant downregulated gene situation during the long-term shock at the 30°C-exposure (figure 1b).
Second, gene expression pathways were clearly different between the two thermal shock conditions (25 and 30°C). A clear pattern of gene expression kinetics with a peak of gene induction just after the shock, followed by a rapid decrease of expression towards inhibition even after 48 h of recovery, was observed in the case of an exposure at 25°C (figure 1a), whereas most genes remained clearly up- or downregulated over the full range of the recovery period depending on the length of the shock in the case of an exposure at 30°C (figure 1b). The number of constantly downregulated genes increased as a function of the length of the shock, indicating that mussels were not able to cope with temperatures above 30°C, at least for the longest exposure time at 1 atm.
(b). Disentangling the relative effects of treatment and genetic components on the variance of gene expression across individuals
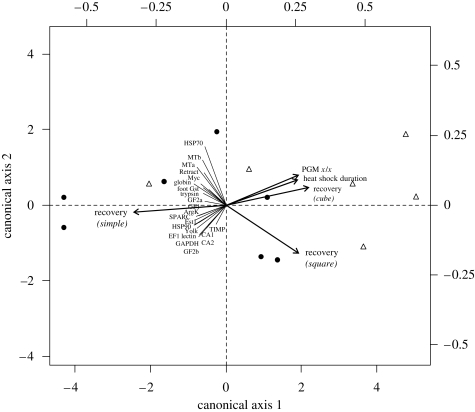
In the first set of experiments (exposure to 25°C), we assessed the part of the observed variance among individual mRNA expression (23 genes) associated with each experimental and genetic factor (figure 2). The first step of the analysis consisted in the selection of significant explanatory variables out of the 10 that were available (heat-shock length, time of recovery, heterozygosity and seven enzyme loci). Using a multivariate forward selection procedure (R-language package ‘packfor’), three variables remained significant (p < 0.05) to explain the variance of the 24 response variables across individuals: recovery time in polynomial form (simple: R2 = 0.125, F = 4.879, p = 0.01; square: R2 = 0.105, F = 4.48, p = 0.018; cube: R2 = 0.103, F = 4.964, p = 0.007), the heat-shock length (R2 = 0.066, F = 3.43, p = 0.033) and the locus Pgm-1 x/x (R2 = 0.088, F = 3.278, p = 0.042). These variables were thus used in the first canonical RDA. The first two axes of the RDA constrained by the two experimental factors (recovery time and length of heat shock) and the genetic background (expression of the Pgm-1 x/x) explained approximately 43.4 per cent (R2adj = 31.7%, F = 3.7, p = 0.001) of the total variance associated with gene expression (RDA-1 = 34.7%, RDA-2 = 4.7%).
Figure 2.
Ordination biplot representing the significant (p < 0.05) effect of experimental (heat-shock duration and recovery) and genetic (PGM-1 genotypes) factors (arrows) on mRNA expression of 23 genes in mussels exposed to thermal challenge (25°C during 30 min and 2 h). Black circles represent individuals exposed for 120 min at 25°C and white triangles represent individuals exposed for 30 min at 25°C at 1 atm. Lines represent the response variables.
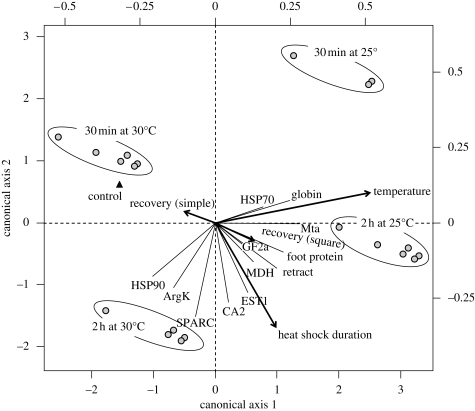
A second set of statistical analyses was conducted to estimate the effect of the thermal threshold on mRNA expression in mussels (figure 3). The data focused on the mRNA expression of 12 genes (§2) using five explanatory variables (heat-shock duration, temperature, recovery time and the loci Pgm-1 and Mdh-2, which yielded the highest R2 values in the first RDA). Three experimental factors were found to be significant (p < 0.05) using the forward selection procedure: the thermal threshold (R2 = 0.282, F = 38.428, p = 0.001), the shock duration (R2 = 0.155, F = 26.7, p = 0.001) and the time of recovery (simple: R2 = 0.037, F = 6.78, p = 0.001; square: R2 = 0.016, F = 3.075, p = 0.042). The RDA performed with this set of significant explanatory variables indicated that the thermal treatment explained 49.0 per cent (R2adj = 46.9%, F = 22.85, p = 0.0001) of the mRNA expression variance observed across individuals. The thermal threshold was mostly associated with the first axis of the ordination (RDA-1 = 31.5%) and the shock length with the second axis (RDA-2 = 15.8%).
Figure 3.
Ordination biplot representing the significant (p < 0.05) effect of experimental (temperature, heat-shock duration and recovery) factors (arrows) on mRNA expression of 12 genes in mussels exposed to thermal challenge (25 and 30°C during 30 and 120 min) at atmospheric pressure. Lines represent the response variables.
(c). Potential correlations between gene expression and genotype
Out of the 10 enzyme loci, only Pgm-1 was contributing to the overall variance of gene expression associated with thermal shocks. For each of the 23 genes, the mean level of mRNA expression was estimated in association with each genotypic class of Pgm-1 (100/100, 100/x and x/x) for the whole set of 25°C heat-shocked mussels (figure S1, electronic supplementary material). All 23 genes clearly displayed the same trend: individuals that did not possess allele 100 had a higher mRNA expression than the other two classes (100/100 or 100/x).
(d). Differential mortality experiments
The period of time prior to which the DL50 was reached increased with decreasing experimental temperature. Experiment durations varied from 28 (at 25°C) to120 h (at 10°C). Dead and surviving individuals were all genotyped at the eight enzyme systems scored in the heat-shock experiments and subsequently used for pairwise genic differentiation tests (exact tests: Genepop v. 3.4) (table S2, electronic supplementary material). The only locus that displayed significant genic differentiation between dead and surviving individuals with increasing acclimatization temperatures is Pgm-1 (table 1). After a multiple test correction, genic differentiation was found to be significant at 15°C only for the Rainbow mussels and for individuals pooled over experiments. Before multiple test correction, the tests were also significant at Menez Gwen for individuals exposed at 20 and 25°C. Difference between batches was only explained by the increase in frequency of allele 95 at the Pgm-1 locus (table S2, electronic supplementary material).
Table 1.
Exact tests of genic differentiation (p) with corresponding standard errors (SE) for Pgm loci between dead and surviving mussels acclimatized at different temperatures at atmospheric pressure using the software Genepop v3.4. The multiple test correction of Holm was applied. Rb, Rainbow; MG, Menez Gwen; significance level: **p < 0.01; ***p < 0.001.
|
p10°C(SE) |
p15°C (SE) |
p20°C (SE) | p25°C (SE) | ||||
|---|---|---|---|---|---|---|---|
| loci | Rb | MG | Rb | MG | MG | MG | pall (SE) |
| Pgm-2 | 0.520 (0.002) | 0.435 (0.002) | 1.000 (0.001) | 1.000 (0.002) | 0.018 ** (0.000) | 1.000 (0.003) | 0.056 (0.003) |
| Pgm-1 | 0.312 (0.002) | 0.115 (0.002) | 0.000 *** (0.000) | 0.312 (0.003) | 0.175 (0.001) | 0.175 (0.001) | 0.000 *** (0.000) |
| all (Fisher's method, Df = 20) (χ2-value multiloci Fisher's test) | 0.384 (21.2) | 0.671 (16.7) | 0.124 (27.4) | 0.977 (9.4) | 0.004 ** (40.8) | 0.892 (12.7) | 0.001 *** (45.5) |
4. Discussion
Understanding how an organism copes with its environmental constraints and by which cellular/physiological mechanisms it adapts to environmental variations is of major interest in biology at all functional levels. Marine organisms inhabiting highly fluctuating reduced environments display adaptive physiological, molecular and cellular responses (Bailly et al. 2003; Berquist et al. 2004). Extreme temperatures (low or high) and temperature-induced anoxia can trigger metabolic depression in aestivating, hibernating or diapausing species mainly at a translational level (Storey & Storey 2004). Effects of temperature on transcription are less well documented and more likely attributable to specific gene cascades that can be up- or downregulated (Storey & Storey 2004). By analysing mRNA expression of different genes involved in various cell functions, here we addressed questions about the threshold tolerance temperature to which the deep-sea vent mussel B. azoricus is able to adapt, knowing that temperature is often negatively correlated to oxygen concentrations in the mixing zone (Johnson et al. 1986; Luther et al. 2001). Based on our data, a 20–25°C temperature increase at 1 atm, even over a brief period of time (ca 30 min), greatly affects mussels by depressing their metabolism and increasing stress proteins mRNA expression. This decrease in gene expression may be slightly dampened across individuals according to genotype, suggesting that diversifying selection may occur depending on the strength of the vent discharge in mussel beds.
(a). Misadaptation to thermal vents or a mean to cope with hypoxia
During the course of experiments, no mortality was detected among mussel groups, but metabolic disorders were observed. Experimentation on deep-sea mussels entail additional negative effects such as decompression and oxidative stress owing to high oxygen level in surface sea-water compared with in situ conditions. Our results showed that the mRNA expression level in control mussel remained constant between the beginning and the end of the experiment, indicating that the differences were essentially due to heat-shock effects and not to the collection practices. The metabolic disorders are mainly linked to an mRNA depression for all genes studied with the exception of metallothioneins and heat-shock proteins. This effect is more pronounced at the highest thermal exposure and heat-shock duration, suggesting that increasing temperature may either block the transcription or alter the integrity of mRNA. Mussels exposed for 120 min at 25 or 30°C were indeed not able to recover an mRNA expression level similar to that of the control mussels (expression levels at least twofold lower in heat-shocked mussels than in control). Most of these genes coincide with genes differentially regulated in another hydrothermal vent mussel, B. thermophilus, in response to a 24 h thermal acclimatization at 10 and 20°C (Boutet et al. 2009) from which very few were upregulated at 20°C, and associated with cell disorders, when compared to mussels conditioned at 10°C. Contrary to the present study, experiments on B. thermophilus were conducted under in situ pressures, indicating that the results obtained were directly correlated with a temperature effect.
(i). Bathymodiolus: a cold stenoecious species that cannot withstand exposures to moderate temperatures?
In marine organisms, a large number of studies have been dedicated to thermal adaptation (Helmuth et al. 2002; Pernet et al. 2007), but have been limited to the analysis of a few genes, encoding stress proteins, often members of HSP families (Buckley et al. 2001; Anestis et al. 2007). To date, very few experiments have focused on the level of gene expression at a transcriptomic scale in response to thermal stress in marine organisms (Buckley et al. 2006; Boutet et al. 2009). But if HSPs were largely involved in the thermal stress response, many other genes were also upregulated. However, none of these studies pointed out the fact that temperature was able to cause a metabolic depression. From our results, the most parsimonious explanation of the decline in gene expression is that deep-sea mussels are not able to survive long-term exposure to in situ temperatures above 20°C and thus need to exploit sulphide/methane sources with low temperature. This fits well with previous ecological observations of vent mussel beds, suggesting that mussels are mainly proliferating at the periphery of the vent emissions and could derive sulphide fluids along cracks and fissures for their own benefit by active filtration (Johnson et al. 1994).
Because a brief exposure to temperature above 20°C can induce the decrease in transcription, one might consider that Bathymodiolus species is a cold stenotherm organism, a situation typifying almost all life in the deep sea. This assumption fits well evolutionary hypotheses, suggesting that deep-sea vent mussels may derive from whale-bones/wood falls deep-sea ancestors (Distel et al. 2000; Lorion et al. 2009) or may have used cold seeps from the continental margins as stepping stones to colonize oceanic ridges (Craddock et al. 1995; Iwasaki et al. 2006). In this context, deep-sea mussels would have benefited from a long history in cold-water masses to develop thioautrophic endosymbiosis (a long evolutionary process involving an array of highly specialized genes) but not enough time to access the thermophilic condition. Experimenting on early developmental stages, Mestre et al. (2009) proposed that deep-sea hydrothermal vent mussels retained the eurytherm character of their shallow-water ancestors when they colonized the deep sea. Our results contradict this hypothesis at least for the postlarval/adult stage.
A second study conducted on another deep-sea mussel species, Bathymodiolus childressi, showed that these seep mussels were not able to cope with strong temperature changes simply because they had lost their inducible heat response capacity (Berger & Young 2006). Such an assumption was mainly based on the lack of HSP70 induction when mussels were experimentally exposed to high temperatures. Based on our experiments, this statement is not valid for the vent mussel species B. azoricus, because (i) the vent environment is highly fluctuating and (ii) the HSP70 expression remained constantly upregulated in heat-shocked individuals. Our RDA results clearly indicate that this response variable was highly correlated to the temperature factor, entailing a clear separation of individuals exposed to a 25°C heat shock from those exposed to a higher 30°C shock. As a consequence, and in contrast to what was observed for the cold seep methanotrophic mussel, one can hypothesize that vent mussels have reactivated and/or conserved their inducible heat-shock response capacity. Because mussels of the genus Bathymodiolus are able to rapidly move using byssal threads to follow or escape environmental conditions (Govenar et al. 2004), we could hypothesize that vent mussels are only exposed to brief periods of moderate-to-high temperatures in natura, and able to reduce their basal metabolism and spare energy while increasing their chaperonin stock (e.g. HSP70 mRNA induction) to limit cell damages.
(ii). Metabolic depression: a means to endure long-term exposure to complete anoxia?
The fact that moderately elevated temperatures could induce a metabolic depression may also be a consequence of the fact that high temperatures and anoxia are highly correlated at hydrothermal vents. In the hydrothermal environment, Bathymodiolus bivalves are subjected to variable conditions, especially temperature and oxygen gradients (Johnson et al. 1994). It is also well established that oxygen concentrations rapidly fall towards zero with temperature above 10°C (Childress & Fisher 1992; Johnson et al. 1994). In our experiments, mussels may have perceived temperature as a signal indicating that in situ conditions are turning to hypoxia or complete anoxia; they may thus have been able to trigger the decrease in mRNA expression in order to spare energy by reducing their metabolism as a response to hypoxia. Metabolic depression seems to be a common mechanism used by terrestrial (hibernation, aestivation) and marine (emersion) organisms in response to oxygen deprivation and/or water limitation (Storey & Storey 2004; Larade & Storey 2007). The main objective of this mechanism is the limitation of ATP use for non-essential pathways. Previous investigations conducted on the response of the anoxia-tolerant marine snail Littorina littorea to short-to-medium-term oxygen deprivation (from hours to a few days) demonstrated that one of the major adaptive mechanism is the suppression of ATP-consuming processes such as transcription and translation (Larade & Storey 2007). However, the level of expression of some constitutive genes, as well as the upregulation of specific genes essential to anoxia survival, is maintained to limit homeostatic disorders (Storey & Storey 2004; Larade & Storey 2007).
(b). Is global gene expression genetically driven between individuals?
Over a long period, the response of populations to a heterogeneous environment mainly depends on both the phenotypic plasticity of the species and/or the maintenance of adaptive polymorphisms by either balancing or diversifying selective processes (Hedrick 2006). There is limited understanding of how differing physiological performances of genotypes among organisms may affect the fitness of individuals, probably because slight changes in physiological performances are difficult to reconcile with the efficiency of an individual to transmit its genome to the next generation. Thus, several authors argued that amino acid polymorphism associated with enzymes involved in metabolic pathways would have no- or little effect on pathway fluxes (Kacser & Burns 1981) and, because only substantial differences in enzyme catalytic properties can generate detectable modifications in flux, most variations are selectively neutral (Hartl et al. 1985). By contrast, numerous studies showed evidence of selection on allozymes (Hawkins et al. 1989; Powers et al. 1991). Among them, functional studies on different allelic forms of the phosphoglucomutase of various invertebrates (Watt et al. 1985; Pogson 1991; Verrelli & Eanes 2001) showed strong evidence for diversifying selection. Cases of thermal selection were also reported at this locus (Nevo et al. 1977; Piccino et al. 2004).
In our experiments, a part of the relatively high inter-individual variance in mRNA expression levels seems to be explained by differences across Pgm genotypes. The first RDA analysis provided evidence that a significant proportion of the mRNA expression variance is explained by differences between Pgm-1 genotypes associated with allele 100 as opposed to the two other less-frequent alleles (105 and 95). These alleles are indeed found in individuals that are prone to display a higher expression pattern of genes following an increase in temperature. Although not significant, a similar trend was also observed between genotypes 100/100 and 120/120 at the Mdh-1 locus. However, linkage disequilibrium analysis did not reveal any allelic covariation between these two loci in the experimented individuals. This situation found for the shocked mussels was also supported by the temperature-induced differential mortality experiments conducted in parallel over one week and for which significant genotypic differences have been observed between dead and surviving individuals at the Pgm-1 locus (increase in frequency of allele 95 in surviving individuals). These results therefore suggest that some genotypes are able to perform better than others under stressful conditions. Such an adaptive process was already showed in the beetle Chrysomela aeneicollis for which differences in Hsp70 expression among PGI genotypes corresponded to differences in the thermal tolerance and reproductive success of individuals (Rank & Dahlhoff 2002). In our study, homozygous individuals carrying either allele 95 or allele 105 were characterized by a higher global gene expression than both homozygous and heterozygous individuals carrying allele 100. The only exceptions are mostly associated with transcripts encoding stress proteins (hsp70, MTs). As the most frequent allele 100 seemed to be counterselected during thermal acclimatization at a pressure of 1 atm (differential mortality experiments), then patterns of global mRNA depression, which are less pronounced for alleles 95 and 105, may be an indication that transcriptional arrest is not an adaptive means to spare energy but rather an indication that animals are in bad condition. Genotypes accentuating this physiological state would therefore be disadvantaged in warmer habitats. Alternatively, this may also indicate that allozyme Pgm100 is less efficient at regulating energy fluxes during anaerobiosis under temperatures exceeding 15°C and, thus, would induce a more pronounced transcriptional arrest to compensate for energy costs.
Conclusion
The main objective of the present study was the identification of molecular response to high-temperature exposure of the hydrothermal vent mussel B. azoricus. Our findings indicated that the variations of mRNA expression observed among individuals are explained by the heat-shock characteristics (temperature and duration), recovery after heat shock and individual genotype at Pgm-1 locus. We also showed that Bathymodiolus could behave as a cold stenoecious species that cannot withstand exposures to moderate temperatures by depressing its transcription as a response to increasing temperature.
Acknowledgements
We thank the crew and pilots of the RV L’Atalante and the ROV Victor6000 for their assistance and technical support during the cruise ATOS 2001. We also thank Pierre-Marie Sarradin, chief scientist of the ATOS 2001 cruise and David R. Dixon, coordinator of the European VENTOX project, who allowed us to collect material. We are particularly indebted to Ana Colaço and Ricardo S. Santos who provided space lab and sea-water facilities at Horta to conduct experiments and to the crew of the RV Archipelago for the recovery of the acoustically retrievable cages. We are also particularly indebted to the Genoscope d’Evry, which performed the sequencing of our Bathymodiolus EST collection, and to Odile Lecompte (IGBMC, Strasbourg) who annotated them. This work was partly funded by the European VENTOX programme (EVK3-1999-00056P), the Fish & Shellfish node of the NoE Marine Genomics Europe (coordination Adenilo Canario) and the Région Bretagne (PRIRE AMETHYST). Pierre Legendre got involved in this research as recipient of a CNRS Associate Research Directorship in UMR CNRS 7144, Station Biologique de Roscoff (April to August 2008).
References
- Anestis A., Lazou A., Pörtner H. O., Michaelidis B.2007Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol 293, R911–R921 (doi:10.1152/ajpregu.00124.20070363-6119/07) [DOI] [PubMed] [Google Scholar]
- Bailly X., Leroy R., Carney S., Collin O., Zal F., Toulmond A., Jollivet D.2003The loss of the hemoglobin H2S-binding function reveals molecular adaptation driven by Darwinian positive selection in annelids from sulphide-free habitats. Proc. Natl Acad. Sci. USA 100, 5885–5890 (doi:10.1073/pnas.1037686100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebianno M. J., Company R., Serafim A., Camus L., Cosson R. P., Fiala-Medoni A.2005Antioxidant systems and lipid peroxidation in Bathymodiolus azoricus from Mid-Atlantic Ridge hydrothermal vent fields. Aquat. Toxicol. 75, 354–373 (doi:10.1016/j.aquatox.2005.08.013) [DOI] [PubMed] [Google Scholar]
- Berger M. S., Young C. M.2006Physiological response of cold-seep mussel Bathymodiolus childressi to acutely elevated temperature. Mar. Biol. 149, 1397–1402 (doi:10.1007/s00227-006-0310-8) [Google Scholar]
- Berquist D. C., Fleckenstein C., Szalai E. B., Knisel J., Fisher C. R.2004Environment drives physiological variability in the cold seep mussel Bathymodiolus childressi. Limnol. Oceanogr. 49, 706–715 [Google Scholar]
- Boutet I., Jollivet D., Shillito B., Moraga D., Tanguy A.2009Molecular identification of differentially regulated genes in the hydrothermal-vent species Bathymodiolus thermophilus and Paralvinella pandorae in response to temperature: a comparative study. BMC Genomics 10, 222 (doi:10.1186/1471-2164-10-222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley B. A., Owen M. E., Hofmann G. E.2001Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J. Exp. Biol. 204, 3571–3579 [DOI] [PubMed] [Google Scholar]
- Buckley B. A., Gracey A. Y., Somero G. N.2006The cellular response to heat stress in the goby Gillichthys mirabilis: a cDNA microarray and protein-level analysis. J. Exp. Biol. 209, 2660–2677 (doi:10.1242/jeb.02292) [DOI] [PubMed] [Google Scholar]
- Cherry R., Desbruyères D., Heyraud M., Nolan C.1992High levels of radioactivity in hydrothermal vent polychaetes. C. R. Acad. Sci. Paris Ser. III 315, 21–26 [Google Scholar]
- Chevaldonné P., Desbruyères D., Le Haître M.1991Time-series of temperature from three deep-sea hydrothermal vent sites. Deep Sea Res. A 38, 1417–1430 (doi:10.1016/0198-0149(91)90014-7) [Google Scholar]
- Childress J. J., Fisher C. R.1992The biology of hydrothermal vent animals: physiology, biochemistry, and autotrophic symbioses. Oceanogr. Mar. Biol. 30, 337–441 [Google Scholar]
- Chung J. S., Zmora N.2008Functional studies of crustacean hyperglycemic hormones (CHHs) of the blue crab, Callinectes sapidus—the expression and release of CHH in eyestalk and pericardial organ in response to environmental stress. FEBS J. 275, 693–704 (doi:10.1111/j.1742-4658.2007.06231.x) [DOI] [PubMed] [Google Scholar]
- Craddock C., Hoeh W. R., Gustafson R. G., Lutz R. A., Hashimoto J., Vrijenhoek R. J.1995Evolutionary relationships among deep-sea mytilids (Bivalvia: Mytilidae) from hydrothermal vents and cold-water methane/sulphide seeps. Mar. Biol. 121, 477–485 (doi:10.1007/BF00349456) [Google Scholar]
- Distel D. L., Baco A. R., Chuang E., Morrill W., Cavanaugh C. M., Smith C.2000Do mussels take wooden steps to deep-sea vents? Nature 403, 725–726 (doi:10.1038/35001667) [DOI] [PubMed] [Google Scholar]
- Duperron S., Bergin C., Zielinski F., Blazejak A., Pernthaler A., McKiness Z. P., DeChaine E., Cavanaugh C. M., Dubilier N.2006A dual symbiosis shared by two mussel species, Bathymodiolus azoricus and Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), from hydrothermal vents along the northern Mid-Atlantic Ridge. Environ. Microbiol. 8, 1441–1447 (doi:10.1111/j.1462-2920.2006.01038.x) [DOI] [PubMed] [Google Scholar]
- Govenar B., Freeman M., Berquist D. C., Johnson G. A., Fisher C. R.2004Composition of a one-year-old Riftia pachyptila community following a clearance experiment: insight to succession patterns at deep-sea hydrothermal vents. Biol. Bull. 207, 177–182 (doi:10.2307/1543204) [DOI] [PubMed] [Google Scholar]
- Hartl D. L., Dykhuizen D. E., Dean A. M.1985Limits of adaptation: the evolution of selective neutrality. Genetics 111, 655–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins A. J. S., Bayne B. L., Day A. J., Rusin J., Worrall C. M.1989Genotype-dependant interrelations between energy metabolism, protein metabolism and fitness. In Reproduction, genetics and distributions of marine organisms (eds Ryland J. S., Tyler P. A.), pp. 283–292 Denmark: Fredensborg [Google Scholar]
- Hedrick P. W.2006Genetic polymorphism in heterogeneous environments: the age of genomics. Annu. Rev. Ecol. Evol. System 37, 67–93 (doi:10.1146/annurev.ecolsys.37.091305.110132) [Google Scholar]
- Helmuth B., Harley C. D., Halpin P. M., O’Donnell M., Hofmann G. E., Blanchette C. A.2002Climate change and latitudinal patterns of intertidal thermal stress. Science 298, 1015–1017 (doi:10.1126/science.1076814) [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Somero G. N.1984Temperature adaptation. In Biochemical adaptations (eds Hochacka P. W., Somero G. N.), pp. 355–449 Princeton, NJ: Princeton University Press [Google Scholar]
- Holm S.1979A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 [Google Scholar]
- Hourdez S., Lallier F. H.2007Adaptations to hypoxia in hydrothermal-vent and cold-seep invertebrates. Rev. Environ. Sci. Biotechnol. 6, 143–159 (doi:10.1007/s11157-006-9110-3) [Google Scholar]
- Iwasaki H., et al. 2006Evolutionary relationships of deep-sea mussels inferred by mitochondrial DNA sequences. Mar. Biol. 149, 1111–1122 (doi:10.1007/s00227-006-0268-6) [Google Scholar]
- Johnson K. S., Beehler C. L., Sakamoto-Arnold C. M., Childress J. J.1986In situ measurements of chemical distributions in a deep-sea hydrothermal vent field. Science 4742, 1139–1141 (doi:10.1126/science.231.4742.1139) [DOI] [PubMed] [Google Scholar]
- Johnson K. S., Childress J. J., Beehler C. L., Sakamoto C. M.1994Biogeochemistry of hydrothermal vent mussel communities: the deep-sea analogue to the intertidal zone. Deep Sea Res. I 41, 993–1011 (doi:10.1016/0967-0637(94)90015-9) [Google Scholar]
- Kacser H., Burns J. A.1981The molecular basis of dominance. Genetics 97, 639–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kádár E., Bettencourt R., Costa V., Santos R. S., Lobo-da-Cunha A., Dando P.2005Experimentally induced endosymbiont loss and re-acquirement in the hydrothermal vent bivalve Bathymodiolus azoricus. J. Exp. Mar. Biol. Ecol. 318, 99–110 (doi:10.1016/j.jembe.2004.12.025) [Google Scholar]
- La Jeunesse I., Elliott M.2004Anthropogenic regulation of the phosphorus balance in the Thau catchment-coastal lagoon system (Mediterraean Sea, France) over 24 years. Mar. Pollut. Bull. 48, 679–687 (doi:10.1016/j.marpolbul.2003.10.011) [DOI] [PubMed] [Google Scholar]
- Larade K., Storey K. B.2007Arrest of transcription following anoxic exposure in a marine mollusc. Mol. Cell. Biochem. 303, 243–249 (doi:10.1007/s11010-007-9468-8) [DOI] [PubMed] [Google Scholar]
- Le Bris N., Zbinden M., Gaill F.2005Processes controlling the physico-chemical micro-environments associated with Pompeii worms. Deep Sea Res. I 52, 1071–1083 (doi:10.1016/j.dsr.2005.01.003) [Google Scholar]
- Legendre P., Legendre L.1998Numerical ecology. 2nd English edn.Amsterdam, the Netherlands: Elsevier [Google Scholar]
- Lorion J., Duperron S., Gros O., Cruaud C., Samadi S.2009Several deep-sea mussels and their associated symbionts are able to live both on wood and on whale falls. Proc. R. Soc. B 276, 177–185 (doi:10.1098/rspb.2008.1101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther G. W., Rozan T. F., Taillefert M., Nuzzio D. B., Di Meo C., Shank T. M., Lutz R. A., Cary S. C.2001Chemical speciation drives hydrothermal vent ecology. Nature 410, 813–816 (doi:10.1038/35071069) [DOI] [PubMed] [Google Scholar]
- McMullin E. R., Bergquist D. C., Fisher C. R.2000Metazoans in extreme environments: Adaptations of hydrothermal vent and hydrocarbon seep fauna. Gravit. Space Biol. Bull 13, 2. [PubMed] [Google Scholar]
- Mestre N. C., Thatje S., Tyler P. A.2009The ocean is not deep enough: pressure tolerances during early ontogeny of the blue mussel Mytilus edulis. Proc. R Soc. B 276, 717–726 (doi:10.1098/rspb.2008.1376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo E., Shimony T., Libni M.1977Thermal selection of allozyme polymorphism in barnacles. Nature 267, 699–700 (doi:10.1038/267699a0) [DOI] [PubMed] [Google Scholar]
- Pernet F., Tremblay R., Comeau L., Guderley H.2007Temperature adaptation in two bivalve species from different thermal habitats: energetics and remodelling of membrane lipids. J. Exp. Biol. 210, 2999–3014 (doi:10.1242/jeb.006007) [DOI] [PubMed] [Google Scholar]
- Piccino P., Viard F., Sarradin P. M., Le Bris N., Le Guen D., Jollivet D.2004Thermal selection of PGM allozymes in newly founded populations of the thermotolerant vent polychaete Alvinella pompejana. Proc. R. Soc. Lond. B 271, 2351–2359 (doi:10.1098/rspb.2004.2852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson G. H.1991Expression of overdominance for specific activity at the phosphoglucomutase-2 locus in the Pacific oyster, Crassostrea gigas. Genetics 128, 133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner H. O.2002Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A 132, 739–761 (doi:10.1016/S1095-6433(02)00045-4) [DOI] [PubMed] [Google Scholar]
- Powers D. A., Lauerman T., Crawford D., DiMichele L.1991Genetic mechanisms for adapting to a changing environment. Annu. Rev. Genet. 25, 629–659 (doi:10.1146/annurev.ge.25.120191.003213) [DOI] [PubMed] [Google Scholar]
- Rank N. E., Dahlhoff E. P.2002Allele frequency shifts in response to climate change and physiological consequences of allozyme variation in a montane insect. Evolution 56, 2278–2289 (doi:10.1043/0014-3820(2002)056(2278:AFSIRT)2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Somero G. N.1995Protein and temperature. Annu. Rev. Physiol. 57, 43–68 (doi:10.1146/annurev.ph.57.030195.000355) [DOI] [PubMed] [Google Scholar]
- Storey K. B., Storey J. M.2004Metabolic rate depression in animals: transcriptional and translational control. Biol. Rev. Camb. Philos. Soc. 79, 207–233 (doi:10.1017/S1464793103006195) [DOI] [PubMed] [Google Scholar]
- Tanguy A., et al. 2008Increasing genomic information in Bivalves by new EST collections in four species: development of new genetic markers for environmental studies and genome evolution. Gene 408, 27–36 (doi:10.1016/j.gene.2007.10.021) [DOI] [PubMed] [Google Scholar]
- Verrelli B. C., Eanes W. F.2001Clinal variation for amino acid polymorphisms at the Pgm locus in Drosophila melanogaster. Genetics 157, 1649–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Cosel R., Comtet T., Krylova E. M.1999Bathymodiolus (Bivalvia: Mytilidae) from hydrothermal vents on the Azores Triple Junction and the Logatchev hydrothermal field, Mid-Atlantic Ridge. Veliger 42, 218–248 [Google Scholar]
- Watt W. B., Carter P. A., Blower S. M.1985Adaptation at specific loci. IV. Differential mating success among glycolytic allozyme genotypes of Colias butterflies. Genetics 109, 157–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks S. J., Currie B., Bakun A.2002Satellite imaging: Massive emissions of toxic gas in the Atlantic. Nature 415, 493–494 (doi:10.1038/415493b) [DOI] [PubMed] [Google Scholar]