Abstract
Evidence accumulates that telomere shortening reflects lifestyle and predicts remaining lifespan, but little is known of telomere dynamics and their relation to survival under natural conditions. We present longitudinal telomere data in free-living jackdaws (Corvus monedula) and test hypotheses on telomere shortening and survival. Telomeres in erythrocytes were measured using pulsed-field gel electrophoresis. Telomere shortening rates within individuals were twice as high as the population level slope, demonstrating that individuals with short telomeres are less likely to survive. Further analysis showed that shortening rate in particular predicted survival, because telomere shortening was much accelerated during a bird's last year in the colony. Telomere shortening was also faster early in life, even after growth was completed. It was previously shown that the lengths of the shortest telomeres best predict cellular senescence, suggesting that shorter telomeres should be better protected. We test the latter hypothesis and show that, within individuals, long telomeres shorten faster than short telomeres in adults and nestlings, a result not previously shown in vivo. Moreover, survival selection in adults was most conspicuous on relatively long telomeres. In conclusion, our longitudinal data indicate that the shortening rate of long telomeres may be a measure of ‘life stress’ and hence holds promise as a biomarker of remaining lifespan.
Keywords: ageing, selective disappearance, oxidative stress, birds, life history, mixed models
1. Introduction
Telomeres are regions of non-coding but highly structured DNA at the end of linear eukaryotic chromosomes, consisting of tandem repeated highly conserved DNA sequence (5′-TTAGGG-3′)n. Telomeres play an important role in the protection of chromosome integrity (Blackburn 1991), and there are indications that telomere length predicts remaining lifespan in humans (Cawthon et al. 2003; Bakaysa et al. 2007; Kimura et al. 2008; but see Aviv 2008), tree swallows Tachycineta bicolor (Haussmann et al. 2005), alpine swifts Apus melba (Bize et al. 2009) and nematodes (Joeng et al. 2004). Moreover, when comparing species, telomere shortening rate is correlated with maximum lifespan, in that short-lived species lose their telomeres at a higher rate (Haussmann et al. 2003), although between species the average telomere length is not correlated with (maximum) lifespan (Haussmann et al. 2003; Seluanov et al. 2007). Telomere shortening rate is accelerated by oxidative stress (von Zglinicki 2002; Tchirkov & Lansdorp 2003). Since oxidative stress is often considered a major agent of senescence (Beckman & Ames 1998), this would provide a mechanistic link explaining associations between telomeres and lifespan, in that shorter telomeres may indicate that the organism experienced higher levels of oxidative stress (Jennings et al. 2000; Monaghan & Haussmann 2006). Thus, telomere length, and perhaps telomere shortening rate in particular, can potentially be used as a proxy for the ‘life stress’ experienced by individual organisms (Epel et al. 2004, 2006; Kotrschal et al. 2007) and hence as a marker of an individual's ‘biological age’. However, more information is required to assess whether telomere length and telomere shortening can be interpreted as proxies for ‘physiological age’. Information from natural populations in particular can contribute to such an assessment, since the fitness consequences of a trait can only be estimated under natural circumstances where animals face the adversities that have shaped their evolution.
Studies on telomere length in relation to age are mostly cross-sectional, where different age classes are represented by different individuals. When individuals with short telomeres are likely to die sooner, individuals with longer telomeres early in life and/or lower telomere shortening rate will be over-represented in older age classes (Haussmann & Mauck 2008a). Relationships between telomere length and ageing obtained using cross-sectional data are therefore likely to be confounded by selective disappearance. More specifically, this would result in estimates of telomere shortening that are lower than the actual within-individual shortening rate. We test this hypothesis using 3 years of telomere data from a colony of free-living jackdaws, and thus test whether short telomeres are associated with higher mortality rate. In addition, we test whether telomere shortening rate is dependent on age and predicts remaining lifespan.
The length of the shortest telomeres in a cell determines when cellular senescence commences (Hemann et al. 2001; di Fagagna et al. 2003; Capper et al. 2007). On functional grounds, one can therefore expect that defensive mechanisms against DNA damage have evolved to act more strongly on chromosomes with short telomeres (Karlseder et al. 2002), resulting in an accumulation of shorter telomeres (Martens et al. 2000). We quantified telomeres in erythrocytes using pulsed-field gel electrophoresis, which yields genome-wide distributions of telomere lengths for each sample (Baird 2006) and hence gives the opportunity to quantify telomere shortening separately for subsets of these size distributions (Haussmann & Mauck 2008b). We use this information to test the hypothesis that telomere shortening rate is lower for short telomeres within individuals.
2. Methods
(a). Study population
We studied a colony of free-living jackdaws in Haren (The Netherlands), a semi-urban environment (see Salomons et al. 2008 for details). Birds were individually marked with colour rings and a metal numbered ring. Estimates of adult age are exact for individual birds first ringed as fledglings or yearlings, the latter distinguishable from older adults through brown plumage colouration. Birds of unknown age were assigned a minimum age of 2 years. This is likely to be a good estimate of their real age, since telomere shortening patterns were not different between individuals for whom the exact age was known or not. Moreover, our primary interest is within-individual telomere shortening rate, for which it is sufficient to know the exact age difference between sampling points. We have studied jackdaws in this colony since 1996, and (minimum) ages of adult birds in this study were 1–12 years.
In the years 2004–2006, adult birds were caught in their nest box using remote-controlled trap doors. All breeding birds were caught twice during the nestling rearing period (when the chicks were 5 and 20 days old, respectively), and most birds were also caught in March, before the breeding season. Jackdaws are highly sedentary by nature and breeding birds generally return to the colony when still alive. Hence the dataset comprised individuals sampled over 1–3 subsequent years. Immediately after capture, a small blood sample (approx. 60 µl) was taken using puncture of the brachial vein. This sample was stored in 2 per cent EDTA buffer at a temperature of 4–7°C, before it was snap-frozen within two weeks in a 40 per cent glycerol solution for permanent storage at −80°C. In total, 48 adults were sampled up to six times, and in total we measured telomere length of 131 samples on 15 gels. In 3 years (2005–2007), we also collected blood samples of 74 individual nestlings (from 29 nests) at both 5 and 30 days after hatching. Telomere length of these samples was assessed using nine gels. Since we were mainly interested in within-individual shortening rates, the different samples of each nestling would always be on the same gel. All animals were handled in strict accordance with good animal practice, and all animal work was conducted under license from the Animal Experiments Committee of the University of Groningen (#D4071).
(b). Telomere length analysis
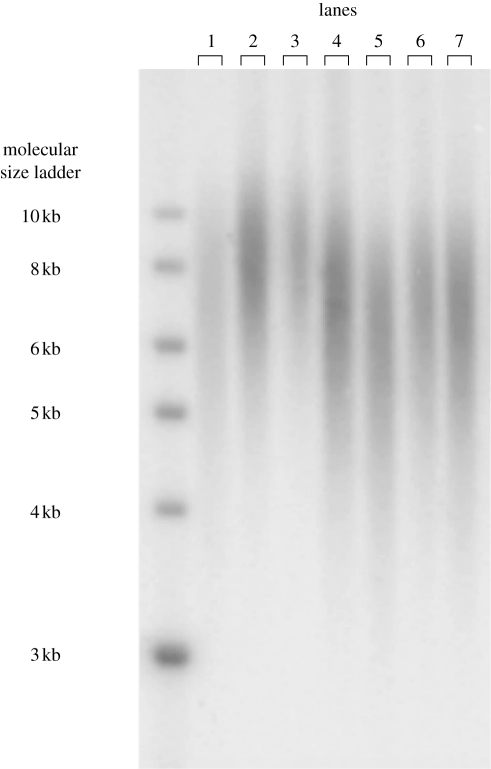
We used erythrocytes as the source of DNA, which was extracted from the nuclei of 5 µl isolated erythrocyte cells using the CHEF Genomic DNA Plug kit (Bio-Rad, Hercules, CA, USA). Cells in the agarose plug were digested overnight with Proteinase K at 50°C. The genomic DNA within half of this plug was digested simultaneously with HindIII (60 U), HinfI (30 U) and MspI (60 U) for ∼18 h in NEB2 buffer (New England Biolabs, Inc, Beverly, MA, USA). Approximately 5 µg of digested DNA from each sample was separated by pulsed-field gel electrophoresis through a 0.8 per cent pulsed-field certified agarose gel (Bio-Rad) at 14°C for 24 h (3 V/cm, initial switch time 0.5 s, final switch time 7.0 s). Gels were dried using a gel dryer (Bio-Rad, model 538) without heating and hybridized overnight with a 32P-endlabelled oligo (5′-CCCTAA-3′)4 that binds to the 3′ overhang of telomeres, thereby avoiding the problem of interstitial telomeres that are prevalent in birds (Class I telomeres; see Delany et al. 2000 for a description of different telomere classes). The radioactive signal of the marker was detected by a phosphor screen (MS, PerkinElmer) and analysed using a phosphor imager (Cyclone™ Storage Phosphor System, PerkinElmer). For size calibration, we used a 32P-labelled size ladder (NEB DNA ladder 1 kb; figure 1).
Figure 1.
Picture of representative pulsed-field gel showing the molecular size ladder and the first seven lanes with smears representing telomeres of individual samples.
Telomere length varies between chromosomes (Lansdorp et al. 1996; Martens et al. 1998; Baird et al. 2003) and, owing to stochastic events, also between the haematopoietic stem cells that generate the erythrocytes. Hence, the data obtained for each sample are a telomere length distribution (a smear; see figure 1) rather than one value (a sharp band). We determined the size distribution of telomeres through densitometry (Haussmann & Mauck 2008b) using the open-source software ImageJ v. 1.38x. We subtracted lane-specific background values based on the lowest signal on the part of the distribution representing the short telomeres, and this point was also taken as the lower boundary of the measurement window over which telomere length was calculated. Determining the upper boundary is more difficult because on the upper side of the distribution the background noise was usually higher and more variable than at the lower end of the distribution. To determine the upper boundary, we used the following formula to first determine threshold background intensity (Y) at the upper side of the distribution
where, for each specific lane, Ip is the peak intensity and Imin the minimum intensity at the side of the distribution representing long telomeres. The upper boundary of the measurement window was taken to be the molecular size at which the signal was first below Y.
Telomere distributions are usually characterized using the average telomere length only, but, as explained above, telomere shortening rate may not be the same for chromosomes with short and long telomeres. We therefore characterized telomere distributions not only by the mean, but also by the mode (running average of three neighbouring pixels) and further quantified every 10th percentile for each sample, and examined telomere shortening separately for each estimate.
Repeatability of telomere length of subsequent samples of the same individual measured within gels was high (for average telomere length: r = 0.90, s.e. = 0.06, F1,35 = 27.6, p < 0.001), while the between-gels coefficient of variation of a standard sample run on all gels was 7.5 per cent.
(c). Statistics
We analysed our data using mixed models, including gel, individual and sample (nested within individual) as random effects. Statistical significance of variables, using the REML estimates, was assessed from the t-ratio. Models were also compared using the Akaike Information Criterion (AIC; Akaike 1981).
Telomere shortening estimates from cross-sectional analyses are a weighted mean of within-individual and between-individuals effects when individuals are sampled repeatedly. We were mainly interested in the telomere shortening rate within individuals. Therefore, the age variable in the model was replaced by two variables, average age (calculated over all samples of an individual) and delta age (the deviation from individual average age for each sample), such that, for each sample, age = average age + delta age. Replacing age with these two terms in the model yields separate estimates for the between-individual effect (average age) and within-individual effect (delta age). Moreover, when the within-individual slope is significantly steeper than the between-individuals slope, this is evidence that individuals with short telomeres disappear from the sample population at a higher rate (Snijders & Bosker 1999). Telomere shortening in relation to survival was further analysed using a variable called ‘returned’, which was coded as follows: all samples collected in a year were coded 1 when that individual returned to breed the next year and coded 0 when that individual did not return the next year. This approach was used instead of capture–recapture methods, as the essence of our analyses is that we test for an association between survival and a trait (telomere length) that changes over time within individuals. This feature is unavailable in a capture–recapture approach.
3. Results and discussion
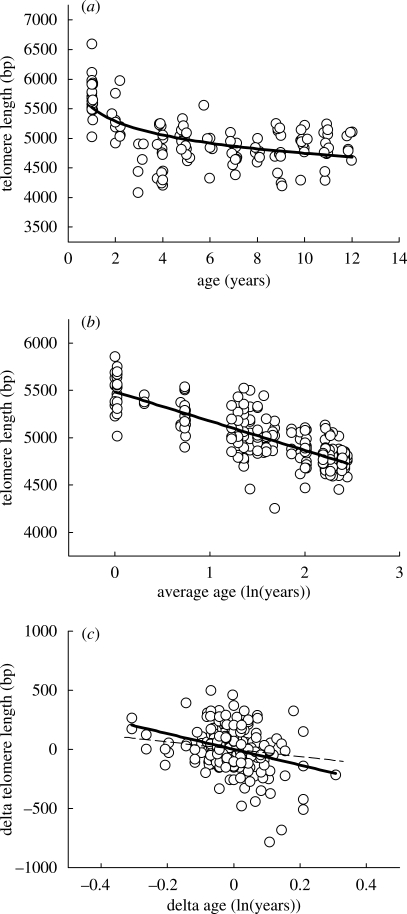
Average telomere length declined with age, but at a decelerating rate (figure 2a). To linearize the model, we used the natural logarithm of age, which yielded the highest fit (AIC = 2708.2) when compared with untransformed age (AIC = 2723.1) and root-transformed age (AIC = 2713.5). Sample sizes for adult data were 131 samples from a total of 48 individuals (see §2 for details). In a cross-sectional analysis, telomere length shortened at a rate of 336 bp/ln(year) (table 1, cross-sectional analysis). On a linear scale, this corresponds to approximately 233, approximately 61 and approximately 33 bp/year for individuals of ages 1, 5 and 10 years old, respectively. Telomere length and telomere shortening rate did not differ between the sexes, which contrasts with the results obtained in humans (Benetos et al. 2001; Terry et al. 2008). Nonlinear telomere shortening with higher rates at young ages was previously reported for birds by Pauliny et al. (2006), but unfortunately they analysed their gels with Telometric (Grant et al. 2001), a computer program which is now known to use an incorrect algorithm that yields strongly biased results (our observations, and see Haussmann & Mauck 2008b). Faster telomere shortening early in life is nevertheless a pattern that is generally observed in birds (Hall et al. 2004), humans (Frenck 1998; Rufer et al. 1999; Zeichner et al. 1999; Sidorov et al. 2004; Aubert & Lansdorp 2008) and other mammals (Brummendorf et al. 2002; Baerlocher et al. 2007). In species such as humans, this could be related to higher cell division rate during the prolonged growth period. However, jackdaws are fully grown at the age of approximately one month, ruling out this hypothesis. Thus, perhaps also in humans, the higher telomere shortening rate early in life is not only because of growth per se but also because of another aspect of being at a younger age such as higher energy turnover (Roberts & Rosenberg 2006; Moe et al. 2009) or increased turnover of haematopoietic stem cells as found in baboons and cats (Brummendorf et al. 2002; Baerlocher et al. 2007). Alternatively, high telomere shortening rate may be a property of long telomere length as the higher amount of guanine makes them particularly vulnerable to oxidative damage (Henle et al. 1999; Oikawa et al. 2001), which could generate the same pattern.
Figure 2.
Cross-sectional estimates of (a) age-related telomere shortening rate follow a log-linear function with a decrease in shortening rate with age. Longitudinal analysis of the same data results in estimates of (b) 309 bp per ln(year) between individuals and (c) 664 bp per ln(year) within individuals. The slope within individuals was significantly steeper than the slope between individuals—for comparison, the between-individuals slope was added to the within-individual graph (dashed line).
Table 1.
(a) Cross-sectional and (b, c) longitudinal analyses of age-dependent telomere shortening (sample sizes are 116 samples from 46 individuals for (a) and (b) and 44 samples from 22 individuals for (c)).
| average telomere length | estimate (standard error) | d.f. | t-ratio | p-value |
|---|---|---|---|---|
| (a) cross-sectional | ||||
| constant | 5520.8 (91.9) | 46.2 | 60.1 | <0.001 |
| ln(age) | −336.3 (46.1) | 91.7 | −7.3 | <0.001 |
| rejected terms | ||||
| sex (female) | −65.5 (73.5) | 39.5 | −0.9 | 0.4 |
| sex * ln(age) | 3.1 (79.1) | 48.8 | 0.04 | 0.97 |
| (b) longitudinal | ||||
| constant | 5484.8 (94.4) | 44.2 | 58.1 | <0.001 |
| average (ln(age)) [between] | −309.4 (47.0) | 81.7 | −6.6 | <0.001 |
| delta ln(age) [within] | −664.0 (162.2) | 81.5 | −4.1 | <0.001 |
| rejected terms | ||||
| sex (female) | −65.3 (72.3) | 39.4 | −0.9 | 0.4 |
| sex * delta ln(age) | 12.6 (314.0) | 78.2 | 0.04 | 0.97 |
| average (ln(age)) * delta ln(age) | 55.9 (267.3) | 120.8 | 0.2 | 0.8 |
| (c) longitudinal (using only birds of known age) | ||||
| constant | 5625.4 (109.3) | 19.6 | 51.5 | <0.001 |
| average (ln(age)) [between] | −367.2 (58.5) | 21.5 | −6.3 | <0.001 |
| delta ln(age) [within] | −554.2 (299.1) | 56.1 | −1.9 | 0.07 |
We partitioned the age variable (in this case the natural log of age) into two terms: average age, to estimate the between-individuals effect (figure 2b); and delta age, to estimate the within-individual effect (figure 2c; see §2 for details). Telomere shortening rate within individuals was significantly higher at 664 bp/ln(year) than the decline between (young and old) individuals (t1,93.9 = 2.1, p = 0.04), which was 309 bp/ln(year). These results did not differ between subsets of data from individuals for which the exact age was known (n = 22) nor for which only an estimate of minimum was available (n = 26; tests of interaction between factor exact/minimum and age differences between (t1,38.2 = −0.88, p = 0.4) and within individuals (t1,74.9 = 0.12, p = 0.9); see also table 1, longitudinal analysis, for tests of the age effect restricted to individuals for whom the exact age was known). The significant difference in slopes between and within individuals is evidence for selective disappearance of individuals with shorter telomere length (van de Pol & Verhulst 2006). The finding that individuals with long telomeres are more likely to return the next year is in agreement with several studies of humans (Cawthon et al. 2003; Bakaysa et al. 2007; Kimura et al. 2008) and two other studies of free-living animals in which this was investigated (Haussmann et al. 2005; Bize et al. 2009). Note, however, that there are also several studies that do not find such a pattern (Martin-Ruiz et al. 2005; Bischoff et al. 2006; Harris et al. 2006), and this issue therefore clearly deserves more study. Note further that in our study telomeres appear to maintain almost constant length later in life when viewed cross-sectionally (figure 2a), but this is apparently caused by selective disappearance of individuals with short telomeres since in the longitudinal analysis there is no evidence that the rate of within-individual telomere shortening depends on (ln-)age (table 1, longitudinal analysis).
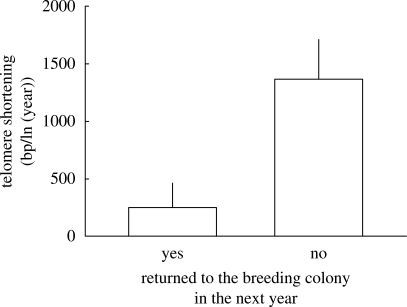
Oxidative stress accelerates telomere shortening, at least in vitro (von Zglinicki et al. 2000; Aviv 2002; von Zglinicki 2002), and evidence accumulates that this also happens in vivo (Cattan et al. 2008; Ilmonen et al. 2008). Since oxidative stress is considered a potentially important agent of senescence, it has been hypothesized that this process may cause the association between telomere length and survival (Haussmann et al. 2005; Vleck et al. 2007). Under this hypothesis, telomere shortening should perhaps be a better predictor of survival than absolute telomere length. We therefore tested the interaction between delta age and a new variable (‘returned’) that indicated whether or not a bird returned to breed the next year. The rate of telomere shortening between years was five times steeper for the individuals that did not return the next year (figure 3; interaction delta age *returned: t1,83.3 = −2.5, p = 0.01; added with main effect to the model in table 1, longitudinal analysis). Thus, telomere shortening is accelerated in the last year before an individual disappears from the colony and presumably dies, suggesting a rapid disintegration of body integrity near the end of life. It is worth noting that this effect is in agreement with data from alpine swifts (Bize et al. 2009) and cross-sectional observations in humans that telomere loss accelerates at high age (Lansdorp 2004), although a direct association between life expectancy and telomere shortening rate has, to our knowledge, not yet been demonstrated in humans.
Figure 3.
Telomere shortening rate (base pairs/ln(year)) (mean ± s.e.) for individuals that did or did not return to the colony the next year.
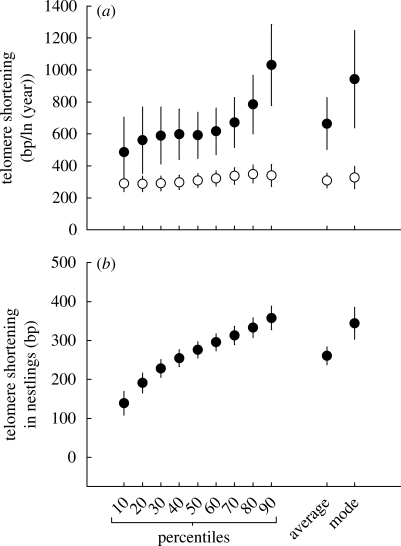
It is the length of shortest telomeres that triggers cellular senescence (Hemann et al. 2001; Capper et al. 2007) and one could hypothesize therefore that short telomeres could be better protected and hence shorten at a lower rate. We therefore repeated the analyses in table 1 (longitudinal) for the other characterizations of the telomere distribution we derived, i.e. the different percentiles of the telomere distribution that each measurement yields. Telomere shortening rate increased with percentile, with the longest telomeres shortening at twice the rate of the shortest telomeres (figure 4; table 2). This is not a regression to the mean effect, since subsequent samples and percentile estimates are statistically independent. Furthermore, the difference between the within- and between-individuals estimates of telomere shortening increased with percentile. Significance level of selective disappearance also increased with percentile, being significant from the 65th percentile onwards and reaching p < 0.01 at the 90th percentile. This indicates that selective disappearance of individuals with short telomeres is most conspicuous on longer telomeres. To our knowledge, there is only one other comparable test: in a human twin study (Kimura et al. 2008), mean telomere length below the 25th and 50th percentiles did not predict mortality better than mean telomere length, but higher percentiles (where we find a clear effect) were not compared.
Figure 4.
(a) The slope (±s.e.) of the relation with age for different estimates of telomere length in adults. The difference between within-individual (black circles) and between-individual (white circles) estimates of telomere shortening in adults shows an accelerated increase. (b) Telomere shortening in the 25 days prior to fledging was much higher compared with adults for all measures used.
Table 2.
Comparison between estimates of age-related telomere shortening derived from cross-sectional and longitudinal analysis, for different parameters characterizing the telomere size distribution (sample size is 116 samples from 46 individuals).
| estimate of telomere length | ln(age) (standard error) | within individuals: delta (ln(age)) (standard error) | between individuals: average (ln(age)) (standard error) |
|---|---|---|---|
| average | −336.3 (46.1) | −664.0 (162.2) | −309.4 (47.0) |
| mode | −357.6 (68.5) | −942.4 (304.1) | −328.2 (69.6) |
| 10th percentile | −301.2 (50.6) | −486.5 (220.4) | −290.1 (51.8) |
| 30th percentile | −309.7 (45.0) | −588.9 (178.4) | −290.6 (45.7) |
| 50th percentile | −332.8 (43.9) | −591.5 (145.2) | −308.5 (45.1) |
| 70th percentile | −369.3 (49.8) | −671.6 (155.6) | −337.4 (51.4) |
| 90th percentile | −382.2 (66.9) | −1031.2 (254.1) | −340.5 (67.7) |
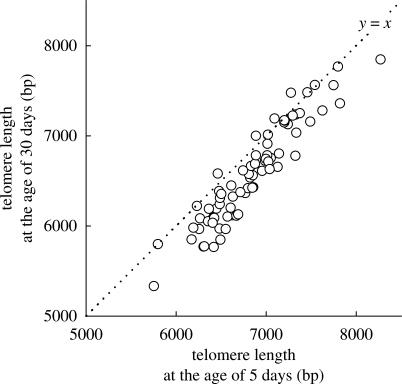
Average telomere length in nestlings decreased by 261 bp between ages 5 and 30 days (n = 74; paired t-test: t = −11.5, d.f. = 73, p < 0.001), and telomere loss was independent of telomere length at day 5. The latter conclusion follows from the finding that the slope of the correlation between telomere lengths at days 5 and 30 was not significantly different from unity (figure 5; b = 1.08, s.e. = 0.05). Telomere length increased in a few nestlings. However, as the activity of telomerase, an enzyme capable of elongating telomeres (Greider & Blackburn 1985), is downregulated in most post-natal somatic tissue (Forsyth et al. 2002; Haussmann et al. 2007), we attribute this to measurement error. Telomere shortening rate in nestlings over the 25-day measurement period (age 5–30 days) was approximately 16 times higher than shortening rates in 1-year-old individuals, and approximately 62 and approximately 115 times higher than in adults at the ages of 5 and 10 years, respectively.
Figure 5.
Nestling telomere length at ages 5 and 30 days plotted against each other. Line indicates equal values ( y = x) and hence when telomeres have shortened between ages 5 and 30 days the data points fall below this line.
Similar to adults, telomere shortening rate within individual nestlings was higher for longer telomeres (figure 4b). Thus, in nestlings, there is an interesting contrast: when comparing between individuals, the telomere shortening rate was independent of telomere length (figure 5), while within individuals the longer telomeres shortened at a higher rate (figure 4). For the adults, the different estimates are more in agreement, since telomere length declined faster early in life (when telomeres are longer) compared with late in life (figure 2a). One possible explanation for the difference between nestlings and adults could be that in adults the haematopoietic stem cells disappear from the bone marrow when their telomeres become too short, whereas this process is unlikely to occur in nestlings owing to the young age (up to 30 days post-hatching) of the majority of stem cells.
4. Conclusions
Cross-sectional analyses yield biased estimates of within-individual effects when the focal trait, in this case telomere shortening, is subject to selective disappearance. However, the effects of within and between individuals can be disentangled using longitudinal data (Snijders & Bosker 1999). This is useful because the biological interpretation of these two effects is different. The within-individual estimate is relevant when one is interested in estimating the telomere loss of individuals, which is clearly the most biologically interesting estimate. The interest in the between-individuals estimate lies primarily in the comparison with the within-individual estimate, as this allows a test of an association between telomere length and selective disappearance from the sample population. Using this approach, we showed that individuals with short telomeres are more likely to disappear from the population and, more specifically, that telomere shortening rate is substantially higher during the last year preceding disappearance from the colony. When telomere shortening rate reflects oxidative stress, this suggests that oxidative stress is higher in the last year of life, because of a higher rate of free radical production, lower antioxidant capacity or a combination of the two. Telomerase activity could also play a role, although, as mentioned above, earlier studies have shown telomerase activity to decline strongly with age very early in life, and to be negligible in adult birds that are not very old. Studies are currently under way to investigate these hypotheses.
We do not take our findings as evidence that there is a direct (causal) effect of telomere length on survival, because it seems at least as likely that its predictive value derives from the link between telomere shortening and oxidative stress or other (DNA) damage-generating mechanisms. This does not, however, detract much of its value, since also in this case telomere length can be used as a biomarker of ageing (von Zglinicki & Martin-Ruiz 2005) and there are few, if any, alternative options available if one aims to predict survival probabilities of individuals. Telomere shortening serves that purpose regardless of the underlying mechanism.
Within individuals, longer telomeres shortened at a higher rate in both adults and nestlings (figure 4), and, to the best of our knowledge, this is the first demonstration of within-individual size-dependent telomere shortening in vivo. The general finding that telomere shortening rate is highest at younger ages suggests that the same occurs between individuals, with individuals with longer telomeres losing base pairs at a higher rate. Note, however, that the observed higher shortening rates in individuals with longer telomeres can also be attributed to other differences between younger and older individuals, such as a decline in metabolic rate (Moe et al. 2009). Furthermore, we found no indication for telomere length-dependent shortening rate when comparing between individual nestlings. Several recent studies did conclude that longer telomeres have higher shortening rates between individuals also (Aviv et al. 2009; Bize et al. 2009; Nordfjäll et al. 2009). Unfortunately, all three studies tested this hypothesis in a way that is known to create a bias in the reported direction called regression to the mean, which can be strong enough to explain the reported correlations.
We can only speculate regarding the mechanisms generating size-dependent telomere shortening, but in addition to the allocation of defensive mechanisms as suggested by Hemann et al. (2001), it seems possible that longer telomeres are intrinsically more vulnerable simply because they form a larger target (op den Buijs et al. 2004). Using other techniques such as T/C-FISH, it is now possible to study telomere shortening for different chromosomes (Perner et al. 2003; Mayer et al. 2006), which would enable a test of the hypothesis that it is the telomere length or shortening rate at specific chromosomes that best predicts mortality. Our finding that selective disappearance with respect to telomere length was most conspicuous for long telomeres (figure 4a) suggests that it is likely that telomere shortening of some chromosomes will be more suitable to predict mortality than others.
Acknowledgements
Data were collected under license of the animal experimentation committee of the University of Groningen. We thank Arjen Wassink for his help with programming ImageJ, and Daniel Promislow and two anonymous referees for valuable comments that improved the manuscript. H.M.S., G.A.M. and S.V. were supported by a NWO Vici-grant to S.V.
References
- Akaike H.1981Likelihood of a model and information criteria. J. Econ. 16, 3–14 [Google Scholar]
- Aubert G., Lansdorp P. M.2008Telomeres and aging. Physiol. Rev. 88, 557–579 (doi:10.1152/physrev.00026.2007) [DOI] [PubMed] [Google Scholar]
- Aviv A.2002Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J. Mol. Med. 80, 689–695 (doi:10.1007/s00109-002-0377-8) [DOI] [PubMed] [Google Scholar]
- Aviv A.2008The epidemiology of human telomeres: faults and promises. J. Gerontol. A Biol. Sci. Med. Sci. 63, 979–983 [DOI] [PubMed] [Google Scholar]
- Aviv A., Chen W., Gardner J. P., Kimura M., Brimacombe M., Cao X. J., Srinivasan S. R., Berenson G. S.2009Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa heart study. Am. J. Epidemiol. 169, 323–329 (doi:10.1093/aje/kwn338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerlocher G. M., Rice K., Vulto I., Lansdorp P. M.2007Longitudinal data on telomere length in leukocytes from newborn baboons support a marked drop in stem cell turnover around 1 year of age. Aging Cell 6, 121–123 (doi:10.1111/j.1474-9726.2006.00254.x) [DOI] [PubMed] [Google Scholar]
- Baird D. M.2006Telomeres. Exp. Gerontol. 41, 1223–1227 (doi:10.1016/j.exger.2006.09.009) [DOI] [PubMed] [Google Scholar]
- Baird D. M., Rowson J., Wynford-Thomas D., Kipling D.2003Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat. Genet. 33, 203–207 (doi:10.1038/ng1084) [DOI] [PubMed] [Google Scholar]
- Bakaysa S. L., Mucci L. A., Slagboom P. E., Boomsma D. I., McClearn G. E., Johansson B., Pedersen N. L.2007Telomere length predicts survival independent of genetic influences. Aging Cell 6, 769–774 (doi:10.1111/j.1474-9726.2007.00340.x) [DOI] [PubMed] [Google Scholar]
- Beckman K. B., Ames B. N.1998The free radical theory of aging matures. Physiol. Rev. 78, 547–581 [DOI] [PubMed] [Google Scholar]
- Benetos A., Okuda K., Lajemi M., Kimura M., Thomas F., Skurnick J., Labat C., Bean K., Aviv A.2001Telomere length as an indicator of biological aging—the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 37, 381–385 [DOI] [PubMed] [Google Scholar]
- Bischoff C., Petersen H. C., Graakjaer J., Andersen-Ranberg K., Vaupel J. W., Bohr V. A., Kolvraa S., Christensen K.2006No association between telomere length and survival among the elderly and oldest old. Epidemiology 17, 190–194 (doi:10.1097/01.ede.0000199436.55248.10) [DOI] [PubMed] [Google Scholar]
- Bize P., Criscuolo F., Metcalfe N. B., Nasir L., Monaghan P.2009Telomere dynamics rather than age predict life expectancy in the wild. Proc. R. Soc. B 276, 1679–1683 (doi:10.1098/rspb.2008.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H.1991Structure and function of telomeres. Nature 350, 569–573 (doi:10.1038/350569a0) [DOI] [PubMed] [Google Scholar]
- Brummendorf T. H., Mak J., Sabo K. M., Baerlocher G. M., Dietz K., Abkowitz J. L., Lansdorp P. M.2002Longitudinal studies of telomere length in feline blood cells: Implications for hematopoietic stem cell turnover in vivo. Exp. Hematol. 30, 1147–1152 (doi:10.1016/S0301-472X(02)00888-3) [DOI] [PubMed] [Google Scholar]
- Capper R., Britt-Compton B., Tankimanova M., Rowson J., Letsolo B., Man S., Haughton M., Baird D. M.2007The nature of telomere fusion and a definition of the critical telomere length in human cells. Gene Dev. 21, 2495–2508 (doi:10.1101/gad.439107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattan V., et al. 2008Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radic. Biol. Med. 44, 1592–1598 (doi:10.1016/j.freeradbiomed.2008.01.007) [DOI] [PubMed] [Google Scholar]
- Cawthon R. M., Smith K. R., O'Brien E., Sivatchenko A., Kerber R. A.2003Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395 (doi:10.1016/S0140-6736(03)12384-7) [DOI] [PubMed] [Google Scholar]
- Delany M. E., Krupkin A. B., Miller M. M.2000Organization of telomere sequences in birds: evidence for arrays of extreme length and for in vivo shortening. Cytogenet. Cell Genet. 90, 139–145 (doi:10.1159/000015649) [DOI] [PubMed] [Google Scholar]
- di Fagagna F. D., Reaper P. M., Clay-Farrace L., Fiegler H., Carr P., von Zglinicki T., Saretzki G., Carter N. P., Jackson S. P.2003A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (doi:10.1038/nature02118) [DOI] [PubMed] [Google Scholar]
- Epel E. S., Blackburn E. H., Lin J., Dhabhar F. S., Adler N. E., Morrow J. D., Cawthon R. M.2004Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17 312–17 315 (doi:10.1073/pnas.0407162101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E. S., Lin J., Wilhelm F. H., Wolkowitz O. M., Cawthon R., Adler N. E., Dolbier C., Mendes W. B., Blackburn E. H.2006Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 31, 277–287 (doi:10.1016/j.psyneuen.2005.08.011) [DOI] [PubMed] [Google Scholar]
- Forsyth N. R., Wright W. E., Shay J. W.2002Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation 69, 188–197 (doi:10.1046/j.1432-0436.2002.690412.x) [DOI] [PubMed] [Google Scholar]
- Frenck R. W.1998The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl Acad. Sci. USA 95, 5607–5610 (doi:10.1073/pnas.95.10.5607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. D., Broccoli D., Muquit M., Manion F. J., Tisdall J., Ochs M. F.2001Telometric: A tool providing simplified, reproducible measurements of telomeric DNA from constant field agarose gels. Biotechniques 31, 1314–1318 [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H.1985Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell 43, 405–413 (doi:10.1016/0092-8674(85)90170-9) [DOI] [PubMed] [Google Scholar]
- Hall M. E., Nasir L., Daunt F., Gault E. A., Croxall J. P., Wanless S., Monaghan P.2004Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. Lond. B 271, 1571–1576 (doi:10.1098/rspb.2004.2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Deary I. J., Maclntyre A., Lamb K. J., Radhakrishnan K., Starr J. M., Whalley L. J., Shiels P. G.2006The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci. Lett. 406, 260–264 (doi:10.1016/j.neulet.2006.07.055) [DOI] [PubMed] [Google Scholar]
- Haussmann M. F., Mauck R. A.2008aTelomeres and longevity: testing an evolutionary hypothesis. Mol. Biol. Evol. 25, 220–228 (doi:10.1093/molbev/msm244) [DOI] [PubMed] [Google Scholar]
- Haussmann M. F., Mauck R. A.2008bNew strategies for telomere-based age estimation. Mol. Ecol. Resour. 8, 264–274 (doi:10.1111/j.1471-8286.2007.01973.x) [DOI] [PubMed] [Google Scholar]
- Haussmann M. F., Winkler D. W., O'Reilly K. M., Huntington C. E., Nisbet I. C. T., Vleck C. M.2003Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc. R. Soc. Lond. B 270, 1387–1392 (doi:10.1098/rspb.2003.2385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann M. F., Winkler D. W., Vleck C. M.2005Longer telomeres associated with higher survival in birds. Biol. Lett. 1, 212–214 (doi:10.1098/rsbl.2005.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann M. F., Winkler D. W., Huntington C. E., Nisbet I. C. T., Vleck C. M.2007Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp. Gerontol. 42, 610–618 (doi:10.1016/j.exger.2007.03.004) [DOI] [PubMed] [Google Scholar]
- Hemann M. T., Strong M. A., Hao L. Y., Greider C. W.2001The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107, 67–77 (doi:10.1016/S0092-8674(01)00504-9) [DOI] [PubMed] [Google Scholar]
- Henle E. S., Han Z. X., Tang N., Rai P., Luo Y. Z., Linn S.1999Sequence-specific DNA cleavage by Fe2+-mediated fenton reactions has possible biological implications. J. Biol. Chem. 274, 962–971 (doi:10.1074/jbc.274.2.962) [DOI] [PubMed] [Google Scholar]
- Ilmonen P., Kotrschal A., Penn D. J.2008Telomere attrition due to infection. PLoS ONE 3, e2143 (doi:10.1371/journal.pone.0002143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings B. J., Ozanne S. E., Hales C. N.2000Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol. Genet. Metab. 71, 32–42 (doi:10.1006/mgme.2000.3077) [DOI] [PubMed] [Google Scholar]
- Joeng K. S., Song E. J., Lee K. J., Lee J.2004Long lifespan in worms with long telomeric DNA. Nat. Genet. 36, 607–611 (doi:10.1038/ng1356) [DOI] [PubMed] [Google Scholar]
- Karlseder J., Smogorzewska A., de Lange T.2002Senescence induced by altered telomere state, not telomere loss. Science 295, 2446–2449 (doi:10.1126/science.1069523) [DOI] [PubMed] [Google Scholar]
- Kimura M., et al. 2008Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am. J. Epidemiol. 167, 799–806 (doi:10.1093/aje/kwm380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrschal A., Ilmonen P., Penn D. J.2007Stress impacts telomere dynamics. Biol. Lett. 3, 128–130 (doi:10.1098/rsbl.2006.0594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp P. M.2004Telomeres and telomerase regulation. In Handbook of stem cells 2 (ed. Lanza R.), pp. 127–137 San Diego, CA: Elsevier Science [Google Scholar]
- Lansdorp P. M., Verwoerd N. P., vandeRijke F. M., Dragowska V., Little M. T., Dirks R. W., Raap A. L., Tanke H. J.1996Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 5, 685–691 (doi:10.1093/hmg/5.5.685) [DOI] [PubMed] [Google Scholar]
- Martens U. M., Zijlmans J. M. J. M., Poon S. S. S., Dragowska W., Yui J., Chavez E. A., Ward R. K., Lansdorp P. M.1998Short telomeres on human chromosome 17p. Nat. Genet. 18, 76–80 (doi:10.1038/ng0198-018) [DOI] [PubMed] [Google Scholar]
- Martens U. M., Chavez E. A., Poon S. S. S., Schmoor C., Landsdorp P. M.2000Accumulation of short telomeres in human fibroblasts prior to replicative senescence. Exp. Cell Res. 256, 291–299 (doi:10.1006/excr.2000.4823) [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C. M., Gussekloo J., van Heemst D., von Zglinicki T., Westendorp R. G. J.2005Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell 4, 287–290 (doi:10.1111/j.1474-9726.2005.00171.x) [DOI] [PubMed] [Google Scholar]
- Mayer S., et al. 2006Sex-specific telomere length profiles and age-dependent erosion dynamics of individual chromosome arms in humans. Cytogenet. Genome Res. 112, 194–201 (doi:10.1159/000089870) [DOI] [PubMed] [Google Scholar]
- Moe B., Rønning B., Verhulst S., Bech C.2009Metabolic ageing in individual zebra finches. Biol. Lett. 5, 86–89 (doi:10.1098/rsbl.2008.0481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P., Haussmann M. F.2006Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53 (doi:10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- Nordfjäll K., Svenson U., Norrback K. F., Adolfsson R., Lenner P., Roos G.2009The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 5, e1000375 (doi:10.1371/journal.pgen.1000375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa S., Tada-Oikawa S., Kawanishi S.2001Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry 40, 4763–4768 (doi:10.1021/bi002721g) [DOI] [PubMed] [Google Scholar]
- op den Buijs J., van den Bosch P. P. J., Musters M. W. J. M., van Riel N. A. W.2004Mathematical modeling confirms the length-dependency of telomere shortening. Mech. Ageing Dev. 125, 437–444 [DOI] [PubMed] [Google Scholar]
- Pauliny A., Wagner R. H., Augustin J., Szep T., Blomqvist D.2006Age-independent telomere length predicts fitness in two bird species. Mol. Ecol. 15, 1681–1687 (doi:10.1111/j.1365-294X.2006.02862.x) [DOI] [PubMed] [Google Scholar]
- Perner S., et al. 2003Quantifying telomere lengths of human individual chromosome arms by centromere-calibrated fluorescence in situ hybridization and digital imaging. Am. J. Pathol. 163, 1751–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. B., Rosenberg I.2006Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol. Rev. 86, 651–667 (doi:10.1152/physrev.00019.2005) [DOI] [PubMed] [Google Scholar]
- Rufer N., Brummendorf T. H., Kolvraa S., Bischoff C., Christensen K., Wadsworth L., Schulzer M., Lansdorp P. M.1999Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 190, 157–167 (doi:10.1084/jem.190.2.157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons H. M., Dijkstra C., Verhulst S.2008Strong but variable associations between social dominance and clutch sex ratio in a colonial corvid. Behav. Ecol. 19, 417–424 (doi:10.1093/beheco/arm149) [Google Scholar]
- Seluanov A., Chen Z. X., Hine C., Sasahara T. H. C., Ribeiro A. A. C. M., Catania K. C., Presgraves D. C., Gorbunova V.2007Telomerase activity coevolves with body mass not lifespan. Aging Cell 6, 45–52 (doi:10.1111/j.1474-9726.2006.00262.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov I. A., Gee D., Dimitrov D. S.2004A kinetic model of telomere shortening in infants and adults. J. Theor. Biol. 226, 169–175 (doi:10.1016/j.jtbi.2003.08.009) [DOI] [PubMed] [Google Scholar]
- Snijders T. A. B., Bosker R. J.1999Multilevel analysis: an introduction to basic and advanced multilevel modeling London, UK: Sage Publications [Google Scholar]
- Tchirkov A., Lansdorp P. M.2003Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia–telangiectasia. Hum. Mol. Genet. 12, 227–232 (doi:10.1093/hmg/ddg023) [DOI] [PubMed] [Google Scholar]
- Terry D. F., Nolan V. G., Andersen S. L., Perls T. T., Cawthon R.2008Association of longer telomeres with better health in centenarians. J. Gerontol. A Biol. Sci. Med. Sci. 63, 809–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pol M., Verhulst S.2006Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 167, 766–773 (doi:10.1086/503331) [DOI] [PubMed] [Google Scholar]
- Vleck C. M., Haussmann M. F., Vleck D.2007Avian senescence: underlying mechanisms. J. Ornithol. 148, S611–S624 (doi:10.1007/s10336-007-0186-5) [Google Scholar]
- von Zglinicki T.2002Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (doi:10.1016/S0968-0004(02)02110-2) [DOI] [PubMed] [Google Scholar]
- von Zglinicki T., Martin-Ruiz C. M.2005Telomeres as biomarkers for ageing and age-related diseases. Curr. Mol. Med. 5, 197–203 (http://dx.doi.org/10.2174/1566524053586545) [DOI] [PubMed] [Google Scholar]
- von Zglinicki T., Pilger R., Sitte N.2000Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic. Biol. Med. 28, 64–74 (doi:10.1016/S0891-5849(99)00207-5) [DOI] [PubMed] [Google Scholar]
- Zeichner S. L., Palumbo P., Feng Y. R., Xiao X. D., Gee D., Sleasman J., Goodenow R., Biggar R., Dimitrov D.1999Rapid telomere shortening in children. Blood 93, 2824–2830 [PubMed] [Google Scholar]