Abstract
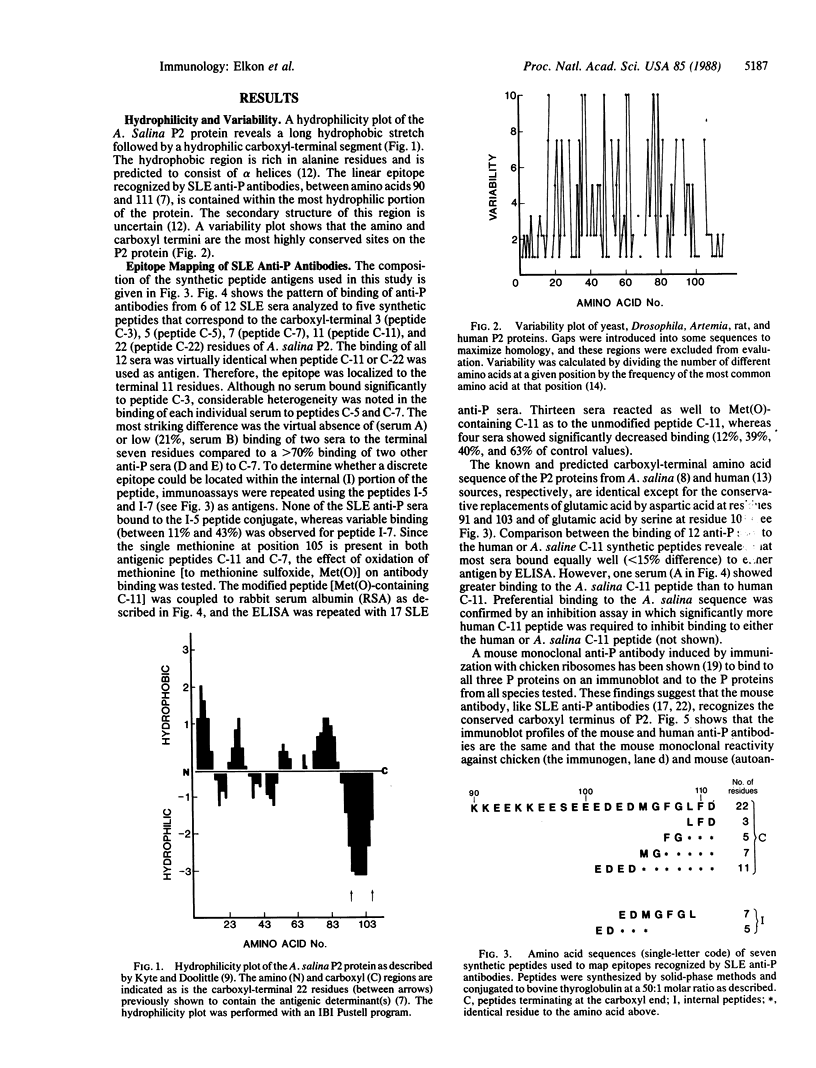
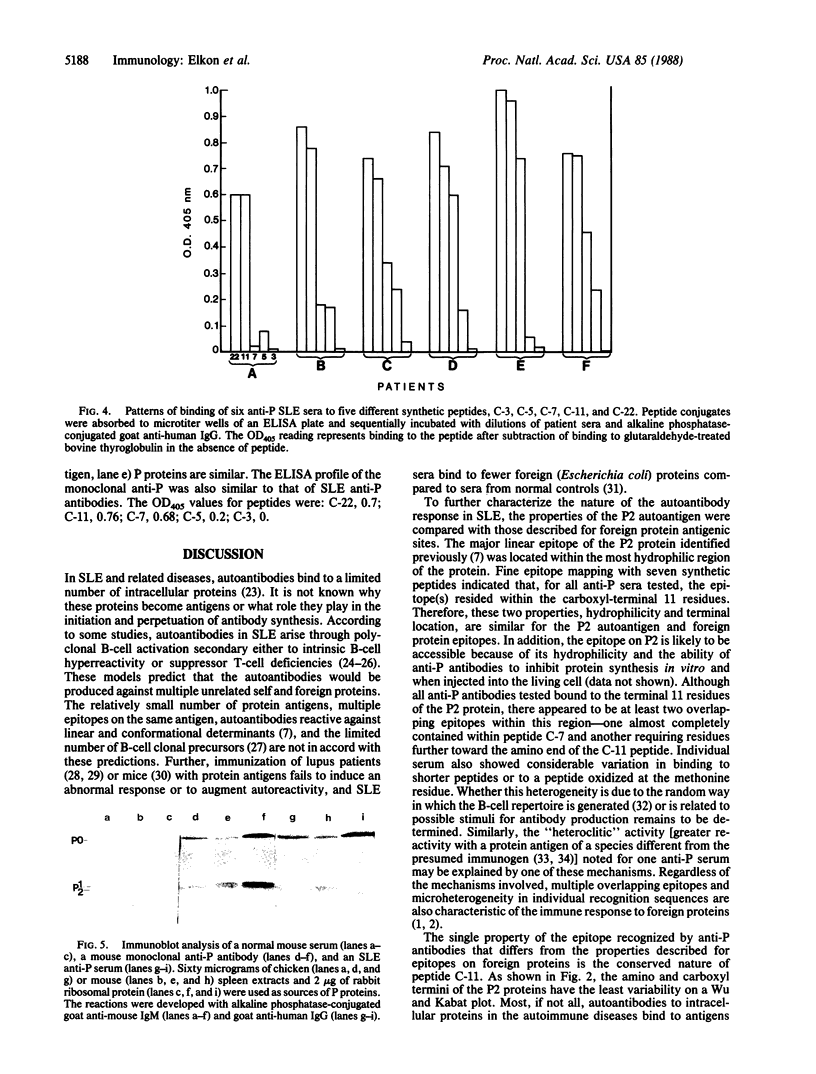
Approximately 15% of patients with systemic lupus erythematosus have autoantibodies that bind to a shared epitope previously shown to be located on the carboxyl-terminal 22 amino acids of three 60S ribosomal proteins, P0, P1, and P2 ("P proteins"). A hydrophilicity plot and fine epitope mapping with seven synthetic peptides revealed that the properties of the antigenic site were similar to certain properties of epitopes on foreign protein antigens--namely, the epitope was located in the most hydrophilic portion of the P2 protein and also in the terminal region of the molecule. However, this site has been highly conserved during evolution. A mouse monoclonal antibody induced by immunization with ribosomal proteins had a fine specificity similar to the lupus antibodies. This finding indicates that a highly conserved region of a lupus autoantigen may also be antigenic in some normal animals. Therefore, lupus autoantibodies may be similar in most, if not all respects, to antibodies produced by immunization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., Pluijms W., Möller W. The primary structure of ribosomal protein eL12/eL12-P from Artemia salina 80 S ribosomes. FEBS Lett. 1979 Aug 1;104(1):85–89. doi: 10.1016/0014-5793(79)81089-3. [DOI] [PubMed] [Google Scholar]
- Bachmann M., Mayet W. J., Schröder H. C., Pfeifer K., Meyer zum Büschenfelde K. H., Müller W. E. Association of La and Ro antigens with intracellular structures in HEp-2 carcinoma cells. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7770–7774. doi: 10.1073/pnas.83.20.7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Elkon K. B. Clinical and serologic associations of the antiribosomal P protein antibody. Arthritis Rheum. 1986 Aug;29(8):981–985. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Golombek S. J., Kaufman L. D., Skelly S., Weissbach H., Brot N., Elkon K. B. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987 Jul 30;317(5):265–271. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- Brodman R., Gilfillan R., Glass D., Schur P. H. Influenzal vaccine response in systemic lupus erythematosus. Ann Intern Med. 1978 Jun;88(6):735–740. doi: 10.7326/0003-4819-88-6-735. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Tan E. M. Human autoantibody-reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987 Dec 1;166(6):1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian C. L., Elkon K. B. Autoantibodies to intracellular proteins. Clinical and biologic significance. Am J Med. 1986 Jan;80(1):53–61. doi: 10.1016/0002-9343(86)90048-3. [DOI] [PubMed] [Google Scholar]
- Creighton W. D., Katz D. H., Dixon F. J. Antigen-specific immunocompetency, B cell function, and regulatory helper and suppressor T cell activities in spontaneously autoimmune mice. J Immunol. 1979 Dec;123(6):2627–2636. [PubMed] [Google Scholar]
- Elkon K. B., Culhane L. Partial immunochemical characterization of the Ro and La proteins using antibodies from patients with the sicca syndrome and lupus erythematosus. J Immunol. 1984 May;132(5):2350–2356. [PubMed] [Google Scholar]
- Elkon K. B., Parnassa A. P., Foster C. L. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985 Aug 1;162(2):459–471. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Skelly S., Parnassa A., Moller W., Danho W., Weissbach H., Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Heckman K. J., Lee J. C., Tan E. M. Identification of ribosomal protein autoantigens. J Immunol. 1985 Oct;135(4):2378–2384. [PubMed] [Google Scholar]
- Hansburg D., Fairwell T., Schwartz R. H., Appella E. The T lymphocyte response to cytochrome c. IV. Distinguishable sites on a peptide antigen which affect antigenic strength and memory. J Immunol. 1983 Jul;131(1):319–324. [PubMed] [Google Scholar]
- Harley J. B., Rosario M. O., Yamagata H., Fox O. F., Koren E. Immunologic and structural studies of the lupus/Sjögren's syndrome autoantigen, La/SSB, with a monoclonal antibody. J Clin Invest. 1985 Aug;76(2):801–806. doi: 10.1172/JCI112037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi T., Mäkelä O. Strain differences in the fine specificity of mouse anti-hapten antibodies. Eur J Immunol. 1973 Jun;3(6):323–330. doi: 10.1002/eji.1830030602. [DOI] [PubMed] [Google Scholar]
- Itoh T. Primary structure of an acidic ribosomal protein YPA1 from Saccharomyces cerevisiae. Isolation and characterization of peptides and the complete amino acid sequence. Biochim Biophys Acta. 1981 Nov 30;671(1):16–24. doi: 10.1016/0005-2795(81)90088-x. [DOI] [PubMed] [Google Scholar]
- Jemmerson R., Margoliash E. Specificity of the antibody response of rabbits to a self-antigen. Nature. 1979 Nov 29;282(5738):468–471. doi: 10.1038/282468a0. [DOI] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Production of autoantibodies by immunization with rabbit myglobin. Immunochemistry. 1978 Jan;15(1):67–70. doi: 10.1016/0161-5890(78)90028-7. [DOI] [PubMed] [Google Scholar]
- Kieber-Emmons T., Kohler H. Evolutionary origin of autoreactive determinants (autogens). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2521–2525. doi: 10.1073/pnas.83.8.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lin A., Wittmann-Liebold B., McNally J., Wool I. G. The primary structure of the acidic phosphoprotein P2 from rat liver 60 S ribosomal subunits. Comparison with ribosomal 'A' proteins from other species. J Biol Chem. 1982 Aug 10;257(15):9189–9197. [PubMed] [Google Scholar]
- Moore G. R., Williams R. J. Comparison of the structures of various eukaryotic ferricytochromes c and ferrocytochromes and their antigenic differences. Eur J Biochem. 1980 Feb;103(3):543–550. doi: 10.1111/j.1432-1033.1980.tb05978.x. [DOI] [PubMed] [Google Scholar]
- Qian S., Zhang J. Y., Kay M. A., Jacobs-Lorena M. Structural analysis of the Drosophila rpA1 gene, a member of the eucaryotic 'A' type ribosomal protein family. Nucleic Acids Res. 1987 Feb 11;15(3):987–1003. doi: 10.1093/nar/15.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter R., Lührmann R. Immunization of mice with purified U1 small nuclear ribonucleoprotein (RNP) induces a pattern of antibody specificities characteristic of the anti-Sm and anti-RNP autoimmune response of patients with lupus erythematosus, as measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8689–8693. doi: 10.1073/pnas.83.22.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich B. E., Steitz J. A. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987 Nov;7(11):4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell dysfunction in systemic lupus erythematosus. Cells involved and in vitro correction. J Clin Invest. 1978 Oct;62(4):789–796. doi: 10.1172/JCI109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Burstein Y., Patchornik A. Selective oxidation of methionine residues in proteins. Biochemistry. 1975 Oct 7;14(20):4497–4503. doi: 10.1021/bi00691a025. [DOI] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shores E. W., Eisenberg R. A., Cohen P. L. Role of the Sm antigen in the generation of anti-Sm autoantibodies in the SLE-prone MRL mouse. J Immunol. 1986 May 15;136(10):3662–3667. [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Alexander H., Houghten R. A., Olson A. J., Lerner R. A., Hendrickson W. A. The reactivity of anti-peptide antibodies is a function of the atomic mobility of sites in a protein. Nature. 1984 Nov 8;312(5990):127–134. doi: 10.1038/312127a0. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Thornton J. M., Sibanda B. L. Amino and carboxy-terminal regions in globular proteins. J Mol Biol. 1983 Jun 25;167(2):443–460. doi: 10.1016/s0022-2836(83)80344-1. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Ramjoué H. P., Kuster H., Liverani D., Gordon J. Monoclonal antibodies against eucaryotic ribosomes. Use to characterize a ribosomal protein not previously identified and antigenically related to the acidic phosphoproteins P1/P2. J Biol Chem. 1982 Nov 10;257(21):12709–12715. [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITEBSKY E., ROSE N. R. Studies on organ specificity. IV. Production of rabbit thyroid antibodies in the rabbit. J Immunol. 1956 Jun;76(6):408–416. [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]
- Williams G. W., Steinberg A. D., Reinertsen J. L., Klassen L. W., Decker J. L., Dolin R. Influenza immunization in systemic lupus eruthematosus. A double-blind trial. Ann Intern Med. 1978 Jun;88(6):729–734. doi: 10.7326/0003-4819-88-6-729. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]



