Abstract
Long-distance dispersal (LDD) of seeds and pollen shapes the spatial dynamics of plant genotypes, populations and communities. Quantifying LDD is thus important for predicting the future dynamics of plants exposed to environmental changes. However, environmental changes can also alter the behaviour of LDD vectors: for instance, increasing air temperature may enhance atmospheric instability, thereby altering the turbulent airflow that transports seed and pollen. Here, we investigate temperature effects on wind dispersal in a boreal forest using a 10-year time series of micrometeorological measurements and a Lagrangian stochastic model for particle transport. For a wide range of dispersal and life history types, we found positive relations between air temperature and LDD. This translates into a largely consistent positive effect of +3°C warming on predicted LDD frequencies and spread rates of plants. Relative increases in LDD frequency tend to be higher for heavy-seeded plants, whereas absolute increases in LDD and spread rates are higher for light-seeded plants for which wind is often an important dispersal vector. While these predicted increases are not sufficient to compensate forecasted range losses and environmental changes can alter plant spread in various ways, our results generally suggest that warming can promote wind-driven movements of plant genotypes and populations in boreal forests.
Keywords: atmospheric instability, boreal forest, global warming, long-distance dispersal, seed dispersal, plant spread
1. Introduction
The long-distance dispersal (LDD) of seeds and pollen shapes many fundamental processes in plant ecology and evolution, such as inter-population gene flow, local adaptation and the regional and continental-scale dynamics of plant species, communities and ecosystems (Ellstrand 1992; Kawecki & Ebert 2004; Neilson et al. 2005; Nathan et al. 2008). LDD also drives many phenomena of applied interest, such as the spread of transgenes and invasive species and the response of plant species to habitat fragmentation (Ellstrand 1992; Kuparinen & Schurr 2007; Damschen et al. 2008). In particular, LDD of seeds is important to the ability of plant species to spread into new areas under future climate change (Fischlin et al. 2007). This is because the spread potential of plant populations depends not only on their reproductive rate and generation time, but also on the distribution of seed dispersal distances (i.e. the dispersal kernel) (Clark 1998; Clark et al. 2001; Thompson & Katul 2008).
Wind is a dispersal vector that can generate LDD of seeds and pollen in many plant species (Kuparinen 2006; Nathan et al. 2008). The LDD of wind-dispersed seeds and pollen is typically promoted by turbulent vertical fluctuations in wind and by coherency in vertical eddy motion that uplifts seeds well above the vegetation canopy (Nathan et al. 2002; Tackenberg 2003; Soons et al. 2004; Nathan & Katul 2005; Nathan et al. 2005). Turbulent wind conditions can be induced by atmospheric instability that, among other factors, depends on temperature differences between the surface and the atmosphere (Monin & Obukhov 1954), which generates the so-called convective (thermal) turbulence (e.g. Tackenberg 2003, Wright et al. 2008). This suggests that distances over which plant propagules are carried by wind can depend on prevailing temperatures and, specifically, that an increase in air temperature might induce an increase in atmospheric instability (see the electronic supplementary material for detailed explanations), thus promoting dispersal. Convective turbulence has been found to be an important mechanism promoting aerial transport of aerial biota such as aphids (Reynolds & Reynolds 2009), pollen and seeds of grassland species (Tackenberg 2003) and, for example, Jacaranda copaia, a common wind-dispersed tree species in a tropical forest (Wright et al. 2008). Yet, no study thus far has investigated whether projected increases in air temperature could substantially alter the LDD and potential spread of wind-dispersed plants (Nathan et al. 2008). Here, we address this question empirically, by combining a 10-year time series of summertime micrometeorological and temperature measurements in a boreal forest (figure 1) with mechanistic models of wind dispersal (Kuparinen et al. 2007) and population spread (Clark et al. 2001). We apply these to predict how a scenario of +3°C warming would alter dispersal and spread of light- and heavy-seeded herbs and trees. This analysis provides the first assessment of how raising air temperatures could affect wind-driven plant movements.
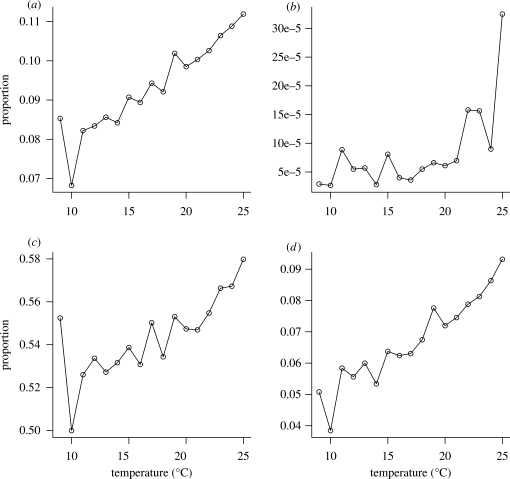
Figure 1.
Relationships between temperature and micrometeorological variables measured from 1998 to 2007 at Hyytiälä, southern Finland. Data are restricted to the vegetation growth period (May–September) and to 4 h periods around solar noon. (a) Proportions of 30 min intervals that are unstable (dark grey, Monin–Obukhov length L < 0 m) and stable (light grey, L > 0 m), respectively. (b) Proportions of unstable 30 min intervals in which L exceeds different threshold values. Atmospheric instability increases as negative values of L approach 0 m. (c) Proportions of unstable 30 min intervals in which friction velocity u* (a measure of ‘windiness’) exceeds different threshold values.
2. Methods
Our study is based on micrometeorological data measured during the vegetation growth period (May–September) of 10 consecutive years (1998–2007) at the Hyytiälä SMEAR II site (65°51' N, 24°17' E) in southern Finland (Vesala et al. 1998). The measurement site is covered by a 40-year-old Scots pine (Pinus sylvestris L.) forest with a canopy height of 14 m. The micrometeorological data are aggregated in half-hourly intervals and measure air temperature, friction velocity (u*: a measure of overall ‘windiness’ reflecting the balance between large-scale pressure gradients driving air movement, the Coriollis force owing to the Earth's rotation and the frictional forces within the soil–plant system) and Monin–Obukhov length (L: reflecting instability/stability in the atmosphere). Positive values of L describe stable atmospheres (e.g. nocturnal conditions) whereas for negative L the instability increases as L approaches 0 m (i.e. buoyancy dominates turbulence production). For large absolute values of L, be L negative or positive, the atmosphere approaches a near-neutral state (Monin & Obukhov 1954) (i.e. turbulence is mechanically generated).
Because atmospheric instability can alter plant LDD in a rather complex manner (Wright et al. 2008; see also the electronic supplementary material), we first aim to characterize temperature-related variation in u* and L and use these to assess whether temperature increases could increase seed dispersal distances and spread rates of plant populations. We restricted the analysis to measurements taken in a 4 h time window around solar noon and conducted separate analyses for each month. In this way, we controlled for seasonal and diurnal covariation between temperature and u* or L, which arise from day/night time or spring/summer/autumn variations in the atmosphere. The majority of measurements represent unstable conditions (L < 0 m), and the frequency of these conditions in the data could be seen to further increase with temperature (figure 1a). To account for the fact that wind-dispersed seeds tend to be released under unstable conditions (Skarpaas et al. 2006; Wright et al. 2008), we only considered measurement intervals where L < 0 m (10 117 intervals). In these data, the frequency of unstable conditions (negative L-values close to 0) generally increases with mean air temperature above the canopy (figure 1b). We found this positive correlation not only for mid-day conditions, but also detected it throughout daytime measurements at Hyytiälä, which suggests that the correlation is robust to rapid transients in atmospheric dynamics. In contrast, no temperature trends were visible in observed u* values (figure 1c). Clearly, such empirical correlations do not imply causation. In the electronic supplementary material, we therefore present the necessary conditions under which positive correlations between air temperature above the canopy and sensible heat arise and can be extrapolated to future warmer atmospheres.
To assess temperature effects on wind dispersal, we split the half-hourly interval measurements of each month into 1°C temperature ranges and subsampled micrometeorological conditions in each month–temperature category for dispersal model runs: we used a random subset of 100 measurements if the number of measurements in a month–temperature category exceeded 100, and all measurements in categories that held between 20 and 100 measurements. Month–temperature categories with less than 20 observations were omitted since we considered the data insufficient for reflecting micrometeorological conditions typical of these categories. The sampled temperature ranges were 3–22°C in May, 9–22°C in June, 12–30°C in July, 10–27°C in August and 5–22°C in September. For the 6997 sets of micrometeorological conditions sampled, we predicted seed dispersal with a Lagrangian stochastic model for inertial particle transport (Kuparinen et al. 2007). The model predicts the flight trajectories of seeds starting from a given release height (h) by simulating the three-dimensional airflows that airborne seeds experience and by calculating seed flight trajectories from these airflows and the seeds' terminal falling velocity (vt). The movement of a seed was simulated until it first hit the ground or reached a downwind distance of 100 km. Canopy interception was neglected owing to the low leaf area at the study site. We parameterized the model for the canopy height (14 m), canopy density (leaf area index LAI = 1.3) and vertical leaf profile measured at the Hyytiälä site (for more details of model structure and parameterization see Kuparinen et al. 2007).
To assess the effect of dispersal traits, we considered four dispersal trait combinations that differ in vt and h. We set vt to either 0.2 or 1.0 m s−1 and refer to these two groups as heavy- and light-seeded species, respectively (while vt depends not only on seed weight, but also on seed surface area, vt and seed weight are typically positively correlated among wind-dispersed plant species) (R. Nathan, unpublished data). The two chosen vt values represent, respectively, a relatively low and a typical (average) value of terminal seed falling velocities of plant species commonly classified as ‘wind dispersed’ (Tackenberg et al. 2003; see Nathan et al. 2008 for a critical review of such classifications). Note that the lower vt is similar to that of fast-falling pollen (such as corn pollen (Aylor 2002)). h was either 1 or 12 m, reflecting herb and tree layers at the Hyytiälä site, respectively. For each of the four dispersal trait combinations, we simulated the dispersal of 10 000 seeds under each of the 6997 sampled micrometeorological conditions. For each dispersal trait combination, the simulations yielded 84 dispersal distributions, corresponding to 84-month–temperature categories. Sets of distributions that shared a month were then combined, each distribution in the combined set weighted by the frequency of the relevant temperature class in that month. In this way, we obtained overall predictions of seed dispersal for a given month and dispersal trait combination.
To predict the potential impact of temperature increase on dispersal at this measurement site, we considered a scenario of a +3°C increase in mid-day air temperature. This increase falls well within the range of mean surface air temperature increases owing to global warming predicted for alternative IPCC emission scenarios (+1.8 to 4.0°C (Meehl et al. 2007)). We implemented the temperature increase scenario by shifting the frequency of each month–temperature category by +3°C. For example, the proportion of temperature measurements between 20 and 21°C under current climate was assumed to equal the proportion of 23–24°C temperatures under increased temperatures. We quantified the uncertainty in dispersal predictions by considering variability in micrometeorological conditions and stochasticity in the seed dispersal model. To represent variability in the frequency of each month–temperature category, we took bootstrap re-samples of the full set of temperature measurements in each month. To reflect variability between micrometeorological conditions within each category, we took bootstrap re-samples of the dispersal model runs in each category. Finally, to represent stochasticity in the proportion of seeds exceeding a certain dispersal distance, we drew binomial random numbers with denominator 10 000 and probability given by the proportion of seeds exceeding the respective distance in the re-sampled model runs.
In the final step of analysis, we assessed how effects of air temperature increases on seed dispersal by wind could affect the spread potential of plant populations. Specifically, we considered a model of plant species spreading by means of extreme dispersal from single reproductive individuals at the population front. This ‘spread by extremes’ model (Clark et al. 2001) produces an upper boundary for expected spread rates in continuous space that can be calculated by combining demographic parameters (net reproductive rate R0 and generation time T) with a marginal density of dispersal distance in one dimension. We transformed simulated distances into marginal distances by assigning each seed a random direction and then projecting its location onto an arbitrarily oriented line (thereby assuming isotropic dispersal). From the resulting marginal distances we then derived empirical density functions. For the ‘herb’ types (h = 1 m) we assumed R0 = 2, and for the ‘tree’ types (h = 12 m) we assumed R0 = 13. These R0 values are the medians for the respective plant types in a demographic meta-analysis (Franco & Silvertown 2004), and are representative of competitive conditions in an established forest (note that under low competition at expanding tree lines trees may have much higher R0; Clark et al. 2001). T of herbs and trees was assumed to be 1 and 20 years, respectively, as typical values for the generation time of these plant types in boreal forests. Since spread rates are proportional to 1/T (Clark et al. 2001), using different T values will rescale the absolute spread rates, but will not alter the relative effects of warming on population spread.
3. Results
For the majority of considered plant types and months, the probability of LDD increased with temperature (see figure 2 for temperature effects on LDD in June), so that the amount of LDD generally increased under the scenario of +3°C warming (figure 3). For trees and light-seeded herbs, the relative increase in LDD frequency was always stronger at larger distances and little uncertainty was associated with these predictions (figure 3). For heavy-seeded herbs, dispersal increased at shorter distances (e.g. dispersal over 10 m increased by 3–17%), whereas at larger distances the outcome varied: for certain months, this dispersal type had the highest expected relative increases in LDD, but it also showed an expected decrease in August and generally high uncertainty around these expectations (figure 3). However, predicted dispersal distances for the heavy-seeded herb were very short (under both present day temperatures and those increased by +3°C the probability of dispersal beyond 20 m was <0.5% for all months), making it unlikely that wind is the predominant LDD vector for this plant type (Nathan et al. 2008). Because heavy-seeded plants had lower absolute probabilities of LDD than light-seeded plants, they also showed much smaller absolute changes in LDD probability: in June the absolute increase in the probability of dispersal over 500 m was 1.1 and 0.4 per cent for light seeds released from 12 and 1 m height, respectively, whereas for heavy seeds the corresponding increases were 0.1 per cent and 0.0003 per cent.
Figure 2.
Relationship between temperature and the proportions of propagules predicted to disperse more than 100 m for different dispersal trait scenarios. Predictions are based on micrometeorological conditions measured in June. (a) Light-seeded herb. (b) Heavy-seeded herb. (c) Light-seeded tree. (d) Heavy-seeded tree.
Figure 3.
Predicted effect of a +3°C temperature increase on long distance dispersal (LDD) by wind for different months and dispersal traits (release height h; terminal falling velocity vt). Dots indicate expected relative changes in the frequency of dispersal beyond 50 m (open circles) and 500 m (filled circles), respectively. Vertical bars are 95 per cent confidence intervals that represent variation in micrometeorological conditions and simulation stochasticity. Note that some confidence intervals are so narrow that they are overlaid by the respective dot.
According to our predictions, the increase in air temperature increases not only LDD but also spread potential of plants: even though spread rates varied strongly between plant types and generally decreased from May to September, they were consistently higher under the +3°C temperature increase scenario (figure 4). For the light-seeded herb, spread rates increased by 35–42 m yr−1 (6.3–9.2%), whereas for the heavy-seeded herb, the increase was only 0.01–0.06 m yr−1 (1.9–6.7%). Light-seeded trees increased their spread rates owing to temperature increase by 27–39 m yr−1 (3.5–6.2%) and heavy-seeded trees by 0.2–0.5 m yr−1 (4.0–8.5%).
Figure 4.
Rates of wind-driven population spread predicted for current temperature distribution (open circles) and a +3°C temperature increase scenario (filled circles). Predictions for herbs (h = 1 m) are given in (a) (vt = 0.2 m s−1) (light-seeded), (b) (vt = 1.0 m s−1) (heavy-seeded), and for trees (h = 12 m) in (c) (vt = 0.2 m s−1) (light-seeded) and (d) (vt = 1.0 m s−1) (heavy-seeded).
4. Discussion
For a wide range of plant types, we found a positive relationship between mean air temperature and the frequency of LDD by wind in a boreal forest (figure 2). This observation suggests that increases in local air temperatures can increase dispersal distances (figure 3) and, consistently, accelerate the spread of wind-dispersed plants (figure 4). According to our results, relative increases in LDD frequency tend to be higher for heavy-seeded plants (figure 3). In contrast, light-seeded plants show the greatest absolute increases in LDD frequency, and the strongest absolute and relative increases in spread rate (figure 4). Another yet interrelated observation emerging from the Hyytiälä data is that climate change driven advancements of flowering and fruiting phenology (Parmesan & Yohe 2003) can increase spread rates of plant populations because wind conditions in spring tend to produce higher spread rates than wind conditions later in the year (figure 4).
The micrometeorological data and models we applied provide detailed information about the effect of temperature on wind dispersal in a boreal forest over a period of 10 years. Yet, our predictions should not be interpreted as precise forecasts. Instead, they indicate the order of magnitude of turbulence-mediated warming effects on LDD and spread potential of wind-dispersed plants in boreal forests. Obtaining precise predictions would require implementing the natural variation in seed terminal velocity, seed release height, timing of seed abscission and the structural properties of the environment affecting winds and thereby wind dispersal. Clearly, the future dispersal and spread of wind-dispersed plants will not only depend on these turbulence-mediated effects but will also be affected by other components of environmental change: global change impacts on wind intensity (Meehl et al. 2007), spatio-temporal variations in canopy structure and density (Nathan & Katul 2005) and seed release dynamics (Skarpaas et al. 2006; Wright et al. 2008) can all affect the wind conditions under which dispersal occurs. Furthermore, rising atmospheric CO2 concentrations may increase the height, leaf area and fecundity of plants (LaDeau & Clark 2001). To quantitatively forecast how these different components of environmental change will interact in their effect on plant spread and dispersal, we first need to understand how each component acts in isolation. The present study is thus a logical first step towards understanding how future changes in the environment might affect the spatial dynamics of plants.
The predicted turbulence-mediated effects of warming on plant population spread are small in comparison to predicted climate-induced shifts of potential species ranges (Thuiller et al. 2005), making it clear that these effects cannot compensate range losses expected under future climate change. In contrast, at a smaller scale, temperature increases can have more pronounced effects on wind-driven plant dynamics: warming can promote the regional-scale spread of light-seeded species (figure 4) and generally increase wind-driven LDD for a wide range of plant types (figure 3). In fragmented populations, such increases in LDD may accelerate the colonization of unoccupied habitat patches (Damschen et al. 2008) and alter the regional dynamics of plant populations (Bohrer et al. 2005). Increased wind dispersal of seeds and pollen may also promote gene flow between populations, thus increasing their genetic diversity and decreasing the risk of inbreeding depression (Ellstrand 1992; Aguilar et al. 2008). Especially along steep environmental gradients, increased gene flow between neighbouring populations can accelerate adaptation to environmental change (Davis & Shaw 2001; Savolainen et al. 2007). An undesired consequence of global warming impacts on dispersal is the expected increase in wind-driven gene flow from genetically modified to conventional plants (Kuparinen & Schurr 2007).
Dispersal and spread of populations are widely viewed as means by which species can buffer negative effects of climate change, but whether future environmental changes can also alter dispersal and spread of plants has so far not been investigated (Nathan et al. 2008). Our study of temperature effects on wind-driven dispersal and spread potential of plants in a boreal forest suggests that increases in local temperatures may indeed promote plant movements. Convective (thermal) turbulence is important not only for LDD of pollen, spores and seeds of plants (Tackenberg 2003, Wright et al. 2008), but also for the long-distance aerial transport of small insects and other airborne biota (Johnson 1969; Reynolds & Reynolds 2009). Therefore, the interplay of temperature and the potential for spread through the air should be considered and, whenever micrometeorological data are available, quantified when predicting movements and dynamics of various airborne biota. To this end, our study provides first indications of the order of magnitude of temperature effect on plant movement at different spatial scales.
Acknowledgements
This study was funded by the Academy of Finland, University of Helsinki and the European Union through Marie Curie Transfer of Knowledge Project FEMMES (MTKD-CT-2006-042261). R.N.'s work was supported by US-NSF (DEB-0453665) ISF-FIRST (1316/15) grants. We thank Ivan Mammarella for providing us with the micrometeorological data and Outi Savolainen and two anonymous reviewers for their valuable comments.
References
- Aguilar R., Quesada M., Ashworth L., Herrerias-Diego Y., Lobo J.2008Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches. Mol. Ecol. 17, 5177–5188 (doi:10.1111/j.1365-294X.2008.03971.x) [DOI] [PubMed] [Google Scholar]
- Aylor D. E.2002Settling speed of corn (Zea mays) pollen. J. Aerosol Sci. 33, 1601–1607 (doi:10.1016/S0021-8502(02)00105-2) [Google Scholar]
- Bohrer G., Nathan R., Volis S.2005Effects of long-distance dispersal for metapopulation survival and genetic structure at ecological time and spatial scales. J. Ecol. 93, 1029–1040 (doi:10.1111/j.1365-2745.2005.01048.x) [Google Scholar]
- Clark J. S.1998Why trees migrate so fast: confronting theory with dispersal biology and the paleorecords. Am. Nat. 152, 204–224 (doi:10.1086/286162) [DOI] [PubMed] [Google Scholar]
- Clark J. S., Lewis M., Hovarth L.2001Invasion by extremes: population spread with variation in dispersal and reproduction. Am. Nat. 157, 537–554 (doi:10.1086/319934) [DOI] [PubMed] [Google Scholar]
- Damschen E. I., Brudvig L. A., Haddad M. N., Levey D. J., Orrock J. L., Tewksbury J. J.2008The movement ecology and dynamics of plant communities in fragmented landscapes. Proc. Natl Acad. Sci. USA 105, 19 078–19 083 (doi:10.1073/pnas.0802037105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. B., Shaw R. G.2001Range shifts and adaptive responses to quaternary climate change. Science 292, 673–679 (doi:10.1126/science.292.5517.673) [DOI] [PubMed] [Google Scholar]
- Ellstrand N. C.1992Gene flow by pollen: Implications for plant conservation genetics. Oikos 63, 77–86 (doi:10.2307/3545517) [Google Scholar]
- Fischlin A., et al. 2007Ecosystems, their properties, goods and services. In Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Parry M. L., Canziani O. F., Palutikof J. P., van der Linden P. J., Hanson C. E.), pp. 211–272 Cambridge, UK: Cambridge University Press [Google Scholar]
- Franco M., Silvertown J.2004A comparative demography of plants based upon elasticities of vital rates. Ecology 85, 531–538 (doi:10.1890/02-0651) [Google Scholar]
- Johnson C. G.1969Migration and dispersal of insects by flight London, UK: Methuen & Co [Google Scholar]
- Kawecki T. J., Ebert D.2004Conceptual issues in local adaptation. Ecol. Lett. 7, 1224–1241 (doi:10.1111/j.1461-0248.2004.00684.x) [Google Scholar]
- Kuparinen A.2006Mechanistic models for wind dispersal. Trends Plant Sci. 11, 296–301 (doi:10.1016/j.tplants.2006.04.006) [DOI] [PubMed] [Google Scholar]
- Kuparinen A., Schurr F. M.2007A flexible modeling framework linking spatio-temporal dynamics of plant genotypes and populations: application to gene flow from transgenic forests. Ecol. Mod. 202, 476–486 (doi:10.1016/j.ecolmodel.2006.11.015) [Google Scholar]
- Kuparinen A., Markkanen T., Riikonen H., Vesala T.2007Modeling air-mediated dispersal of spores, pollen and seeds in forested areas. Ecol. Mod. 208, 177–188 (doi:10.1016/j.ecolmodel.2007.05.023) [Google Scholar]
- LaDeau S. L., Clark J. S.2001Rising CO2 levels and the fecundity of forest trees. Science 292, 95–98 (doi:10.1126/science.1057547) [DOI] [PubMed] [Google Scholar]
- Meehl G. A., et al. 2007Global climate projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.) Cambridge, UK: Cambridge University Press [Google Scholar]
- Monin A. S., Obukhov A. M.1954Basic laws of turbulent mixing in the ground layer of the atmosphere. Tr. Geofiz Inst. Akad. Nauk SSSR 151, 163–187 [Google Scholar]
- Nathan R., Katul G. G.2005Foliage shedding in deciduous forests lifts up long-distance seed dispersal by wind. Proc. Natl Acad. Sci. USA 102, 8251–8256 (doi:10.1073/pnas.0503048102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R., Katul G., Horn H. S., Thomas S. M., Oren R., Avissar R., Pacala S. W., Levin S. A.2002Mechanisms of long-distance dispersal of seeds by wind. Nature 418, 409–413 (doi:10.1038/nature00844) [DOI] [PubMed] [Google Scholar]
- Nathan R., et al. 2005Long-distance biological transport processes through the air: can nature's complexity be unfolded in silico? Divers. Distrib. 11, 131–137 (doi:10.1111/j.1366-9516.2005.00146.x) [Google Scholar]
- Nathan R., Schurr F. M., Spiegel O., Steinitz O., Trakhtenbroth A., Tsoar A.2008Mechanisms of long-distance seed dispersal. Trends Ecol. Evol. 23, 638–647 (doi:10.1016/j.tree.2008.08.003) [DOI] [PubMed] [Google Scholar]
- Neilson R. P., Pitelka L. F., Solomon A. M., Nathan R., Midgley G. F., Fragoso J. M., Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Neilson R. P., Pitelka L. F., Solomon A. M., Nathan R., Midgley G. F., Fragoso J. M. V., Lischke H., Thompson K.2005Forecasting regional to global plant migration in response to climate change. Bioscience 55, 749–759 (doi:10.1641/0006-3568(2005)055[0749:FRTGPM]2.0.CO;2) [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Reynolds A. M., Reynolds D. R.2009Aphid aerial density profiles are consistent with turbulent advection amplifying flight behaviours: abandoning the epithet ‘passive’. Proc. R. Soc. B 276, 137–143 (doi:10.1098/rspb.2008.0880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen O., Pyhäjärvi T., Knürr T.2007Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 38, 595–619 (doi:10.1146/annurev.ecolsys.38.091206.095646) [Google Scholar]
- Skarpaas O., Auhl R., Shea K.2006Environmental variability and the initiation of dispersal: turbulence strongly increases seed release. Proc. R. Soc. B 273, 751–756 (doi:10.1098/rspb.2005.3366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons M. B., Heil G. W., Nathan R., Katul G. G.2004Determinants of long-distance seed dispersal by wind in grasslands. Ecology 85, 3056–3068 (doi:10.1890/03-0522) [Google Scholar]
- Tackenberg O.2003Modelling long-distance dispersal of plant diaspores by wind. Ecol. Monogr. 73, 173–189 (doi:10.1890/0012-9615(2003)073[0173:MLDOPD]2.0.CO;2) [Google Scholar]
- Tackenberg O., Poschlod P., Bonn S.2003Assessment of wind dispersal potential in plant species. Ecol Monogr. 73, 191–205 (doi:10.1890/0012-9615(2003)073[0191:AOWDPI]2.0.CO;2) [Google Scholar]
- Thompson S., Katul G. G.2008Plant propagation fronts and wind dispersal: An analytical model to upscale from seconds to decades using superstatistics. Am. Nat. 171, 468–479 (doi:10.1086/528966) [DOI] [PubMed] [Google Scholar]
- Thuiller W., Lavorel S., Araújo M. B., Sykes M. T., Prentice I. C.2005Climate change threats to plant diversity in Europe. Proc. Natl Acad. Sci. USA 102, 8245–8250 (doi:10.1073/pnas.0409902102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesala T., et al. 1998Long-term field measurements of atmosphere–surface interactions in boreal forest combining forest ecology, micrometeorology, aerosol physics and atmospheric chemistry. Trends Heat Mass Moment Transf. 4, 17–35 [Google Scholar]
- Wright S. J., Trakhtenbrot A., Bohrer G., Detto M., Katul G. G., Harvitz N., Muller-Landau H. C., Jones F. A., Nathan R.2008Understanding strategies for seed dispersal by wind under contrasting atmospheric conditions. Proc. Natl Acad. Sci. USA 105, 19 084–19 089 [DOI] [PMC free article] [PubMed] [Google Scholar]