Abstract
Courtship is well known for its positive effects on mating success. However, in polyandrous species, sexual selection continues to operate after copulation. Cryptic female choice is expected under unpredictable mating rates in combination with sequential mate encounters. However, there are very few accounts of the effects of courtship on cryptic female choice, and the available evidence is often correlative.
Mature Argiope bruennichi females are always receptive and never attack or reject males before mating, although sexual cannibalism after mating occurs regularly. Still, males usually perform an energetic vibratory display prior to copulation. We tested the hypothesis that beneficial effects of courtship arise cryptically, during or after mating, resulting in increased paternity success under polyandry. Manipulating courtship duration experimentally, we found that males that mated without display had a reduced paternity share even though no differences in post-copulatory cannibalism or copulation duration were detected. This suggests that the paternity advantage associated with courtship arose through female-mediated processes after intromission, meeting the definition of cryptic female choice.
Keywords: sexual selection, sexual conflict, polyandry, Araneae, sexual cannibalism
1. Introduction
Courtship displays are often energy demanding and hence costly to perform. Numerous studies on a variety of taxa confirmed that these costs are balanced by positive effects of courtship on mating success (Andersson 1994).
Particularly in polyandrous mating systems, sexual selection continues to operate after mating through the forces of sperm competition and cryptic female choice, so that pre-copulatory factors such as access to mates are not sufficient to characterize selection on courtship displays (Lewis & Austad 1994; Danielsson 2001; Pizzari et al. 2002; Demary & Lewis 2007). Attempts to disentangle the different episodes of selection between courtship and fertilization are still rare and often difficult because traits may be correlated and because sperm competition and cryptic female choice cannot be separated, for example, because sperm from different males is stored in a single location (Birkhead & Møller 1998; Wagner & Harper 2003). Unambiguous accounts of cryptic female choice are particularly infrequent and the available evidence is heterogeneous even in very well studied insect systems such as Tribolium beetles (Edvardsson & Arnqvist 2000; Fedina & Lewis 2006, 2008).
Here we use a polyandrous spider to experimentally test the hypothesis that courtship intensity influences male relative paternity through the process of cryptic female choice (Eberhard 1996). Entelegyne spiders, and particularly the genus Argiope, provide ideal systems to overcome many of the methodological problems encountered in insects or other taxa (Eberhard 2004). The sexually cannibalistic spider Argiope bruennichi is well studied in terms of its sexual behaviour (Fromhage et al. 2003; Schneider et al. 2005; Nessler et al. 2007; Uhl et al. 2007). Morphology and behaviour of A. bruennichi provide the following advantages: (i) females possess paired, independent spermathecae that are filled in separate copulations, hence ejaculates of different males do not need to interact physically; (ii) males have paired intromittent organs (the pedipalps) that are used individually and in a fixed insertion pattern, so that ejaculates within a given spermatheca can be attributed to a specific male; (iii) courtship is no prerequisite for mating because females accept males regardless, even without courtship; and (iv) males shorten or drop courtship if rivals are present on the webs of females, enabling us to manipulate courtship experimentally. In contrast to previous studies, we directly manipulate behaviour and not indirectly, for example, through the female's perception of courtship (Edvardsson & Arnqvist 2000). Encounters between rivals on the web of a female are aggressive, and after a brief tussle, both males rush into genital contact with the female and skip further courtship. Meanwhile the female is passive, never actively rejecting a suitor, and often assuming a characteristic mating position with her body lifted off the web at a 90° angle. In spiders, males face a risk of being mistaken as prey when approaching a female. Courtship may transmit signals that prevent this outcome. Female A. bruennichi are indeed very cannibalistic during and after mating, whereas pre-copulatory cannibalism is extremely rare. Here, we test whether the duration of the courtship performance influences the probability of cannibalism prior or during copulation. Male reproductive success with a given female is maximal if he can fill both of her spermathecae with his sperm. In order to achieve this, a male must survive its first copulation in order to court again and use the second pedipalp to fill the second spermatheca. Stoltz et al. (2008) observed that in the redback spider Latrodectus hasselti females punished short courtship performances of competing males by cannibalizing short-courting males after their first copulation. Thus, unlike longer-courting males, the affected males were unable to inseminate both spermathecae and therefore had decreased paternity success. However, in that study, courtship was not manipulated but covaried with body size, leaving the possibility that females selected male quality traits correlated with courtship vigour.
We manipulated the courtship behaviour of males on webs of virgin females by introducing a rival male that was unable to mate. The rival was unmated but had both pedipalps amputated (eunuch), resulting in a strong motivation to copulate without being able to achieve genital contact. Hence, even if the eunuch was first at the female genital opening, only the intact, focal male could insert his pedipalp. With this method, we induced the focal male to abbreviate or skip courtship, independent of his quality. We compared the paternity success of these males with control males that mated in the absence of a direct rival. A second male was later introduced to each female and relative paternity was assessed through the use of irradiated males (sterile-male technique, Parker 1970). We tested whether our experimental manipulation of courtship affected cryptic female choice through copulation duration, sexual cannibalism or relative paternity.
2. Material and methods
(a). Study animals: courtship and mating behaviour
Argiope bruennichi (Araneidae; SCOPOLI, 1772) is common in sunny and open meadows throughout most of Europe. The larger females reach a body length of 14–20 mm while the protandric males are 4–6 mm. The mating season starts in July and usually lasts two to three weeks. Males actively search for mature females. Upon encountering a web, a male will approach the female while plucking the threads with his legs, thereby identifying himself as a potential mate. By the time he reaches the female, the male starts courting by drumming his forelegs on the dorsal and the ventral side of the female's body, including her legs. Argiope bruennichi females are generally receptive and do not attack males before mating. As soon as the male approaches, the female signals her willingness to copulate by lifting her body from the web at a right angle, while exposing the genital openings by erecting her scape (a sclerotized plate that covers the genital openings). Towards the end of the courtship performance, which takes approximately 5 min, the male drums the female's ventral side near the epigyne with his pedipalps until copulation starts. As in all entelegyne spiders, the females of A. bruennichi possess two genital openings that are connected through paired insemination ducts with two independent spermathecae. In these spermathecae, the sperm is stored until oviposition. Corresponding to the females’ paired sexual organs, the males possess paired pedipalps that they use to transfer their sperm through the female genital pores. Argiope bruennichi males use only one of their pedipalps at a time, always inserting the right pedipalp into the female's right genital pore and the left pedipalp into the female's left genital pore. As soon as the male achieves genital contact, the female attacks him and attempts to wrap him in silk (post-insemination sexual cannibalism). Usually males try to escape, but between 70 and 80 per cent of males are caught nevertheless (Fromhage et al. 2003; Nessler et al. 2007). The copulation (i.e. insertion) lasts only a few seconds. In most cases, the tip of the embolus of the male's pedipalp breaks off during copulation (Nessler et al. 2007). The broken-off piece blocks the female genital opening so that future copulations into the same opening are at least shortened, if not prevented. Consequently, males copulating into a used insemination duct obtain a reduced paternity share (Schneider et al. 2006), leading to a first-male advantage in A. bruennichi.
(b). Rearing conditions and measurements
Penultimate-instar males and females were collected in June 2007 from their webs on meadows south of Hamburg, Germany. All mating experiments took place in a laboratory at the University of Hamburg from 4 July until 27 July 2007.
The males were housed in 250 ml plastic cups, fed on a diet of Drosophila two times a week and sprayed with water 4 days per week. Cotton wool put into a hole in the plastic cup served to regulate the moisture level inside. Subadult females were kept in similarly prepared 500 ml plastic cups and were fed with two to three Calliphora on 2 days and watered 4 days a week. After their final moult, adult females were transferred to perspex frames (30 × 30 × 6 cm), where they built typical orb webs. Mating trials were conducted in these frames. Adult males remained in the plastic cups until the start of the mating experiment.
(c). Male and female phenotypes
Both males and females were weighed immediately after their last moult on an electronic balance (accuracy 0.01 mg). Age was measured in days since the final moult. Male and female body sizes were measured as the length of tibia plus patella of the first pair of legs, using a microscope and the Leica IM v. 4.0 image software. Despite efforts to randomize the distribution of males and females into the treatment groups, we found undesired differences in body parameters between treatments: first males and females in the treatment group were heavier than in the controls. All other parameters (age and size) did not differ between treatment groups (table 1).
Table 1.
Comparison of age (d), weight (mg) and size (mm) of females and first males between the control and the treatment group (mean ± s.e. (n)). Significant differences are given in bold.
| control | treatment | ||
|---|---|---|---|
| courtship by first male | no courtship by first male | p | |
| female age | 4.28 ± 0.45 (32) | 4.36 ± 0.45 (33) | 0.85 |
| female weight | 181.79 ± 10.87 (32) | 222.79 ± 10.84 (34) | 0.005 |
| female size | 6.74 ± 0.14 (32) | 7.02 ± 0.14 (33) | 0.17 |
| first male age | 11.44 ± 0.71 (32) | 11.56 ± 0.67 (34) | 0.84 |
| firstmale weight | 14.36 ± 0.56 (32) | 16.78 ± 0.75 (30) | 0.01 |
| first male size | 4.20 ± 0.08 (28) | 4.45 ± 0.09 (34) | 0.06 |
We removed body weight of males and females from the final model because they were not significant and did not improve model fit.
(d). Experimental design
We conducted double-mating trials and assigned males randomly to one of the two groups. In the control group (with courtship, n = 32), one virgin female was successively mated with two different males that were free to court as long as they wanted. In the treatment group (shortened courtship by first male, n = 34), a virgin female was first mated with a male that shortened his courtship considerably because he had to compete with a rival. Afterwards, the female mated with a second male that was free in his courtship behaviour and hence performed a longer courtship than the manipulated first male. The designated rivals in the treatment were virgin males that had their sexual organs amputated (eunuchs). Pedipalps were removed by squeezing the femur with tweezers until the male ectomized the appendage without losing haemolymph. The males’ behaviour and health were not noticeably affected by the treatment, as has been shown in the past (Rovner 1967; Nessler et al. 2007). Thirteen eunuchs were produced in this way prior to the start of the mating experiments. To minimize the number of males handled in this way, every eunuch was used on average in two to three trials. As eunuchs never mated, their behaviour and motivation did not change across trials. Mating trials were started by placing the test males in one and the eunuchs in the other corner of the female's web in quick succession and random order. Trials were terminated after the second male's copulation. All males were removed from the females' chelicerae immediately after copulation and were preserved in alcohol. Consumption of the male body has no effect on paternity (Schneider et al. 2006) or on clutch size (Fromhage et al. 2003).
During every mating trial, we recorded (i) courtship duration, defined as the time between the first physical contact of male and female and the beginning of copulation; (ii) copulation duration, the time from insertion to removal of the pedipalp; (iii) into which insemination duct the male copulated; and (iv) the occurrence of sexual cannibalism, here defined as the capture but not the (experimentally prevented) consumption of the male. While recording courtship duration, the stopwatch was halted when males interrupted their courtship for longer than 1 min, and restarted when the male resumed courtship again. Males that stayed inactive in the web without performing courtship for more than half an hour were replaced by a new male (n = 8).
In total, we conducted 71 double-mating trials with 71 females and 142 males, excluding the eunuchs. Of these 71 females, three died without laying an egg sac and two others built empty egg sacs, so that in the end five females and the corresponding males were excluded from the dataset. The remaining 66 females were either mated with a first male that performed a normal courtship (n = 32, control) or with a first male under competitive pressure that performed a shortened courtship (n = 34, treatment). All second males showed an undisturbed, and therefore normal long courtship display.
(e). Paternity assignment
The sterile male method (Parker 1970) was used to determine the P2 value. For this purpose, mature males were randomly assigned to the normal group (N) or the sterilized group (S). The males from the S group were irradiated with 40 Gy of X-rays (200 kVp with 0.8 mm Be and 0.5 mm Cu filtering; RS225, Gulmay Medical Ltd, Camberley, UK; dose rate 0.8 to 1.2 Gy min−1) at the Laboratory of Radiobiology and Experimental Radiooncology, University Medical Center Hamburg—Eppendorf. The irradiation does not affect the male's ability to fertilize eggs but inhibits embryogenesis and results in undeveloped eggs. Irradiated males did not differ in courtship duration, copulation duration or cannibalism from untreated males (all p-values > 0.2) (see also Schneider et al. 2006).
In both groups, the females were mated alternately with a normal male first and a sterilized male second or vice versa. There were no order effects on P2 resulting from the sterilization procedure (Mann–Whitney U-test: Z = − 0.14, p = 0.89).
The paternity share of each male was determined by assigning the undeveloped eggs to the sterile male and the developed eggs to the normal male. To estimate the proportion of undeveloped eggs in a natural clutch, a control group was used. In this group, the females were mated with two normal males (NN, n = 5) and the mean hatching success in such a control group for A. bruennichi was 92.30 ± 2.56% (Schneider et al. 2006). We adopted this correction factor for this study, so that 8 per cent of the undeveloped eggs were assigned to the normal males and not to the sterile ones.
In addition to the experimental trials, females were mated with two sterile males (SS, n = 5) in order to check the effectiveness of sterilization; we found no developed eggs in clutches fertilized by two sterile males.
Mated females were transferred back into their plastic cups and fed and watered as described above. Within four weeks after copulation, A. bruennichi females lay their eggs inside a protective egg sac. We weighed females' first eggs sacs, placed them in small plastic containers with holes to ensure aeration and stored them in an incubator at 25°C. Four weeks later, at a stage when it is possible to distinguish between undeveloped eggs and hatched spiderlings, we preserved the egg sacs in alcohol. We then examined them under a microscope to determine the relative paternity share of second males (P2). The females laid 64 first egg sacs in total, 15.6 ± 0.6 days after copulation (two females did not produce egg sacs). On average, the egg sacs weighed 126.85 ± 4.55 mg and contained 214.69 ± 8.16 eggs. Less than half of those eggs (0.48 ± 0.05%) developed into spiderlings; the rest remained undeveloped.
(f). Statistics
Data were analysed with the statistical program JMP 5.1.2. All tests are two-tailed (α = 0.05) and non-parametric tests were used where the requirements of a parametric test (normal distribution of residuals) were not achieved through transformation of data. Paternity shares were generally arcsine transformed. Descriptive statistics are given as mean ± s.e. Sample sizes may differ because not all data are available for all observations.
3. Results
(a). Treatment effects on copulation duration and cannibalism of first males
As intended, courtship displays of first males with a eunuch rival were considerably shorter than in the control group (without rival; χ2 = 52.17, p < 0.001, table 2).
Table 2.
Comparison of courtship duration (s), copulation duration (s) and cannibalism of all males that inseminated an unused spermatheca in the control and the treatment group (mean ± s.e. (n); one-way ANOVA).
| treatment |
control |
||||
|---|---|---|---|---|---|
| first male (with rival) | second male (without rival) | first male (without rival) | second male (without rival) | p | |
| courtship duration | 47.71 + 11.35 (34) | 252.41 + 19.48 (34) | 306.58 + 49.29 (31) | 216 + 25.48 (32) | <0.001 |
| copulation duration | 7.41 + 0.64 (34) | 7.3 + 1.4 (20) | 6.59 + 0.55 (32) | 5.75 + 0.59 (20) | 0.47 |
| cannibalism | 76.5% (34) | 61.1% (18) | 56.3% (32) | 55.0% (20) | 0.13 |
Shortened courtship display of males under competition did not translate into shorter copulation durations (one-way ANOVA, F3,102 = 0.86, p = 0.47, first males: Z = − 1.3, p = 0.19, table 2). Males that did not court had an increased risk of cannibalism, although the difference was not significant (all groups: χ2 = 5.6, p = 0.13, first males: χ2 = 3.03, p = 0.08, table 2). Cannibalized males copulated longer (F1,65 = 3.14, p = 0.02), independent of the treatment (F1,65 = 3.14, p = 0.63), corroborating results from previous studies (Schneider et al. 2006).
(b). Treatment effects on copulation duration and cannibalism of second males
Copulation duration and rates of cannibalism of second males did not differ between treatment and control groups (copulation duration: one-way ANOVA, F1,64 = 0.5, p = 0.48; cannibalism: χ2-test; χ2 = 0.5, p = 0.48).
However, the behaviour and performance of a second male strongly depended on whether he copulated into a used or an unused spermatheca. Twenty-six of 63 second males copulated into the previously inseminated spermatheca and the strong effects of mating plugs on their copulation duration and their paternity success overrode the effects of cannibalism and treatment (table 3). The probability that a second male mated into a used side was independent of the treatment and we discarded these cases from subsequent analyses.
Table 3.
Comparison of copulation duration (s) and paternity success of second males that inseminated either a used or unused spermatheca (Mann–Whitney U-test).
| used spermatheca | unused spermatheca | p | |
|---|---|---|---|
| copulation duration | 3.04 ± 0.33 (26) | 6.78 ± 0.80 (37) | <0.0001 |
| paternity success | 0.17 ± 0.05 (25) | 0.48 ± 0.05 (35) | <0.0001 |
(c). The influence of courtship and cannibalism on relative paternity (P2)
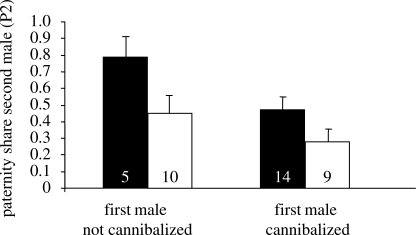
A two-factorial ANOVA (F1,37 = 5.70, R2 = 0.25, p = 0.007) revealed a significant influence of the treatment (courtship versus no courtship by first male; F = 7.65, p = 0.009) and the cannibalization of the first male (F = 6.81, p = 0.01) on P2 (figure 1). The relative paternity of the second male was significantly lower if the first male was cannibalized than if he was not, independent of the treatment. The interaction term was not significant and there was no significant effect of the cannibalization of the second male on P2, and we excluded these factors from the final model.
Figure 1.
The mean paternity share of second males (P2) influenced by the cannibalization and courtship performance of first males that either courted (control) or did not (treatment). Only males that copulated into unused spermathecae were considered (mean ± s.e.); sample sizes are given in the bars. Shaded bar, treatment group; unshaded bar, control group.
4. Discussion
Our data support the hypothesis that courtship directly increases a male's paternity success under polyandry, independent of male quality and of male copulation success. Hence, an influence of the courtship display carries over to episodes that occur after the actual copulation and after the male has vanished.
Interestingly, the effect of courtship on paternity in A. bruennichi appears not to be mediated by measures such as prolonged copulations, because short-courting and long-courting males did not differ in copulation duration. Unlike in some other spiders (e.g. Snow & Andrade 2004), copulation duration in A. bruennichi is directly related to sperm transfer and relative paternity success (Schneider et al. 2006). This suggests that the courtship display influences paternity success via a different mechanism from that shown for sexual cannibalism, which increases paternity success by prolonging copulation (Elgar 1998; Elgar & Schneider 2004, this study). We found that courtship decreased the likelihood of sexual post-insemination cannibalism but unlike expectations and other findings (Stoltz et al. 2008, 2009), the effect was not significant. Argiope bruennichi generally shows a higher incidence of cannibalism after the first insertion in non-competitive contexts than most other cannibalistic spider species and the stereotyped attack behaviour may leave little scope for variation.
Males can influence the risk of cannibalism by modulating the duration of copulation, and they use this depending on the local availability of females (Nessler et al. in press). Early termination of copulation increases the probability of survival, while remaining in copula for more than 10 s invariably leads to cannibalism. The absence of a difference in copula duration in our study shows that males did not use this strategy in this study. A strategic use of sperm supplies of individual pedipalps independent of survival and copulation duration as an adaptation to the risk of sperm competition (Parker 1998) is, however, not an option in these spiders because each pedipalp is charged shortly after maturation and then used only once, limiting males to a maximum of two copulations—one per pedipalp. Consequently, males gain nothing by saving sperm for the future. Given that short-courting males did not copulate shorter and therefore presumably did not transfer less sperm than long-courting males, it seems likely that our treatment influenced how much of the received sperm is stored and/or used by the female. This cryptic bias by the female may be directly affected by the length of courtship or indirectly by the presence of a rival. By reducing the amount of sperm stored by her first suitor, she has more scope for up- or downregulating sperm numbers stored from a subsequent male (Ward 2007).
In the absence of material benefits (e.g. food gifts, paternal care, access to resources), polyandrous females may produce fitter offspring through biasing paternity towards males with higher genetic quality, variability/compatibility (Bernasconi et al. 2003; Zeh & Zeh 2003) or with better sperm (Pai & Yan 2002). Courtship could provide a female with information about a male's genetic quality and females may base their decision as to which male to choose as a principal sire for her offspring on the courtship performance (Tallamy et al. 2003). However, the duration of courtship in our experiment was context dependent and independent of male phenotypic quality or condition, suggesting that courtship duration alone may not be a very reliable indicator of genetic quality in a competitive situation.
Alternatively, courtship could be a means for the male to manipulate the female's physiology, with females either suffering no cost from this or having not yet evolved resistance (Holland & Rice 1998). The male would therefore induce the female to prefer his own sperm over that of a rival for fertilization, without providing genetic benefits. For example, males may exploit pre-existing perceptual mechanisms of females in order to increase the probability of being preferred by the females (sensory exploitation, West-Eberhard 1979; Ryan & Keddy-Hector 1992). The predatory females of web-building spiders rely on seismic signals in their web to detect and catch their prey, and the male vibratory courtship displays are likely adapted to the females' sensory susceptibility. In addition, males directly touch females while drumming with their forelegs on the females' body during courtship. As spiders are chemosensitive (Bristowe & Locket 1926), the transfer of contact pheromones between male and female during courtship appears possible. Such manipulation through male pheromones has been already shown for the desert spider Agelenopsis aperta; Becker et al. (2005) revealed that the female's catalepsis during mating was induced by the male's emission of a volatile pheromone and they presumed the drumming of the pedipalps to direct the volatile chemical towards the female. A similar effect was recorded for the abdominal stroking that males perform in a coreid bug (Leptoglossus clypealis), where stroking helps to disperse a sex pheromone that serves as a female attractant and aphrodisiac (Wang & Millar 2000). Such an aphrodisiac could be transferred from the A. bruennichi male to the female during vibratory courtship as well, making the female more receptive to the received sperm.
The proximate mechanisms responsible for this courtship-related paternity bias in A. bruennichi are unknown. Diverse mechanisms have been suggested for other organisms such as selective ejection of sperm (Pizzari & Birkhead 2000), or digestion (e.g. Vreys et al. 1997) and differential positioning of ejaculates (Ward 1998; Fedina & Lewis 2006). The paired storage organs of A. bruennichi females and other spiders may facilitate female control over paternity (Snow & Andrade 2005). The presence of extremes in second-male paternity (P2 = 0 or P2 = 1) might be generated by selective activation of a particular spermatheca, although this possibility has been considered unlikely (Berendonck & Greven 2005).
Despite the demonstrated positive effect of courtship on paternity success, all males in A. bruennichi dramatically shorten or skip courtship as soon as a rival appears on the scene. The reason may be that the benefits of mating with a virgin and placing a plug in at least one of her spermathecae ensures around 50 per cent paternity, and even more if the rival fails to use the unused copulatory duct. Being the second to mate is unlikely to result in more than 50 per cent paternity, and may be much less if the male mates into the previously used and likely plugged side. Strangely, A. bruennichi males do not preferentially use a virgin over a plugged genital opening (Schneider et al. 2006, own observations).
In conclusion, the results of this study show that male courtship per se, independent of male quality, increases paternity success through cryptic processes under polyandry. The observed patterns meet broader definitions of cryptic female choice (Arnqvist & Rowe 2005). However, the mechanisms behind the bias and whether the positive effect of courtship on paternity is under female or male control require further study.
Acknowledgements
We are very grateful to Dr I. Brammer at the Laboratory of Radiobiology and Experimental Radiooncology, University Medical Center Hamburg—Eppendorf for his help with irradiating the spiders. We thank T. Dirks and A. Taebel-Hellwig who helped with the maintenance of the spiders and S. Nessler, L. Fromhage and M. Taborsky for providing advice with the data analysis and the preparation of the manuscript.
References
- Andersson M.1994Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- Arnqvist G., Rowe L.2005Sexual conflict Princeton, NJ: Princeton University Press [Google Scholar]
- Becker E., Riechert S., Singer F.2005Male induction of female quiescence/catalepsis during courtship in the spider Agelenopsis aperta. Behaviour 142, 57–70 (doi:10.1163/1568539053627767) [Google Scholar]
- Berendonck B., Greven H.2005Genital structures in the entelegyne widow spider Latrodectus revivensis (Arachnida; Araneae; Theridiidae) indicate a low ability for cryptic female choice by sperm manipulation. J. Morphol. 263, 118–132 (doi:10.1002/jmor.10296) [DOI] [PubMed] [Google Scholar]
- Bernasconi G., Paschke M., Schmid B.2003Diversity effects in reproductive biology. Oikos 102, 217–220 (doi:10.1034/j.1600-0706.2003.12598.x) [Google Scholar]
- Birkhead T. R., Møller A. P.1998Sperm competition and sexual selection London, UK: Academic Press [Google Scholar]
- Bristowe W. S., Locket G. H.1926The courtship of British lycosid spiders and its probable significance. Proc. Zoolog. Soc. 317 [Google Scholar]
- Danielsson I.2001Antagonistic pre- and post-copulatory sexual selection on male body size in a water strider (Gerris lacustris). Proc. R. Soc. Lond. B 268, 77–81 (doi:10.1098/rspb.2000.1332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demary K. C., Lewis S. M.2007Male courtship attractiveness and paternity success in Photinus greeni fireflies. Evolution 61, 431–439 (doi:10.1111/j.1558-5646.2007.00033.x) [DOI] [PubMed] [Google Scholar]
- Eberhard W. G.1996Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press [Google Scholar]
- Eberhard W. G.2004Why study spider sex: special traits of spiders facilitate studies of sperm competition and cryptic female choice. J. Arachnol. 32, 545–556 (doi:10.1636/0161-8202(2004)032[0545:WSSSST]2.0.CO;2) [Google Scholar]
- Edvardsson M., Arnqvist G.2000Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc. R. Soc. Lond. B 267, 559–563 (doi:10.1098/rspb.2000.1037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgar M. A.1998Sexual selection and sperm competition in arachnids. In Cannibalism (eds Elgar M. A., Crespi B. J.), pp. 128–155 Oxford, UK: Oxford University Press [Google Scholar]
- Elgar M. A., Schneider J. M.2004The evolutionary significance of sexual cannibalism. Adv. Study Behav. 34, 135–163 (doi:10.1016/S0065-3454(04)34004-0) [Google Scholar]
- Fedina T. Y., Lewis S. M.2006Proximal traits and mechanisms for biasing paternity in the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae). Behav. Ecol. Sociobiol. 60, 844–853 (doi:10.1007/s00265-006-0228-7) [Google Scholar]
- Fedina T. Y., Lewis S. M.2008An integrative view of sexual selection in Tribolium flour beetles. Biol. Rev. 83, 151–171 (doi:10.1111/j.1469-185X.2008.00037.x) [DOI] [PubMed] [Google Scholar]
- Fromhage L., Uhl G., Schneider J. M.2003Fitness consequences of sexual cannibalism in female Argiope bruennichi. Behav. Ecol. Sociobiol. 55, 60–64 (doi:10.1007/s00265-003-0656-6) [Google Scholar]
- Holland B., Rice W. R.1998Chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7 (doi:10.2307/2410914) [DOI] [PubMed] [Google Scholar]
- Lewis S. M., Austad S. N.1994The relationship between sperm precedence and male olfactory attractiveness. Behav. Ecol. 5, 219–224 [Google Scholar]
- Nessler S. H., Uhl G., Schneider J. M.2007Genital damage in the orb-web spider Argiope bruennichi increases paternity success. Behav. Ecol. 18, 174–181 (doi:10.1093/beheco/arl074) [Google Scholar]
- Nessler S. H., Uhl G., Schneider J. M.In press Scent of a woman—the effect of female presence during male maturation on sexual cannibalism in an orb-weaving spider (Araneae: Araneidae). Ethology [Google Scholar]
- Pai A., Yan G.2002Polyandry produces sexy sons at the cost of daughters in red flour beetles. Proc. R. Soc. Lond. B 269, 361–368 (doi:10.1098/rspb.2001.1893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. A.1970Sperm competition and its evolutionary consequences in the insects. Biol. Rev. Camb. Philos. Soc. 45, 525–567 [Google Scholar]
- Parker G. A.1998Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 3–54 London, UK: Academic Press [Google Scholar]
- Pizzari T., Birkhead T. R.2000Female feral fowl eject the sperm of subdominant males. Nature 405, 787–789 (doi:10.1038/35015558) [DOI] [PubMed] [Google Scholar]
- Pizzari T., Froman D. P., Birkhead T. R.2002Pre- and post-insemination episodes of sexual selection in the fowl, Gallus g. domesticus. Heredity 88, 112–116 (doi:10.1038/sj.hdy.6800014) [DOI] [PubMed] [Google Scholar]
- Rovner J.1967Copulation and sperm induction by normal and palpless male in Linyphiid spiders. Science 157, 835 (doi:10.1126/science.157.3790.835) [DOI] [PubMed] [Google Scholar]
- Ryan M. J., Keddy-Hector A.1992Directional patterns of female mate choice and the role of sensory biases. Am. Nat. 139, 4–35 [Google Scholar]
- Schneider J. M., Fromhage L., Uhl G.2005Extremely short copulations do not affect hatching success in Argiope bruennichi (Araneae, Araneidae). J. Arachnol. 33, 663–669 (doi:10.1636/S03-32.1) [Google Scholar]
- Schneider J. M., Gilberg S., Fromhage L., Uhl G.2006Sexual conflict over copulation duration in a cannibalistic spider. Anim. Behav. 71, 781–788 (doi:10.1016/j.anbehav.2005.05.012) [Google Scholar]
- Snow L. S. E., Andrade M. C. B.2004Pattern of sperm transfer in redback spiders: implications for sperm competition and male sacrifice. Behav. Ecol. 15, 785–792 (doi:10.1093/beheco/arh080) [Google Scholar]
- Snow L. S. E., Andrade M. C. B.2005Multiple sperm storage organs facilitate female control of paternity. Proc. R. Soc. B 272, 1139–1144 (doi:10.1098/rspb.2005.3088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz J. A., Elias D. O., Andrade M. C. B.2008Female reward courtship by competing males in a cannibalistic spider. Behav. Ecol. Sociobiol. 62, 689–697 (doi:10.1007/s00265-007-0493-0) [Google Scholar]
- Stoltz J. A., Elias D. O., Andrade M. C. B.2009Male courtship effort determines female response to competing rivals in redback spiders. Anim. Behav. 77, 79–85 (doi:10.1016/j.anbehav.2008.09.012) [Google Scholar]
- Tallamy D. W., Darlington M. B., Pesek J. D., Powell B. E.2003Copulatory courtship signals male genetic quality in cucumber beetles. Proc. R. Soc. Lond. B 270, 77–82 (doi:10.1098/rspb.2002.2198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G., Nessler S. H., Schneider J.2007Copulatory mechanism in a sexually cannibalistic spider with genital mutilation (Araneae: Araneidae: Argiope bruennichi). Zoology 110, 398–408 (doi:10.1016/j.zool.2007.07.003) [DOI] [PubMed] [Google Scholar]
- Vreys C., Steffanie N., Gevaerts H.1997Digestion of spermatophore contents in the female genital system of the hermaphroditic flatworm Dugesia gonocephala (Tricladida, Paludicola). Invert. Biol. 116, 286–293 (doi:10.2307/3226860) [Google Scholar]
- Wagner W. E., Harper C. J.2003Female life span and fertility are increased by the ejaculates of preferred males. Evolution 57, 2054–2066 [DOI] [PubMed] [Google Scholar]
- Wang Q. A., Millar J. G.2000Mating behavior and evidence for male-produced sex pheromones in Leptoglossus clypealis. Ann. Entomol. Soc. Am. 93, 972–976 (doi:10.1603/0013-8746(2000)093[0972:MBAEFM]2.0.CO;2) [Google Scholar]
- Ward P. I.1998A possible explanation for cryptic female choice in the yellow dung fly, Scathophaga stercoraria (L.). Ethology 104, 97–110 [DOI] [PubMed] [Google Scholar]
- Ward P. I.2007Postcopulatory selection in the yellow dung fly Scathophaga stercoraria (L.) and the mate-now-choose-later mechanism of cryptic female choice. Adv. Study Behav. 37, 343–369 (doi:10.1016/S0065-3454(07)37007-1) [Google Scholar]
- West-Eberhard M. J.1979Sexual selection, social competition and evolution. Proc. Am. Philos. Soc. 123, 222–234 [Google Scholar]
- Zeh J. A., Zeh D. W.2003Toward a new sexual selection paradigm: polyandry, conflict and incompatibility. Ethology 109, 929–950 (doi:10.1046/j.1439-0310.2003.00945.x) [Google Scholar]