Abstract
Latitudinal gradients in diversity are among the most striking features in ecology. For terrestrial species, climate (i.e. temperature and precipitation) is believed to exert a strong influence on the geographical distributions of diversity through its effects on energy availability. Here, we provide the first global description of geographical variation in the diversity of marine copepods, a key trophic link between phytoplankton and fish, in relation to environmental variables. We found a polar–tropical difference in copepod diversity in the Northern Hemisphere where diversity peaked at subtropical latitudes. In the Southern Hemisphere, diversity showed a tropical plateau into the temperate regions. This asymmetry around the Equator may be explained by climatic conditions, in particular the influence of the Inter-Tropical Convergence Zone, prevailing mainly in the northern tropical region. Ocean temperature was the most important explanatory factor among all environmental variables tested, accounting for 54 per cent of the variation in diversity. Given the strong positive correlation between diversity and temperature, local copepod diversity, especially in extra-tropical regions, is likely to increase with climate change as their large-scale distributions respond to climate warming.
Keywords: latitudinal gradient, copepods, temperature, species richness, diversity, environmental factors
1. Introduction
The latitudinal gradient in species richness is a well-known ecogeographical pattern in terrestrial ecology where there is a cline in species diversity from high to low latitudes (e.g. Pianka 1966; Gray et al. 1997; Gaston & Blackburn 2000). While this latitudinal pattern seems to hold true for most major terrestrial and some marine groups (Macpherson 2002), the latitudinal gradient appears to be less consistent in the marine environment (Clarke 1992; Roy et al. 2000). Global-scale studies on the distribution of diversity among fish (Boyce et al. 2008) and planktonic species, e.g. pteropods (Pierrot-Bults 1997), tintinnid ciliates (Dolan et al. 2006), foraminifera (Rutherford et al. 1999) and euphausiids (Reid et al. 1978) show a polar–tropical difference in diversity with peaks at some distance away from the Equator. Exceptions to the classical diversity gradient occur among molluscs, which exhibit hotspots of diversity rather than latitudinal clines (Crame & Clarke 1997) and turtles and marine mammals, which show inverse latitudinal gradients (Proches 2001; Stephens & Wiens 2003). A meta-analytical study by Hillebrand (2004) compared 198 published marine gradients and concluded that the strength and the slope of latitudinal gradients of marine biota are clearly subjected to regional, habitat and taxonomic features.
Appreciating the patterns of diversity and their causes and consequences is central to understanding evolutionary and ecological processes (Levin 1992). Historical processes such as tectonics and glaciations are undoubtedly important in the origin of the latitudinal cline (Crame & Clarke 1997). However, contemporary climatic and ecological processes play an important role in the maintenance of geographical variations in diversity. Copepods are ubiquitous and the most numerous metazoans in pelagic communities (Turner 2004b) and they are a key group that transfers energy from phytoplankton to higher trophic levels. Previous studies on patterns in zooplankton diversity have been based upon broad extant taxonomic groups (MacPherson 2002), fossil records (Rutherford et al. 1999) or have only been quantified over a short latitudinal range (Turner 1981). While distributions of copepod diversity have been considered at both regional and basin scales (Woodd-Walker 2001; Beaugrand et al. 2002a; Beaugrand 2004; Piontkovski et al. 2006), the environmental controls of spatial variations of copepod diversity at the global scale have not been examined. Such a study is now possible as high-quality data on copepod composition have become available for large areas of the ocean and over long temporal scales, with most organisms identified at the species level.
At the core of most of the hypotheses that try to explain contemporary patterns of diversity is a link between the abiotic environment and species diversity (Kerr 2001; Turner 2004a). In particular, species–energy hypothesis (Wright 1983; Currie 1991), emphasizing the importance of available energy for the regulation of diversity, have received widespread support. However, there is no consensus on the ultimate mechanisms linking available energy to diversity patterns. Since local species diversity appears sensitive to recent global warming (Beaugrand et al. 2008; Hiddink & Hofstede 2008), it is especially timely to understand the drivers of diversity in key trophic groups at the large scale to help anticipate the likely responses of marine ecosystems to climate change. Features of the marine environment that are likely to affect patterns of copepod diversity are: temperature (Rutherford et al. 1999), chlorophyll a (chl a; e.g. Connell & Orias 1964; Roy et al. 1998) and seasonality in these environmental variables (Begon et al. 1996; Rex et al. 2000; Woodd-Walker et al. 2002) and chemical and physical properties of the ocean (Ruddiman 1969), all of which are influenced by climate. Predicting the patterns of diversity is not only an intellectual problem, but also an urgent practical task (Pimm & Brown 2004). Indeed, understanding the mechanisms underlying these patterns may be among the most important challenges biologists will have to face in the twenty-first century (Willig & Bloch 2006).
The objectives of the present paper are: (i) to provide a global description of copepod diversity; (ii) to investigate the relationships between latitudinal variations in copepod diversity across a variety of biomes and environmental variables, these being ocean temperature, chl a, seasonality in environmental variables, chemical and physical properties of the ocean; and (iii) to use a statistical model based on generally available environmental variables to forecast copepod diversity in regions where sampling is currently poor or non-existent.
2. Material and methods
(a). Biological datasets
Our analysis of global-scale variability of copepod diversity required the construction of a representative database of copepod taxonomic composition that covered large spatial and temporal scales. The data came from sampling programmes that covered several geographical regions and latitudinal ranges. In order to allow temporal averaging of abundances and reduce noise due to patchiness of zooplankton spatial distributions and seasonality, we selected datasets that were temporally extensive (i.e. more than 5 years). Comparing diversity among datasets can be biased when the samples are collected with different methodologies. Visual inspection of some datasets confirmed that when larger mesh sizes (>330 µm) were used in surveys, there was skewness towards larger copepod taxa. Since there is no uniform mathematical solution to standardize taxonomic composition data across datasets, we decided to avoid possible biases by including in our database only samples collected with a mesh size <330 µm. Using this rule, we selected seven datasets (electronic supplementary material, table S1), which extended over a latitudinal range from 86.5° N to 46.5° S. The sampling stations covered well the Atlantic Ocean and its adjacent seas, and two regions of the Pacific Ocean (figure S1 in the electronic supplementary material). To our best knowledge, no information on copepods that satisfies the above criteria is presently available for the Indian or the Southern Oceans.
To allow the inter-comparison of zooplankton datasets, the database was homogenized for taxonomic identification. Even if most individuals had been identified to the species level, the taxonomic resolution was not always uniform across datasets. To resolve this problem, individuals that were not consistently identified to the species level in all datasets were reduced to the genus level prior to determining diversity. This loss in taxonomic resolution for some species is unlikely to affect the calculations of diversity since genus included in the database tend to have low genus : species ratios, i.e. each genus was represented by relatively few species.
(b). Environmental datasets
We created a complementary database of 10 environmental variables (see table S2 in the electronic supplementary material) to examine their relationships with copepod diversity.
Ocean temperature data were obtained from the World Ocean Atlas 2005 (WOA05) (Locarnini et al. 2006). Monthly climatology data of upper ocean chl a concentration were retrieved and calculated from the satellite SeaWiFS (Sea-viewing Wide Field-of-view Sensor) (1997–2005) on a grid of 1° longitude × 1° latitude. Monthly surface climatology data of chemical variables (objectively analysed mean values of nitrate, silicate, phosphorus, oxygen and salinity) with a 1° global spatial resolution were retrieved from the WOA05 (Antonov et al. 2006; Garcia et al. 2006a,b).
Ocean surface currents data covering a latitudinal range from 60° N to 60° S were downloaded from the OSCAR data access system of the National Oceanic and Atmospheric Organisation. Global mixed layer depth (MLD) climatologies were obtained from the Pierre Simon Laplace Institute; the MLD used in this analysis was based on the temperature criterion of ΔT = 0.2°C (de Boyer Montégut et al. 2004). Bathymetry data were used to test the covariation of diversity with bottom topography; the data originated from a global ocean bathymetry map (1° longitude × 1° latitude) (Smith & Sandwell 1997). This dataset is among the most complete, high-resolution image of sea floor topography currently available.
(c). Data analyses
The data were analysed in five steps.
(i) Pre-processing of data. The biological and environmental datasets were reorganized to create a global common grid (1° latitude × 1° longitude) of copepod composition data and environmental variables. The mean abundance of each taxon was calculated per year for each cell of the grid, and mean values of all environmental variables were calculated for every geographical cell. In order to test the relationships between seasonal environmental stability and the spatial variation in taxonomic richness, the coefficient of variation (CV) was computed for each environmental variable (except bathymetry) as follows:
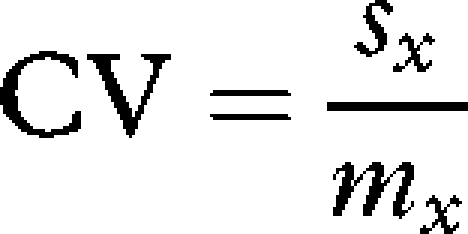 |
2.1 |
where mx and sx are the mean and standard deviation, respectively, of environmental variable x (e.g. ocean temperature) calculated on a 12-month basis. The resulting data matrix consisted of 433 geographical cells with yearly zooplankton taxonomic composition data and corresponding values for 19 environmental variables (i.e. 10 mean values and 9 CV values).
(ii) Computing diversity. The choice of a diversity index for a given investigation depends on the nature of the data (Pianka 1966) and the aims and scale of the study. The uneven taxonomic resolution of the data in our study would have biased the calculation of indices that are based on relative frequencies of taxa. In large-scale studies, indices weighted towards species richness are more useful for detecting differences between sites than the indices that emphasize the evenness component of diversity (Magurran 2004). Indeed, even though the calculation of species richness is sensitive to sample size and results in systematic underestimation of copepod diversity, it is still a satisfactory estimator that can be used for comparisons between sites with low spatial resolution (Beaugrand & Edwards 2001). For these reasons, taxonomic richness (i.e. the number of taxa present per geographical cell) was selected for the present study. This avoided the potential confounding effects of differences in sampling protocols on taxonomic richness. In the vertical plane, the gridded mean zooplankton abundance data were integrated over the epipelagic layer (0–200 m), and yearly taxonomic richness was calculated for each geographical cell. The resulting values were time averaged over all observed years.
(iii) Latitudinal pattern in copepod diversity. A statistical test was performed to analyse the variation of diversity between 30° S and 30° N latitude. The dataset was divided in three latitudinal bands (30° S to 10° S, 10° S to 10° N, 10° N to 30° N), and the resulting subsets were compared using the non-parametric Kruskal–Wallis test to examine if their medians differed. The non-parametric Mann–Whitney U test was used on each band pair to identify which latitudinal bands were significantly different.
(iv) Correlation analyses. Correlative methods have been effective in examining possible global change impacts on species (Kerr et al. 2007). Diversity data were first log-transformed to normalize them, and the Pearson correlation coefficient was calculated for each pair of the 19 environmental variables. When several tests of significance are carried out simultaneously, the probability of a type I error becomes larger than the nominal value α (Legendre & Legendre 1998). Therefore, the sequential Bonferroni adjustment was performed to test the significance of the correlation coefficients at the table-wide α-level (Holm 1979; Rice 1989). First, the p-values for n tests were ranked in ascending order. The adjusted probability values (pi′) were then computed by pi′ = α/(1+n−i), where α was chosen at the 0.05 significance level and i the rank number from the unadjusted p-values. Finally, each adjusted pi′ was compared with the unadjusted α significance level. To account for spatial autocorrelation, the degrees of freedom were recalculated (n*) to indicate the minimum number of samples needed to maintain a significant relationship at p = 0.05 (Beaugrand et al. 2008). Because the relationship between temperature and diversity along a latitudinal gradient can be masked by the effect of latitude, we calculated partial correlation coefficients between temperature and diversity while controlling for the linear effect of latitude.
(v) Global empirical modelling of copepod diversity. A multiple linear regression model was built to forecast copepod diversity on a global scale. Multicollinearity among explanatory variables can often distort the results of a multiple linear regression. Ridge regression can be used to counter this problem and provides better estimates of the ‘true’ regression coefficients than those obtained by ordinary least squares (Legendre & Legendre 1998). Environmental variables that showed significantly strong correlations with species diversity (outcome from step 4) were selected as initial predictor variables, i.e. ocean temperature, salinity and chl a. A stepwise selection was performed to retain those variables which contributed significantly to the model. In addition, standard regression coefficients (b′) were calculated on standardized environmental variables to assess the relative importance of the explanatory variables xj and their confidence intervals were calculated to further validate the retention of variables in the model. In order to predict global copepod diversity, the residuals of the regression model must not be spatially autocorrelated. In order to check for spatial autocorrelation, a semi-variogram of the residuals was calculated and the values of semi-variance were regressed against the distance classes. The absence of a significant slope, b = 0, indicates that there is no spatial autocorrelation.
3. Results
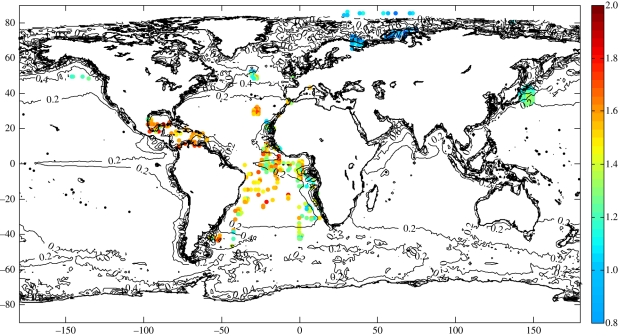
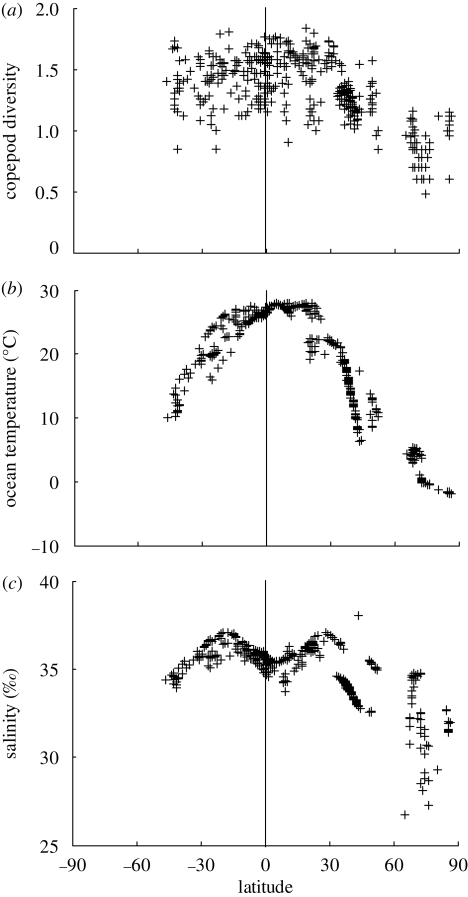
Geographical variations of mean copepod diversity (calculated as taxonomic richness) showed a clear latitudinal pattern in diversity, with higher diversity in low latitudes and lower diversity in high latitudes (figure 1). The latitudinal variation was not a constant cline, but exhibited instead a hump-shaped relationship (figure 2a). In the Northern Hemisphere, there was a peak in diversity at 20° latitude followed by a sharp decrease north of 40° N, and in the Southern Hemisphere, a plateau extending from the Equator into the beginning of extratropical regions. The Kruskal–Wallis test showed a significant difference (p < 0.005) between the three latitudinal bands 30° S to 10° S (tropical), 10° S to 10° N (equatorial), 10° N to 30° N (tropical), and the Mann–Whitney U test identified differences between the equatorial and tropical regions in the Northern Hemisphere (p < 0.05) and between the two tropical regions (p < 0.001). This confirmed the asymmetry in the diversity-latitude relationship between the Northern and Southern Hemispheres and the lower diversity over the Equator. The longitudinal variation in diversity was also investigated (electronic supplementary material, figure S3), but no significant pattern was visible in the Atlantic or the Pacific.
Figure 1.
Spatial distribution of copepod diversity per geographic cell (1° longitude × 1° latitude). A total of 433 values are displayed, corresponding to 13713 individual samples from seven datasets (see electronic supplementary material, table S1) that cover a latitudinal range from 86.5° N to 46.5° S. The colour of each circle (see vertical scale bar) indicates the mean number of species per standardized area. No data that satisfied our selection criteria were found in the South Pacific or the Indian Oceans. Contour lines of mean chl a concentrations were added to the map using an interval of 0.2 mg m−3.
Figure 2.
(a) Mean copepod diversity as a function of latitude (by convention latitudes S are shown negative). Each point (433 grid cells) represents the log-transformed number of species (log) present in each square on a 1° × 1° degree geographical grid. The vertical line corresponds to the Equator. There is a hump-shaped distribution of diversity in the Northern Hemisphere with a peak around 20° latitude followed by a sharp decrease north of 40° latitude. (b) Ocean temperature as a function of latitude. Each point indicates mean temperature (data source in table S1 in the electronic supplementary material) per grid cell corresponding to the grid cells with copepod diversity data. The shape of the distribution of temperature over latitudes is similar to that of copepod diversity in the upper panel, in particular the peak at 20° N followed by a sharp decline to 40° N. (c) Salinity as a function of latitude. Each point indicates mean salinity (data source in table S1 in the electronic supplementary material) per grid cell corresponding to the grid cells with copepod diversity data. The shape of the latitudinal change is not symmetrical on the two sides of the Equator. The Inter Tropical Convergence Zone (ITCZ) in the Northern Hemisphere reduces salinity between the Equator and 20° N and coincides with an area of relatively lower diversity.
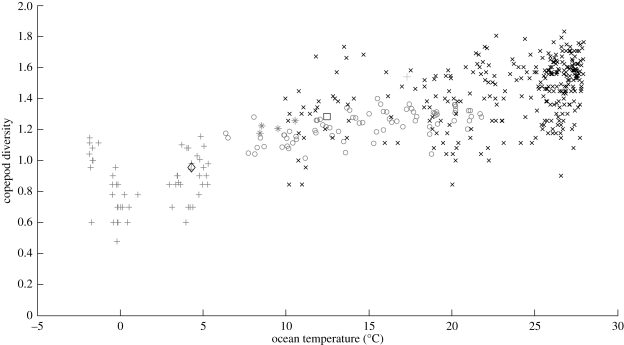
Latitudinal variations of gridded diversity of all datasets and temperature are shown in figure 3. The correlation coefficient between copepod diversity and temperature was the highest (r = 0.76, p < 0.001), and indicated that ocean temperature explained 54 per cent of the total variation in copepod diversity. Relationships between log-transformed copepod diversity and environmental variables are summarized in table 1.
Figure 3.
Relationship between gridded log-transformed (log of taxonomic richness) copepod diversity and temperature. The zooplankton datasets used for the analysis are identified by different marker codes (below). There was a significant linear correlation between copepod diversity and temperature (r = 0.76 and p < 0.001). The partial correlation controlling for the effects of latitude was significant (r = 0.45 and p < 0.005). Thin plus, Barents and Kara Sea; square, Western English Channel; circle, east of Japan; cross, tropical and South Atlantic; diamond, White Sea; thick plus, Point B; star, Station P and adjacent sites.
Table 1.
Correlation (Bravais–Pearson) between log-transformed copepod diversity and both mean environmental variables and an index of their seasonal variations. n = number of geographical cells used to calculate the correlation; n* = number of geographical cells needed to obtain a p-value of 0.05. N/A = not applicable. Zooplankton datasets used for the analysis are shown in electronic supplementary material, figure S1. **p < 0.01. ***p < 0.001.
| correlation between diversity (log of taxonomic richness) and mean values of environmental variables |
correlation between diversity (log of taxonomic richness) and an index of seasonal variations of environmental variables |
|||||
|---|---|---|---|---|---|---|
| environmental variable | correlation coefficient (r) | degrees of freedom (n−2) | n* | correlation coefficient (r) | degrees of freedom (n−2) | n* |
| ocean temperature (°C) | 0.7630*** | 431 | 6 | −0.0081 | 431 | N/A |
| salinity (psu) | 0.6599*** | 431 | 10 | −0.4385*** | 431 | 25 |
| station depth (m) | −0.3357*** | 406 | 35 | N/A | N/A | N/A |
| ocean surface currents (m s−1) | −0.0421 | 329 | N/A | 0.1673** | 329 | 138 |
| MLD (m) | −0.4219*** | 286 | 23 | −0.3322*** | 286 | 36 |
| chl a (mg m−3) | −0.5327*** | 394 | 14 | −0.3913*** | 394 | 26 |
| oxygen (ml l−1) | −0.7682*** | 431 | 6 | −0.4590*** | 431 | 19 |
| nitrate (µmol l−1) | −0.2949*** | 431 | 45 | −0.0546 | 431 | N/A |
| phosphate (µmol l−1) | −0.3285*** | 431 | 37 | 0.1206 | 431 | N/A |
| silicate (µmol l−1) | −0.4134*** | 431 | 23 | −0.3570*** | 431 | 31 |
The partial correlation between diversity and ocean temperature when controlling for latitude was significant (rrich,temp = 0.45, p < 0.005), whereas the partial correlation between diversity and latitude when controlling for the linear effect of ocean temperature was not significant (rrich,lat = −0.04, p > 0.05). This indicated that the relationship between diversity and temperature held independently of the effect of latitude. This shows that the link between diversity and ocean temperature is not owing to the link between temperature and latitude at the large scale.
There were also strong relationships between diversity and environmental factors other than temperature. This is illustrated in the scatterplots of log-transformed diversity versus environmental variables for cases were the variables were significantly related (electronic supplementary material, figure S1; see also table 1). The sequential Bonferroni adjustment procedure confirmed that the individually significant correlation coefficients in table 1 were also significant at the table wide α-level (pi′ = 0.05). Spatial variations in diversity were strongly and inversely correlated with chl a and salinity, indicating high diversity of copepods in areas where concentrations of chl a were low and salinity high.
Oxygen concentration was the chemical variable with the strongest correlation with diversity. Nutrients showed overall weak but significant relationships with diversity. MLD, as a proxy for water column stability, was negatively correlated with diversity, and shallower areas were more diverse than deeper waters. Correlations with seasonal variations of environmental variables were weaker than with mean values. There was a negative correlation of diversity with the variability of chl a concentration, indicating that regions with more stable productivity regimes at the seasonal scale held a larger number of taxa. The variation in MLD was negatively and significantly correlated with diversity.
In an initial stage, a ridge regression was performed to counter the effects of multicollinearity between predictor variables on the results of the regression coefficients. However, the ridge trace diagram (not shown here) showed that the regression coefficients were stable starting from k = 0, where the regression coefficients estimates are equal to the standardized ordinary least squares multiple regression coefficients. This result indicated that the regression coefficients were not affected by collinearity among predictor variables and the regression parameters could be estimated by the least-squares method. The final model (R2 = 0.6, p < 0.05) included three explanatory variables: ocean temperature (T), chl a (C) and salinity (S):
| 3.1 |
The model in equation (3.1) was used to forecast copepod diversity at the global scale.
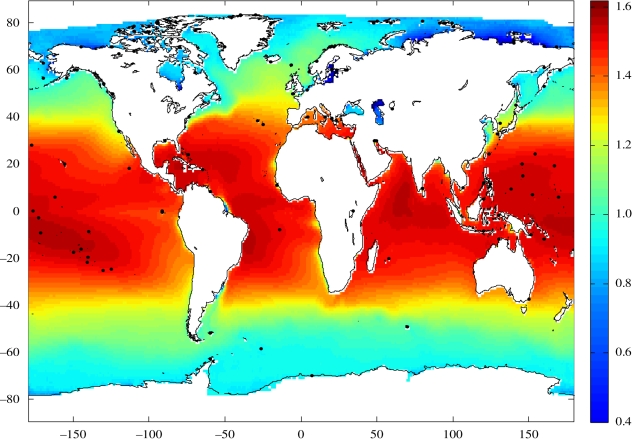
Even though the coefficient of multiple determination (R2) of the model did not show a large increase when salinity and chl a were added, the significant p-values (p < 0.05) of the ordinary regression coefficients suggested that these variables should be retained in the model. The standard regression coefficients implied that all three variables should be kept in the model and that ocean temperature contributed the most to the estimated ŷ values. The results of the regression on the values of the semi-variogram suggested that the residuals from the final multiple regression model were not spatially auto-correlated, so that regression equation (3.1) could be used for predicting global copepod diversity. Diversity calculated using equation (3.1) (figure 4) peaked in the Northern Hemisphere above the Equator in the Atlantic basin, and in the Southern Hemisphere in the Indo-pacific regions. A marked decline in diversity occurred around 35° in both hemispheres. Low diversity regions include upwelling areas on the Eastern coasts of the Atlantic and Pacific basins.
Figure 4.
World ocean copepod diversity (D) (log of taxonomic richness, see vertical scale bar) predicted from ocean temperature (T), salinity (S) and chl a (C) using the equation: D = 0.2597 + 0.0189T + 0.0214S − 0.0798C (environmental variables were gridded on 1° longitude × 1° latitude).
4. Discussion
Three limitations must be considered before interpreting our results. Firstly, it is likely that our calculated diversity underestimated real diversity. According to Beaugrand & Edwards (2001), taxonomic richness is by far the most sensitive index for the calculation of zooplankton data. Using data from the Continuous Plankton Recorder (CPR) surveys and based on species accumulation curves, these authors estimated that even when one CPR sample was used, taxonomic richness was the best indicator for discrimination in space and time, a result also obtained by Magurran (2004). Despite the well known underestimation of true diversity by taxonomic richness, we believe that our spatial comparisons are satisfactory, but our values of diversity should not be interpreted as reflecting the true epipelagic diversity of copepods. Secondly, even if we had tried to construct an extensive database of copepod taxonomic composition data, we could not fully assess the variability of diversity over all ocean basins because the relevant data did not exist in some oceans (e.g. Southern Ocean, Indian Ocean) or were not available for some regions, especially in the Southern Hemisphere (e.g. South Pacific, figure 1), remembering that one of our criteria for the inclusion of datasets in our database was at least 5 years of sampling (see §2). Thirdly, we took measures to standardize the data across datasets, but methodological differences may nevertheless have introduced biases in the calculation of taxonomic richness. Mesh sizes, for example, varied across datasets (ranging from 167 to 330 µm). Therefore, abundance data in some areas might not have reflected the whole community because the mesh size was not appropriate for the wide size range of zooplankton organisms to be collected, and the smallest taxa may have been missed (Hopcroft et al. 2005). Despite these possible biases, a consistent latitudinal pattern emerged from the collated data. The diversity patterns found in this study can serve as a foundation for future genetic analysis to investigate true diversity in those areas where diversity seems to reach a plateau. In previous studies, genetic methods have also been useful to reveal cryptic speciation in copepods (Goetze 2003).
Our results show two interesting features. First, the latitudinal variation in copepod diversity did not exhibit a continuous decline from the Equator to the Poles (figure 2). Second and perhaps more important, the shape of the latitudinal change was not symmetrical on the two sides of the Equator. Diversity peaked at subtropical latitudes in the Northern Hemisphere and showed a plateau in the Southern Hemisphere where diversity remained high from the Equator to the beginning of the temperate regions. This spatial pattern, where the peak in diversity was displaced from the Equator, is consistent with latitudinal variations found for some other marine taxa, e.g. foraminifera (Rutherford et al. 1999), tintinnids (Dolan et al. 2006) and fish (Worm et al. 2005; Boyce et al. 2008), and also in the terrestrial environment, e.g. aphids, sawflies and birds (Gaston & Blackburn 2000). Longitudinal variation in copepod diversity was also investigated but no significant pattern was found (electronic supplementary material, figure S3).
All aspects of the climate system, such as winds, clouds and temperature, influence the supply of energy to organisms. The asymmetry of the relationship between diversity and latitude around the Equator could be attributed to the climatic conditions prevailing in that region, in particular the incoming radiation and heat. The Inter-Tropical Convergence Zone (ITCZ) is a major area of cloud formation and instability in the tropics (Robinson & Henderson-Sellers 1999). High precipitation causes a negative evaporation–precipitation budget and thus lower salinities in the tropics (Toledo et al. 2007). The ITCZ moves over the oceans from 5° N to 10° N in July to close to the Equator in January (Robinson & Henderson-Sellers 1999), which especially affects the northern side of the Equator. The asymmetry in the incoming radiation and amount of precipitation between the two hemispheres is reflected in the relationships of temperature and salinity with latitude and is mirrored by the latitudinal variation in diversity (figure 2). Our results (table 1) also show that copepod diversity is higher in areas of high salinity. The peak in diversity around 20° N (figure 2a) coincides with a peak in salinity in the same area. In contrast to the ITCZ, the subtropical areas are characterized by higher salinities, corresponding to little cloud formation and, hence, excess evaporation over precipitation.
Among the variables tested in our study, temperature was the environmental factor that showed the highest correlation with copepod diversity. The large-scale relationship between copepod diversity and temperature in this study agrees with findings at smaller scales (Beaugrand et al. 2002a; Beaugrand 2004; Mackas et al. 2007; Piontkovski et al. 2006). Different forms of energy influence diversity at different levels of the food web and the influence of temperature on this suite of mechanisms is all-pervasive and complex (Clarke & Gaston 2006). The effects of temperature on diversity can be direct or indirect. Indirect relationships between temperature and diversity exist where temperature and radiant energy limits, for example, the abundance of plants and therefore decreases the availability of chemical energy to higher trophic levels. Several species–energy hypotheses share the common theme of temperature but differ in the ultimate pathways by which energy availability leads to a change in species diversity. One class of explanations, often referred to as the ‘more individuals’ hypothesis, suggest that warmer regions can support greater biomass and thus more species can coexist at abundances that enable them to maintain viable populations (Wright et al. 1993; Turner 2004a). Other hypotheses, e.g. the ‘evolutionary speed’ hypothesis (Rohde 1992), propose that temperature promotes mutation through the effects on generation times and metabolic rates and hence controls rates of speciation and extinction of populations (Allen et al. 2002; Brown et al. 2004). Furthermore, Gillooly & Allen (2007) emphasize that two forms of energy, kinetic and chemical potential energy, should be separated since they regulate diversity in different ways. Where kinetic energy is thought to influence biodiversity through its effects on individual metabolic rate, chemical potential energy influences speciation rates through their effects on total community abundance (Allen et al. 2007). Temperature will most probably affect copepod diversity in direct and indirect ways, and the combined mechanism of the more individuals and evolutionary speed hypotheses may be the most plausible explanation for the regulation of copepod diversity. Copepods are ectotherms with short generation times, so increasing temperatures could rapidly affect diversity in a direct way through the influence on metabolic rates of individuals but also indirectly on the population abundance and diversity.
Our study has also shown that factors other than temperature were involved in the regulation of large-scale pattern in copepod diversity. Chl a can be considered to be a proxy for the chemical energy available to copepods. The significant inverse relationship of diversity with chl a indicated that copepod diversity is high in oligotrophic regions. Previous studies have also pointed out high species diversity of copepods in oligotrophic systems, where productivity is limited by nutrients (McGowan 1990; Longhurst 2007). Recent studies suggest an influence of oxygen concentration on spatial and temporal changes in ectotherms (Pörtner 2001). Areas with higher copepod diversity are characterized by lower oxygen concentrations. The strong negative relationship of diversity and oxygen is not surprising since spatial changes in mean ocean temperature and surface oxygen concentration are also highly and inversely correlated (Beaugrand et al. 2008). The generally negative correlations between diversity and the various indices of seasonality of environmental variables agree with widespread evidence that more species co-occur in areas with lower seasonal changes in physical properties and primary productivity (Woodd-Walker et al. 2002). A stable physical structure of the near-surface ocean provides higher vertical niche availability than less stable conditions (Rutherford et al. 1999), and hence can support a higher number of species. In this study, however, correlations of diversity with seasonal variations in environmental variables were generally weaker than with variables describing mean conditions.
A first global map of copepod diversity was produced using a statistical model based on ocean temperature, salinity and chl a (figure 4). Our ocean-wide representation of diversity made it possible to infer zooplankton richness in areas where time series of species abundance data are presently non-existent (there are often only short-term or one-time sampling data in these areas) or not available. Clear differences in diversity are shown between tropical and polar regions, with high diversity in tropical regions and a marked transition zone between 30° and 40° latitude (in both hemispheres) in the Atlantic and Pacific Oceans. Upwelling regions were characterised by high productivity but low zooplankton diversity, e.g. Benguela and Humboldt upwelling regions. Similar maps of global patterns of pelagic diversity as a function of temperature have been proposed for foraminifera (Rutherford et al. 1999) and fish (Worm et al. 2005; Boyce et al. 2008). Increased data sharing would facilitate the validation of such maps.
Climate and energy provide a framework for explaining the latitudinal variation in pelagic species diversity and can act on evolutionary and biogeographical processes to determine patterns of species richness (Wiens & Donoghue 2004). In the present study, more than 50 per cent of the variance in copepod diversity was accounted for by ocean temperature. This strong co-variation is probably mediated through the effects of the climate system on the availability of energy to the organisms. A clear argument is the coinciding asymmetry in diversity and ocean temperature and salinity, most probably caused by the ITCZ. This result can be used for examining how climate change could influence global diversity patterns and forecast responses of copepod diversity. Climate change is expected to reduce the number of species globally (Thomas et al. 2004), but at the regional scale species richness might either increase or decrease (Menendez et al. 2006). In the North Atlantic, the link between large-scale patterns of pelagic diversity and increased ocean temperature has already led to northwards expansions of species bringing about local diversity increases (Beaugrand et al. 2002b; Hiddink & Hofstede 2008).
Acknowledgements
We are grateful to the institutes and research programmes that shared their valuable data with us. In particular, the Tohoku National Fisheries Institute who kindly provided us with the ODATE collection. We also thank David L. Mackas for sharing data and Todd O'Brien for helping us with the acquisition of additional datasets. This research was funded by the EU Marie Curie EST project METAOCEANS (MEST-CT-2005-019678).
References
- Allen A. P., Brown J. H., Gillooly J. F.2002Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297, 1545–1548 (doi:10.1126/science.1072380) [DOI] [PubMed] [Google Scholar]
- Allen A. P., Gillooly J. F., Brown J. H.2007Recasting the species-energy hypothesis: the different roles of kinetic and potential energy in regulating biodiversity. In Scaling biodiversity (eds Storch D., Marquet P. A., Brown J. H.), pp. 283–299 New York, NY: Cambridge University Press [Google Scholar]
- Antonov J. I., Locarnini R. A., Boyer T. P., Mishonov A. V., Garcia H. E.2006. In World Ocean Atlas 2005, volume 2: salinity (ed. Levitus S.), 182 pp., Washington, DC: NOAA Atlas NESDIS 62, US Government Printing Office [Google Scholar]
- Beaugrand G.2004Monitoring marine plankton ecosystems. Description of an ecosystem approach based on plankton indicators. Mar. Ecol. Prog. Ser. 269, 69–81 (doi:10.3354/meps269069) [Google Scholar]
- Beaugrand G., Edwards M.2001Differences in performance among four indices used to evaluate diversity in planktonic ecosystems. Oceanol. Acta 24, 467–477 (doi:10.1016/S0399-1784(01)01157-4) [Google Scholar]
- Beaugrand G., Edwards M., Brander K., Luczak C., Ibanez F.2008Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic. Ecology Letters 11, 1157–1168 [DOI] [PubMed] [Google Scholar]
- Beaugrand G., Ibanez F., Lindley A. J., Reid P. C.2002aDiversity of calanoid copepods in the North Atlantic and adjacent seas: species associations and biogeography. Mar. Ecol. Prog. Ser. 232, 179–195 (doi:10.3354/meps232179) [Google Scholar]
- Beaugrand G., Reid P. C., Ibanez F., Lindley J. A., Edwards M.2002bReorganization of North Atlantic marine copepod biodiversity and climate. Science 296, 1692–1694 (doi:10.1126/science.1071329) [DOI] [PubMed] [Google Scholar]
- Begon M., Harper J. L., Townsend C. R.1996Ecology: individuals, populations and communities Oxford, UK: Blackwell Science Ltd [Google Scholar]
- Boyce D. G., Tittensor D. P., Worm B.2008Effects of temperature on global patterns of tuna and billfish richness. Mar. Ecol. Prog. Ser. 355, 267–276 (doi:10.3354/meps07237) [Google Scholar]
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B.2004Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (doi:10.1890/03-9000) [Google Scholar]
- Clarke A.1992Is there a latitudinal diversity cline in the sea? Trends Ecol. Evol. 7, 286–287 (doi:10.1016/0169-5347(92)90222-W) [DOI] [PubMed] [Google Scholar]
- Clarke A., Gaston K. J.2006Climate, energy and diversity. Proc. R. Soc. B 273, 2257–2266 (doi:10.1098/rspb.2006.3545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crame J. A., Clarke A.1997The historical component of marine taxonomic diversity gradients. In Marine Biodiversity: patterns and processes (eds Ormond R. F. G., Gage J. D., Angel M. V.), pp. 258–273 New York, NY: Cambridge University Press [Google Scholar]
- Connell J. H., Orias E.1964The ecological regulation of species diversity. Am. Nat. 98, 399–414 (doi:10.1086/282335) [Google Scholar]
- Currie D. J.1991Energy and large-scale patterns of animal and plant species richness. Am. Nat. 137, 27–49 (doi:10.1086/285144) [Google Scholar]
- de Boyer Montégut C., Madec G., Fischer A. S., Lazar A., Iudicone D.2004Mixed layer depth over the global ocean: an examination of profile data and a profile-based climatology. J. Geophys. Res. 109, 1–20 [Google Scholar]
- Dolan J. R., Lemee R., Gasparini S., Mousseau L., Heyndrickx C.2006Probing diversity in the plankton: using patterns in Tintinnids (planktonic marine ciliates) to identify mechanisms. Hydrobiologia 555, 143–157 (doi:10.1007/s10750-005-1112-6) [Google Scholar]
- Garcia H. E., Locarnini R. A., Boyer T. P., Antonov J. I.2006a. In World Ocean Atlas 2005, volume 3: dissolved oxygen, apparent oxygen utilization, and oxygen saturation (ed. Levitus S.), 342 pp., Washington, DC: NOAA Atlas NESDIS 63, US Government Printing Office [Google Scholar]
- Garcia H. E., Locarnini R. A., Boyer T. P., Antonov J. I.2006b. In World Ocean Atlas 2005, Volume 4: Nutrients (phosphate, nitrate, silicate) (ed. Levitus S.), 396 pp. Washington, DC: NOAA Atlas NESDIS 64, US Government Printing Office [Google Scholar]
- Gaston J. K., Blackburn T. M.2000Pattern and process in macroecology Oxford, UK: Blackwell Science Ltd [Google Scholar]
- Gillooly J. F., Allen A. P.2007Linking global patterns in biodiversity to evolutionary dynamics using metabolic theory. Ecology 88, 1890–1894 (doi:10.1890/06-1935.1) [DOI] [PubMed] [Google Scholar]
- Goetze E.2003Cryptic speciation on the high seas; global phylogenetics of the copepod family Eucalanidae. Proc. R. Soc. B 270, 2321–2331 (doi:10.1098/rspb.2003.2505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. S., Poore G. C. B., Ugland K. I., Wilson R. S., Olsgard F., Johannessen O.1997Coastal and deep sea benthic diversities compared. Mar. Ecol. Prog. Ser. 159, 97–103 (doi:10.3354/meps159097) [Google Scholar]
- Hiddink J. G., Hofstede R.2008Climate induced increases in species richness of marine fishes. Glob. Change Biol. 14, 453–460 (doi:10.1111/j.1365-2486.2007.01518.x) [Google Scholar]
- Hillebrand H.2004Strength, slope and variability of marine latitudinal gradients. Mar. Ecol. Prog. Ser. 273, 251–267 (doi:10.3354/meps273251) [Google Scholar]
- Holm S.1979A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 [Google Scholar]
- Hopcroft R. R., Clarke C., Nelson R. J., Raskoff K. A.2005Zooplankton communities of the Arctic's Canada Basin: the contribution by smaller taxa. Polar Biol. 28, 198–206 (doi:10.1007/s00300-004-0680-7) [Google Scholar]
- Kerr J. T.2001Global biodiversity patterns: from description to understanding. Trends Ecol. Evol. 16, 424–425 (doi:10.1016/S0169-5347(01)02226-1) [Google Scholar]
- Kerr J. T., Kharouba H. M., Currie D. J.2007The macroecological contribution to global change solutions. Science 316, 1581–1584 (doi:10.1126/science.1133267) [DOI] [PubMed] [Google Scholar]
- Legendre P., Legendre L.1998Numerical ecology Amsterdam, The Netherlands: Elsevier Science [Google Scholar]
- Levin S. A.1992The problem of pattern and scale in ecology. Ecology 73, 1943–1967 (doi:10.2307/1941447) [Google Scholar]
- Locarnini R. A., Mishonov A. V., Antonov J. I., Boyer T. P., Garcia H. E.2006. In World Ocean Atlas 2005, Volume 1: Temperature. (ed. Levitus S.), 182 pp., Washington, DC: NOAA Atlas NESDIS 61, US Government Printing Office [Google Scholar]
- Longhurst A.2007Ecological geography of the sea London, UK: Academic Press [Google Scholar]
- Mackas D. L., Batten S., Trudel M.2007Effects on zooplankton of a warmer ocean: recent evidence from the Northeast Pacific. Progr. Oceanogr. 75, 223–252 (doi:10.1016/j.pocean.2007.08.010) [Google Scholar]
- Macpherson E.2002Large-scale species-richness gradients in the Atlantic Ocean. Proc. R. Soc. Lond. B 269, 1715–1720 (doi:10.1098/rspb.2002.2091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magurran A. E.2004Measuring biological diversity Oxford, UK: Blackwell Science Ltd [Google Scholar]
- McGowan J. A.1990Species dominance–diversity patterns in oceanic communities. In The Earth in transition (ed. Woodwell G. M.), pp. 395–421 New York, NY: Cambridge University Press [Google Scholar]
- Menendez R., Megías A. G., Hill J. K., Braschler B., Willis S. G., Collingham Y., Fox R., Roy D. B., Thomas C. D.2006Species richness changes lag behind climate change. Proc. R. Soc. B 273, 1465–1470 (doi:10.1098/rspb.2006.3484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka E. R.1966Latitudinal gradients in species diversity: a review of concepts. Am. Nat. 100, 33–46 (doi:10.1086/282398) [Google Scholar]
- Pierrot-Bults A. C.1997Biological diversity in oceanic macrozooplankton: more than counting species. In Marine biodiversity: patterns and processes (eds Ormond R. F. G., Gage J. D., Angel M. V.), pp. 69–93 New York, NY: Cambridge University Press [Google Scholar]
- Pimm S. L., Brown J. H.2004Domains of diversity. Science 304, 831–833 (doi:10.1126/science.1095332) [DOI] [PubMed] [Google Scholar]
- Piontkovski S. A., O'Brien T. D., Umani S. F., Krupa E. G., Stuge T. S., Balymbetov K. S., Grishaeva O. V., Kasymov A. G.2006Zooplankton and the North Atlantic oscillation: a basin-scale analysis. J. Plankt. Res. 28, 1039–1046 (doi:10.1093/plankt/fbl037) [Google Scholar]
- Pörtner H. O.2001Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137–146 (doi:10.1007/s001140100216) [DOI] [PubMed] [Google Scholar]
- Proches S.2001Back to the sea: secondary marine organisms from a biogeographical perspective. Biol. Linn. Soc. 74, 197–203 (doi:10.1006/bijl.2001.0565) [Google Scholar]
- Rice W. R.1989Analyzing tables of statistical tests. Evolution 43, 223–225 (doi:10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- Reid J., Brinton E., Fleminger A., Venrick E. L., McGowan J. A.1978Ocean circulation and marine life. In Advances in oceanography (eds Charnock H., Sir Deacon G.), pp. 65–130 New York, NY: Plenum Press [Google Scholar]
- Rex M. A., Stuart C. T., Coyne G.2000Latitudinal gradients of species richness in the deep-sea benthos of the North Atlantic. Proc. Natl Acad. Sci. 97, 4082–4085 (doi:10.1073/pnas.050589497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. J., Henderson-Sellers A.1999Contemporary climatology. Essex, UK: Pearson Education Ltd [Google Scholar]
- Rohde K.1992Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65, 514–527 (doi:10.2307/3545569) [Google Scholar]
- Roy K., Jablonski D., Valentine J. W.2000Dissecting latitudinal diversity gradients: functional groups and clades of marine bivalves. Proc. R. Soc. Lond. B 267, 293–299 (doi:10.1098/rspb.2000.0999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K., Jablonski D., Valentine J. W., Rosenberg G.1998Marine latitudinal diversity gradients: tests of causal hypotheses Proc. Natl Acad. Sci. USA 95, 3699–3702 (doi:10.1073/pnas.95.7.3699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddiman W. F.1969Recent planktonic foraminifera: dominance and diversity in North Atlantic surface sediments. Science 164, 1164–1167 (doi:10.1126/science.164.3884.1164) [DOI] [PubMed] [Google Scholar]
- Rutherford S., D'Hondt S., Prell W.1999Environmental controls on the geographic distribution of zooplankton diversity. Nature 400, 749–753 (doi:10.1038/23449) [Google Scholar]
- Smith W. H. F., Sandwell D. T.1997Global sea floor topography from satellite altimetry and ship depth soundings. Science 277, 1956–1962 (doi:10.1126/science.277.5334.1956) [Google Scholar]
- Stephens P. R., Wiens J. J.2003Explaining species richness from continents to communities: the time-for-speciation effect in emydid turtles. Am. Nat. 161, 112–128 (doi:10.1086/345091) [DOI] [PubMed] [Google Scholar]
- Thomas C. D., et al. 2004Extinction risk from climate change. Nature 427, 145–148 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- Toledo F. A. L., Costa K. B., Pivel M. A. G.2007Salinity changes in the western tropical South Atlantic during the last 30 kyr. Global Planet. Change 57, 383–395 (doi:10.1016/j.gloplacha.2007.01.001) [Google Scholar]
- Turner J. T.1981Latitudinal patterns of Calanoid and Cyclopoid copepod diversity in estuarine waters of Eastern North America. J. Biogeogr. 8, 369–382 (doi:10.2307/2844757) [Google Scholar]
- Turner J. R. G.2004aExplaining the global biodiversity gradient: energy, area, history and natural selection. Basic Appl. Ecol. 5, 435–448 (doi:10.1016/j.baae.2004.08.004) [Google Scholar]
- Turner J. T.2004bThe importance of small planktonic copepods and their roles in pelagic marine food webs. Zool. Stud. 43, 255–226 [Google Scholar]
- Wiens J. J., Donoghue M. J.2004Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644 (doi:10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- Willig M. R., Bloch C. P.2006Latitudinal gradients of species richness: a test of the geographic area hypothesis at two ecological scales. Oikos 112, 163–173 (doi:10.1111/j.0030-1299.2006.14009.x) [Google Scholar]
- Woodd-Walker R. S.2001Spatial distributions of copepod genera along the Atlantic Meridional transect. Hydrobiologia 453/454, 161–170 (doi:10.1023/A:1013140606293) [Google Scholar]
- Woodd-Walker R. S., Ward P., Clarke A.2002Large-scale patterns in diversity and community structure of surface water copepods from the Atlantic Ocean. Mar. Ecol. Prog. Ser. 236, 189–203 (doi:10.3354/meps236189) [Google Scholar]
- Worm B., Oschlies A., Lotze H. K., Myers R. A.2005Global patterns of predator diversity in the open oceans. Science 309, 1365–1369 (doi:10.1126/science.1113399) [DOI] [PubMed] [Google Scholar]
- Wright D. H.1983Species–energy theory: an extension of species–area theory. Oikos 41, 496–506 (doi:10.2307/3544109) [Google Scholar]
- Wright D. H., Currie D. J., Maurer B. A.1993Energy supply and patterns of species richness on local and regional scales. In Species diversity in ecological communities (eds Ricklefs R. E., Schluter D.), pp. 66–74 Chicago, IL: Chicago University Press [Google Scholar]