Abstract
A major goal of evolutionary biology is to identify the causes of diversification and to ascertain why some evolutionary lineages are especially diverse. Evolutionary biologists have long speculated that polyphenism—where a single genome produces alternative phenotypes in response to different environmental stimuli—facilitates speciation, especially when these alternative phenotypes differ in resource or habitat use, i.e. resource polyphenism. Here, we present a series of replicated sister-group comparisons showing that fishes and amphibian clades in which resource polyphenism has evolved are more species rich, and have broader geographical ranges, than closely related clades lacking resource polyphenism. Resource polyphenism may promote diversification by facilitating each of the different stages of the speciation process (isolation, divergence, reproductive isolation) and/or by reducing a lineage's risk of extinction. Generally, resource polyphenism may play a key role in fostering diversity, and species in which resource polyphenism has evolved may be predisposed to diversify.
Keywords: adaptive radiation, extinction, key innovation, phenotypic plasticity, replicated sister-group comparison, speciation
1. Introduction
How do new species arise, and why are some groups of organisms more species rich than others? In this article, we consider a possible key facilitator of both speciation and species richness: the widespread tendency of individual organisms to produce discrete, alternative phenotypes in response to different environmental stimuli, a phenomenon known as polyphenic development or polyphenism (sensu Mayr 1963).
Evolutionary biologists generally agree that speciation begins when populations become differentiated genetically and isolated reproductively (see recent reviews in Schluter 2000; Coyne & Orr 2004; Bolnick & Fitzpatrick 2007). But what factors cause such divergence, and why do some lineages seem especially prone to undergo this process? Divergence (and subsequent reductions in gene flow between populations) may be triggered when one population disperses and colonizes a new habitat or when two populations become isolated through a vicariance event (i.e. when a single population is split into two by a new physical barrier). Alternatively, divergence may arise as an adaptive response to disruptive selection acting to minimize potentially costly interactions among individuals within populations, such as intraspecific resource competition (Maynard Smith 1966; Rosenzweig 1978; Wilson & Turelli 1986; Wilson 1989; Dieckmann & Doebeli 1999; Dieckmann et al. 2004). This process, termed ‘adaptive speciation’ (sensu Dieckmann et al. 2004), may account for much biological diversity (reviewed in Schluter 2000; Dieckmann et al. 2004; Rundle & Nosil 2005; Bolnick & Fitzpatrick 2007).
When evaluating the causes of speciation, research has focused primarily on genetic and ecological mechanisms (e.g. Schluter 2000; Coyne & Orr 2004; Rundle & Nosil 2005; Grant & Grant 2008). By contrast, the role of development has been largely overlooked. This omission is surprising, given that many of life's most spectacular bouts of diversification—adaptive radiations—appear to be triggered by key innovations stemming from developmental changes (Simpson 1944; Simpson 1953; Heard & Hauser 1995; Schluter 2000). The evolution of a key innovation, especially one that enables individuals to exploit resources in a novel way, can cause a lineage to diversify into many descendent species (Niklas et al. 1983; Bambach 1985; reviewed in Schluter 2000).
Here, we explore whether polyphenic development facilitates adaptive lineage splitting and speciation. Polyphenic development arises when a single genome produces two or more alternative phenotypes in response to different environmental stimuli (Moran 1992; West-Eberhard 2003). In essence, individuals with identical genomes express distinctively different adaptive phenotypes in response to environmental cues that determine which genes, and, thereby, which of several alternative developmental pathways, will be expressed (Nijhout 2003). Alternative phenotypes that arise through this process often differ markedly not only in morphology, but also in behaviour, ecology and physiology (Nijhout 2003; West-Eberhard 2003).
We specifically focus on resource polyphenism, which we define as the occurrence within a single population of environmentally triggered alternative phenotypes showing differential resource use. Such polyphenism has been documented in diverse organisms, from viruses to salamanders (table 1). Resource polyphenism has long been viewed as a critical, early stage in the speciation process (Maynard Smith 1966; Felsenstein 1981; West-Eberhard 1989, 2003, 2005; Meyer 1993; Wimberger 1994; Smith & Skúlason 1996; Skúlason et al. 1999; Stauffer & Gray 2004; Mallet 2008). Historically, this belief has been grounded in the observation that phenotypic differences between alternative morphs are often comparable to those normally seen between species (e.g. Liem & Kaufman 1984; Hendry et al. 2006; Calsbeek et al. 2007). Indeed, many populations that differ in the expression of resource polyphenism possess some of the same characteristics as species, including ecological and genetic differences and even partial reproductive isolation (Meyer 1993; Wimberger 1994; Smith & Skúlason 1996; Skúlason et al. 1999; Hendry 2009), suggesting that they are clear forerunners of species (West-Eberhard 2005; Mallet 2008; Hendry 2009).
Table 1.
Resource polyphenisms in selected taxa and the nature of the ecological segregation between alternative resource-use morphs.
| organism | nature of the ecological differences | references |
|---|---|---|
| viruses | ||
| lambda bacteriophage | lysis versus lysogeny reproduction | Ptashne (1986) |
| ciliates | ||
| Tetrahymena vorax | bacterivore versus carnivore niches | Ryals et al. (2002) |
| Lembadion bullinum | non-cannibal versus cannibal niches | Kopp & Tollrian (2003) |
| rotifers | ||
| Asplanchna sieboldi | non-cannibal versus cannibal niches | Gilbert (1973) |
| insects | ||
| geometrid moth caterpillars (Nemoria arizonaria) | different host plants | Greene (1989) |
| fish | ||
| numerous species | benthic versus limnetic niches | Kornfield & Taylor (1983), Robinson & Wilson (1994), Skúlason et al. (1999) and Robinson & Parsons (2002) |
| amphibians | ||
| spadefoot toad tadpoles (genus Spea) | omnivore versus carnivore niches | Pfennig (1990) |
| tiger salamander larvae (Ambystoma tigrinum) | planktivore versus cannibal niches | Collins & Cheek (1983) |
| long-toed salamander larvae (Ambystoma macrodactylum) | planktivore versus cannibal niches | Walls et al. (1993) |
| ringed salamander larvae (Ambystoma annulatum) | planktivore versus cannibal niches | Nyman et al. (1993) |
| Asian salamander larvae (Hynobius retardatus) | omnivore versus carnivore niches | Michimae & Wakahara (2002) |
Furthermore, the ability to express alternative resource-use morphs facultatively in response to different environmental conditions may itself represent a key innovation that triggers an adaptive radiation (West-Eberhard 2003). Such phenotypic plasticity may facilitate peak shifts on the adaptive landscape that would not be possible via the accumulation of genetic changes (Pál & Miklos 1999; Price et al. 2003; Schlichting 2004; Pfennig et al. 2006). If this plasticity is accompanied by genetic assimilation (Waddington 1953) or genetic accommodation (West-Eberhard 2003), speciation and adaptive radiation can result (West-Eberhard 2003).
In addition to possibly promoting the formation of new species, resource polyphenism might increase diversity indirectly by reducing extinction risk. In other words, polyphenism may maintain existing species. Any factor that reduces extinction risk should also tend to promote species richness, because reduced extinction (i) leads to more species in a clade, each of which provides additional opportunity for speciation, and (ii) gives the clade more time to diversify. Thus, reduced extinction could indirectly lead to greater species richness through greater number of lineages and more time to diversify per lineage.
Polyphenism may play a largely underappreciated role in lessening a lineage's risk of extinction (Bradshaw 1965), thereby providing them with more opportunity to diversify. In particular, because polyphenic development provides a mechanism whereby an organism can respond rapidly and adaptively to environmental change (reviewed in West-Eberhard 1989), organisms in which resource polyphenism has evolved should be less sensitive to changing environments than those in which it has not evolved. Moreover, because polyphenism enables organisms to adjust quickly to variable environments, such organisms should also be able to occupy a wider range of habitat types. An ability to invade diverse habitats may thereby reduce a lineage's risk of extinction; organisms that are more widely distributed appear to be less vulnerable to extinction (Jablonski 1986), presumably because they are less susceptible to deterioration of any one habitat or part of their geographical range. Thus, polyphenic development may promote diversification in two ways: by facilitating speciation, and by reducing extinction.
Despite these longstanding arguments that phenotypic plasticity in general—and polyphenism in particular—foster diversification, there are relatively few empirical tests of these ideas (see Losos et al. 2000; Pfennig & Murphy 2002; Gomez-Mestre & Buchholz 2006; Parsons & Robinson 2006; Ledón-Rettig et al. 2008; Wund et al. 2008). Yet, such tests are critically needed if we are to clarify the causes of diversification and establish whether (and why) some taxa are predisposed to diversify.
In the present study, we sought to fill these gaps. In particular, we present a comparative analysis aimed at evaluating whether evolutionary lineages in which resource polyphenism has evolved tend to be more species rich, as would be predicted if there were a causal relationship between resource polyphenism and diversification. We also examine whether taxa expressing resource polyphenism occupy more diverse habitats. We did so because taxa with broader ranges may be less likely to go extinct and therefore have more opportunity to diversify.
Before presenting these tests, however, we begin by discussing how resource polyphenism arises. Our goal here is to illustrate the continuity between the evolution of resource polyphenism and speciation by describing how a prime agent of adaptive speciation—intraspecific resource competition (see above)—also favours resource polyphenism.
2. Evolution and development of resource polyphenism
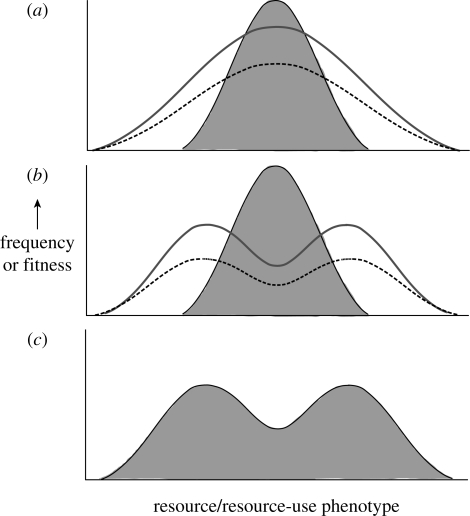
Resource polyphenism has long been viewed as an adaptive response to lessen intraspecific competition for resources (reviewed in Smith & Skúlason 1996). To understand why, consider a population experiencing intense intraspecific competition for a continuously varying resource. In such a situation, disruptive selection should favour individuals with extreme resource-use traits, because such individuals specialize on less common, but underused, resources on either end of the resource gradient (figure 1). This process is driven by negative frequency-dependent selection, in which rare resource-use phenotypes have a fitness advantage because of decreased competition with more common forms (Pfennig 1992; Day & Young 2004; Rueffler et al. 2006). Moreover, if such selection persists, it may promote the evolution of alternative phenotypes within the same population that specialize on different resource types (Smith & Skúlason 1996).
Figure 1.
How resource polyphenism evolves as an adaptive response to minimize intraspecific competition. In each graph, the shaded area represents a hypothetical population's distribution of resource-use phenotypes (shown as a quantitative trait), the dashed line shows the fitness associated with different phenotypes, and the heavy grey line represents the distribution of resource types. (a) Initially, in a population that exploits a range of resource types that are normally distributed (e.g. a range of prey sizes), selection will favour those individuals that use the most common resource type (e.g. prey of intermediate size). (b) Over time, as these individuals exploit it, this resource type becomes depleted. Individuals that use the intermediate resource type will therefore experience more severe competition than those that use more extreme, but underexploited, resource types (e.g. very small or very large prey items). Eventually, individuals that use the intermediate resource type will have lower fitness than those that use extreme resource types, causing disruptive selection. (c) Such selection can promote the evolution of alternative phenotypes within the same population that specialize on different resource types. Moreover, if individuals can assess environmental cues that reliably predict the likely success of each morph, then environmental induction of resource-use phenotype—i.e. resource polyphenism—is expected to evolve. Modified from Martin & Pfennig (2009).
Generally, however, the evolution of resource polyphenism requires more than intraspecific competition for resources. In particular, in order for resource polyphenism to evolve, there must also be sufficient ecological opportunity in the form of alternative resources underused by other species (sensu Simpson 1953; Schluter 2000). Because resource polyphenism entails the evolution of a novel resource-use phenotype, an ‘open niche’ must be available for this new resource-use phenotype to occupy. Without underused resources, niche width expansion, and thus, the evolution of resource polyphenism, is not feasible. When underused resources are present, by contrast, a population can expand the range of resources that it uses as an adaptive response to lessen intraspecific competition, thereby enabling resource polyphenism to evolve (Martin & Pfennig in press; figure 1).
As predicted by theory, resource polyphenism occurs most often in situations where intraspecific competition is intense, underused resources are present, and there is a relaxation of interspecific competition (the latter two factors combine to increase ecological opportunity). For example, lakes in recently glaciated regions of the Northern Hemisphere are relatively resource poor, which serves to increase intraspecific competition, and relatively species poor, which serves to increase ecological opportunity. In such lakes, many species of fishes produce two, sympatric, environmentally induced morphs: a benthic morph, which specializes on macroinvertebrates in the littoral zone, and a limnetic morph, which specializes on plankton in the open water (reviewed in Robinson & Wilson 1994; Wimberger 1994; Skúlason et al. 1999; Robinson & Parsons 2002). Similarly, environmentally induced trophic morphs occur among amphibians that inhabit environments where intraspecific competition is intense, underused resources are present, and there are few heterospecific competitors (e.g. Collins 1981; Pfennig 1990; Walls et al. 1993; Michimae & Wakahara 2002).
The environmental cues that induce resource polyphenism are often associated with intraspecific competition and ecological opportunity, as would be expected if these factors favour the evolution of resource polyphenism. For example, many alternative resource-use morphs are induced by tactile cues associated with an increased density of conspecifics (e.g. Collins & Cheek 1983; Hoffman & Pfennig 1999) or by the handling and/or ingestion of under-exploited resources (e.g. Gilbert 1973; Pfennig 1990; Day et al. 1994; Padilla 2001; Michimae & Wakahara 2002). Moreover, in many systems, which morph an individual expresses depends on its size or condition at the point in development when morph determination takes place. For example, in tiger salamanders (Maret & Collins 1997) and spadefoot toad tadpoles (Frankino & Pfennig 2001), larger individuals are more likely to develop into the more robust cannibal or carnivore morph. Such condition dependence makes adaptive sense: larger individuals are more likely to succeed at preying on larger food items (e.g. Frankino & Pfennig 2001).
Because polyphenism is environmentally induced, it is sometimes regarded as ‘non-genetic polymorphism’ (e.g. Jablonka & Lamb 1995, p. 238). However, in most examples that have been studied thoroughly, the magnitude and direction of the plastic response to the environment (the norm of reaction) is genetically variable, suggesting that polyphenism is subject to natural selection and evolutionary change (Schlichting & Pigliucci 1998; West-Eberhard 2003; Windig et al. 2004). This is certainly true for resource polyphenism. For example, there is considerable variation in the degree to which resource polyphenism is inducible by the environment among species of spadefoot toads, and, within species, among populations (e.g. Pfennig & Murphy 2002). Indeed, even within a single population, different sibships vary in propensity to produce alternative tadpole phenotypes (Pfennig 1999).
Greater sensitivity to environmental cues should be favoured by natural selection whenever the expected fitness of a particular phenotype is strongly influenced by environmental features that can be assessed reliably (Charnov & Bull 1977; Lively 1986; Pfennig 1990; West-Eberhard 2003). Generally, polyphenism is expected to be favoured over strictly genetically determined polymorphism when individuals can assess environmental cues that reliably predict the likely success of one of the morphs (for the basic theory, see Charnov & Bull 1977; Lively 1986).
Having reviewed how resource polyphenism arises, we now turn to polyphenic development's possible role in speciation. In particular, we present the results of a comparative analysis in which we addressed two questions: First, are evolutionary lineages in which resource polyphenism has evolved more species rich, as would be expected if there were a causal relationship between resource polyphenism and speciation? Second, do evolutionary lineages in which resource polyphenism has evolved tend to have broader geographical ranges?
3. Methods
(a). Resource polyphenism and species richness
If resource polyphenism promotes speciation, as evolutionary biologists have long suspected (see §1), then it should leave a signature on patterns of species richness in different clades. In particular, clades in which resource polyphenism has evolved should be more species rich than closely related clades in which resource polyphenism has not evolved.
One way to address such an issue is to compare the species richness of sister taxa, one of which contains the trait in question and the other which does not. By sister taxa, we mean two taxonomic groups (e.g. genera) that are derived from an immediate common ancestor and are therefore each other's closest relatives. Because sister taxa are of the same age, then any differences between them in species richness must be because of a difference in the rate of diversification and not age (Futuyma 2005). Moreover, if a character (such as the presence of resource polyphenism) is consistently associated with high diversity in a number of independently evolving clades, then the data would suggest that this character is associated with (and possibly caused) the higher rate of diversification.
Such replicated sister-group comparisons do, however, have a number of important limitations. First, as with all such comparative analyses, the data are merely correlational. Thus, although such analyses can be used to detect a relationship between some trait of interest and speciation, it is neither possible to determine the direction of causation, nor to rule out entirely uncontrolled, confounding variables (Barraclough et al. 1999). Second, in order to strengthen the case that the trait of interest actually caused the higher level of diversification in a particular clade, one should show that the trait is basal within the clade. However, this may not be possible in all cases (unless all members of the clade possess the trait), either because of poor resolution of the phylogenies, or because of incomplete information regarding the distribution of the trait of interest in each clade (especially if the trait is expressed facultatively). These limitations notwithstanding, replicated sister-group comparisons can be a powerful approach for testing hypotheses about the evolution of species richness within groups. Indeed, replicated sister-group comparisons have been widely used to study the factors that promote speciation (Read & Nee 1995; Barraclough et al. 1999; Barraclough & Nee 2001).
We performed such an analysis to explore the possible relationship between resource polyphenism and species richness in various fishes and amphibian taxa (resource polyphenism is relatively common in these two classes; table 1). We identified five major clades: centrarchid fish, salmonid fish, Neotropical cichlids, spadefoot toads and mole salamanders (see appendix A). Each clade contains two or more species known to have resource polyphenism (for illustrations of representative resource polyphenisms in these clades, see figure 2). Major clades with only one known polyphenic species (e.g. smelt, sticklebacks and Hynobius salamanders) were excluded from our analysis because low taxon sampling for polyphenism confounded our ability to assign a likely node for pairwise comparison.
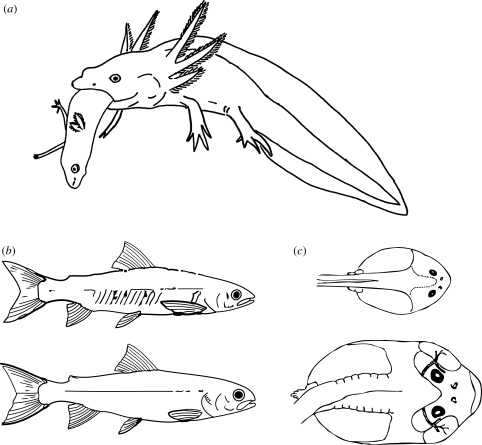
Figure 2.
Examples of resource polyphenism in fishes and amphibians. (a) In the southwestern USA, tiger salamanders (Ambystoma tigrinum) breed in temporary ponds that lack a top predator, such as fish. Competition for resource is frequently intense, and larvae often succumb before metamorphosis, particularly in rapidly drying ponds. In these ponds, the larvae often occur as two discrete morphs that differ in resource use. Typical larvae have a relatively small head and feed on small invertebrates. Under crowded conditions, however, some individuals develop a more robust head and larger teeth. These induced individuals are highly cannibalistic, occupying the formerly vacant top-predator niche. Here, a cannibal morph larva is shown engulfing a typical-morph larva. (b) In many lakes in recently glaciated regions of the Northern Hemisphere, many species of fish, such as Arctic charr, Salvelinus alpinus (shown here) occur as discrete morphological, behavioural and life history morphs that specialize on alternative ecological niches: a limnetic morph (upper fish) and a benthic morph (lower fish). The limnetic morph forages on zooplankton in the open water of the lake, whereas the benthic morph forages on invertebrates in the littoral zone of the lake. (c) In the southwestern USA, spadefoot toad tadpoles (genus Spea) frequently occur within the same ephemeral ponds as two environmentally induced ecomorphs: an omnivore morph (upper tadpole), which feeds mostly on detritus and plankton on the pond bottom, and a large-headed carnivore morph (lower tadpole), which feeds mostly on large anostracan fairy shrimp in the water column.
Sister clades with no known polyphenic species were identified using molecular phylogenies incorporating both mitochondrial and nuclear DNA data (appendix A). Whenever possible, we focused on comparisons between well-resolved nodes and avoided pairwise comparisons with sister clades containing many rare, endangered or otherwise poorly studied groups. We also attempted to avoid comparisons to clades with sympatric trophic phenotypes similar to known polyphenisms in their close relatives (McCart 1970; Meyer 1993). The Integrated Taxonomic Information System online database (http://www.itis.gov) and Fishbase (www.fishbase.org) were used to generate data on the number of species in each clade, except in cases where recent molecular phylogenies re-assigned species, as was the case for the heroine and cichlasomatine cichlids (Smith et al. 2008).
We treated the presence of polyphenism within a clade as a binary character, the number of species as a continuous character, and mapped these states onto pairs of sister clades. We then tested for a correlation between the presence of polyphenic species and the number of species in a clade using the Maddison pairwise comparison method in the Mesquite software package (Maddison 2000; Maddison & Maddison 2007).
(b). Resource polyphenism and geographical range
We found that lineages in which resource polyphenism has evolved tend to be more species rich (see §4a). We therefore sought to determine if the presence of a resource polyphenism might promote speciation by reducing the risk of extinction. Our underlying assumption was that any factor that reduces an evolutionary lineage's extinction risk should also tend to cause that lineage to become more diverse, because reduced extinction (i) leads to more species in a clade, each of which provides additional opportunity for speciation, and (ii) gives the lineage more time to diversify. Thus, reduced extinction could indirectly lead to greater species richness through greater numbers of lineages and more time to diversify per lineage.
As we noted in §1, one way in which resource polyphenism might increase species persistence is by increasing the geographical range over which the species occurs. Generally, evolutionary lineages with broader geographical ranges appear to be less vulnerable to extinction (Jablonski 1986).
For this analysis, we used data for the two amphibian clades only (Ambystoma versus Dicamptodon and Spea versus Scaphiopus), because data on geographical ranges was incomplete for the three fish clades. We approached this problem in two ways. First, we used a replicated sister-group comparison to test whether species within Ambystoma have wider geographical ranges than do species within its sister genus Dicamptodon (the former genus has evolved resource polyphenism, whereas the latter genus has not). Specifically, we compared the number of biotic provinces occupied by species of Ambystoma to that occupied by species of Dicamptodon. We obtained ranges for each species from field guides (Conant & Collins 1998; Stebbins 2003) or the worldwide web (AmphibiaWeb 2009). We overlaid each species' range onto a map of biotic provinces of North America (obtained from Rockwell 1998) and tallied the number of biotic provinces in which each species has been documented. We reasoned that the number of biotic provinces that a species occupies would reflect more accurately its ability to colonize diverse habitats than would the size of its geographical range (e.g. a species with a large range might actually occupy a lower diversity of habitats than one that has a smaller range but that exists in more diverse habitat types). Moreover, polyphenism is expected to go hand in hand with diverse habitat use, but not necessarily with large ranges. We used a two-tailed t-test to contrast the number of biotic provinces occupied by species of Ambystoma to that occupied by species of Dicamptodon. We also conducted a separate analysis within Ambystoma where we compared the number of biotic provinces occupied by the three species of Ambystoma in which resource polyphenism has been documented (A. tigrinum, A. macrodactylum and A. annulatum) to that occupied by the 24 species of Ambystoma in which resource polyphenism has not been documented. For this analysis, we used a one-tailed, non-parametric Wilcoxon Rank Sums test (a one-tailed test was justified because we found that the genus Ambystoma occupies more biotic provinces than does its sister genus Dicamptodon in which resource polyphenism has not evolved; see §4a).
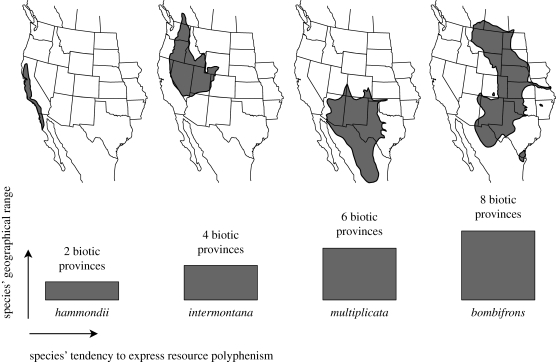
The second way in which we determined if polyphenic lineages have wider ranges was to compare the number of biotic provinces occupied by the four species of Spea. Resource polyphenism has been documented in all four species, but its expression varies from species to species, with Sp. bombifrons being the most prone to produce the distinctive carnivore morph, Sp. multiplicata being the second most prone, Sp. intermontana being the third most prone and Sp. hammondii being the least prone (D. Pfennig, unpublished data; J. Arendt 2001, personal communication). We asked if there was a significant relationship between the frequency of carnivore morph production and the number of biotic provinces a species occupies. We calculated the number of biotic provinces that each species occupies as before, and then used a non-parametric Spearman rank order correlation coefficient to determine if there was a significant relationship between the degree of expression of resource polyphenism in a particular species and the number of biotic provinces that it occupies.
4. Results
(a). Resource polyphenism and species richness
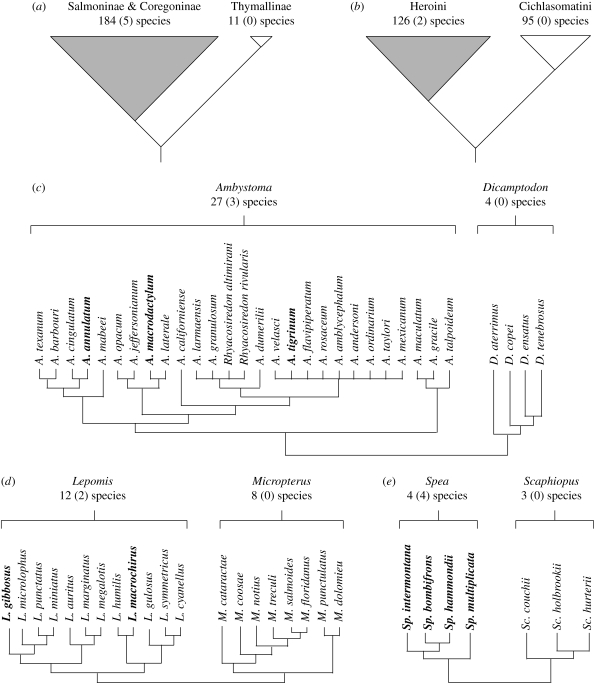
All five clades in which resource polyphenism has evolved are more species rich than their sister clades in which resource polyphenism has not evolved (figure 3). The overall trend was statistically significant (p = 0.03) and robust to randomization of character state across the phylogeny (Read & Nee 1995).
Figure 3.
Five replicated sister-group comparisons showing the species richness of different fishes and amphibian taxonomic groups in which resource polyphenism has evolved compared with that of their sister taxa in which resource polyphenism has not evolved. Shown are comparisons for (a) two subfamilies of salmonid fish; (b) two tribes of cichlid fish; (c) two genera of salamanders; (d) two genera of sunfish; and (e) two genera of spadefoot toads. In each case, the taxonomic group in which resource polyphenism has evolved is shown to the left of its sister group in which resource polyphenism has not evolved. The species richness of each clade is shown along with the number of species in which resource polyphenism has been documented (in parentheses). For clades in (c), (d) and (e), bold type signifies the species in which resource polyphenism has been documented (for the species not in bold type, we cannot say with certainty whether they are, or are not, polyphenic). For all five comparisons, the clade containing resource polyphenism is more species rich than its sister clade. For sources of information, see appendix.
(b). Resource polyphenism and geographical range
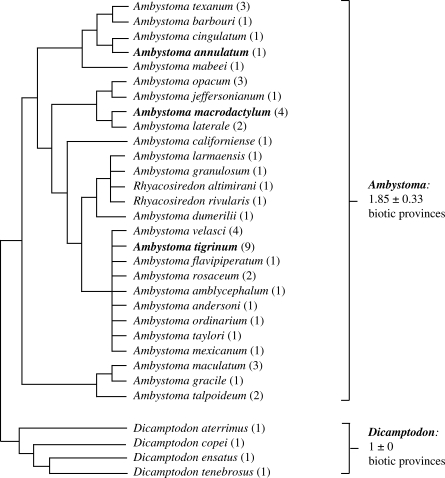
As predicted, lineages in which resource polyphenism has evolved tend to occur in more diverse habitat types than comparable lineages that lack resource polyphenism. In particular, we found that different species of Ambystoma (a genus in which resource polyphenism has evolved) occupy a greater number of biotic provinces than did species of Dicamptodon (a genus in which resource polyphenism has apparently not evolved; mean ± s.e.m. number of biotic provinces occupied by Ambystoma: 1.85 ± 0.33; number of biotic provinces occupied by Dicamptodon: 1 ± 0; p = 0.016, two-tailed t-test; figure 4). We also found that the three species of Ambystoma in which resource polyphenism has been documented occupy a greater number of biotic provinces than do the 24 species of Ambystoma in which resource polyphenism has not been documented (mean ± s.e.m. number of biotic provinces occupied by species of Ambystoma in which resource polyphenism has been documented: 4.67 ± 2.33; number of biotic provinces occupied by species of Ambystoma in which resource polyphenism has not been documented: 1.5 ± 0.88; p = 0.033; one-tailed Wilcoxon Rank Sums test; figure 4).
Figure 4.
A replicated sister-group comparison of the number of biotic provinces occupied by species of Ambystoma salamanders (a genus in which resource polyphenism has evolved) compared to that occupied by species of Dicamptodon (a genus in which resource polyphenism has not evolved). The number of biotic provinces that each species is known to inhabit is given in parentheses following each species' name.
Finally, there was a significant, positive relationship between the degree to which resource polyphenism is expressed in different species of spadefoot toads and the number of biotic provinces that each species occupies (p < 0.0001; Spearman rank order correlation coefficient = 1.0; figure 5).
Figure 5.
Relationship between geographical range and the expression of resource polyphenism in spadefoot toads (genus Spea). The four species that comprise the genus are arranged according to their tendency to express a resource polyphenism among their larvae (Sp. hammondii is least likely and Sp. bombifrons is most likely). The height of the bars is proportional to the number of biotic provinces that each species occupies (the geographical range of each species is shown above each bar).
5. Discussion
We found that fishes and amphibian taxa in which resource polyphenism has evolved are more species rich than their sister taxa in which resource polyphenism has not evolved (figure 3). These data are therefore consistent with the hypothesis that resource polyphenism increases species richness.
These findings must be interpreted with caution, however, for at least two reasons. First, for most of the clades in our analysis that contain resource polyphenism, we cannot say if the resource polyphenism evolved before or after the clade diversified. This uncertainty arises because resource polyphenism has been documented in only a minority of species in most clades (spadefoot toads were the sole exception). Such a pattern is not surprising for two reasons. First, resource polyphenism tends to be expressed only under certain environmental conditions, and most species have not been studied sufficiently thoroughly to assess whether or not they can produce a resource polyphenism. Second, as we explain in detail below, a pattern in which clades contain a mix of polyphenic and non-polyphenic species is exactly what is predicted under the hypothesis that polyphenism promotes speciation through the differential fixation of one of a series of morphs in different populations. Regardless of the reason for this pattern, for most of the clades in our dataset, we cannot preclude the possibility that resource polyphenism arose after the clade diversified. If resource polyphenism did evolve after a clade underwent an adaptive radiation, then, clearly, resource polyphenism would have played no role in the clade's adaptive radiation. Nevertheless, it is striking that in five independent comparisons, the clade in which resource polyphenism has been detected is more species rich than its sister clade in which resource polyphenism has not been reported. The probability of finding this overall pattern by chance was low (0.55 or 0.03, where 0.5 is the probability of finding such a pattern in any one clade).
A second reason why our findings must be interpreted cautiously is that they are correlational. Although our results are consistent with the notion that there is a relationship between resource polyphenism and species richness, they do not actually permit us to ascribe a causal link. Some uncontrolled factor may account for both the presence of the resource polyphenism and greater species richness. For example, given that taxa in which resource polyphenism has evolved tend to occur in more diverse habitats (see §4b and also below), the greater habitat diversity that these species inhabit might have promoted both the evolution of resource polyphenism and speciation.
Despite these caveats, our results nevertheless point to an association between resource polyphenism and species richness, as has been long predicted (see §1). These findings also suggest that resource polyphenism may represent a key innovation (sensu Simpson 1944, 1953; Schluter 2000) that enables a lineage in which it evolves to occupy a substantially new ecological niche. As noted in §1, the invasion of a novel niche is often associated with an increase in diversity (Niklas et al. 1983; Bambach 1985).
As further evidence that resource polyphenism enables species to occupy new niches, we also found that polyphenic taxa tend to occur in more diverse habitats than closely related non-polyphenic taxa (see §4b and figures 4 and 5). Again, as with the species richness data, these data are correlational and the direction of causation is unclear. On the one hand, such a pattern could arise if species that evolve resource polyphenism are able to invade more diverse habitats and, therefore, wider geographical ranges because of the presence of a resource polyphenism. On the other hand, this pattern could arise if species that occupy more diverse habitats are more likely to evolve resource polyphenism. For example, species that occupy diverse environments may be more likely to encounter the conditions that favour resource polyphenism (see §2). Although our data are consistent with the predictions we described at the outset, further work is needed to evaluate polyphenism's role in species' ranges and habitat use. Regardless of which of the above two scenarios is correct, our data indicate that species with resource polyphenism have broader habitat ranges.
Given the association between resource polyphenism and species richness (figure 3), we now turn to the important issue of how resource polyphenism might actually promote species richness. The species richness of a particular clade reflects the difference within that clade between the rate of speciation (i.e. the ‘birth’ of new species) and the rate of extinction (i.e. the ‘death’ of existing species). Resource polyphenism might affect both processes. In particular, resource polyphenism might increase a clade's species richness by increasing the rate of speciation or by decreasing the rate of extinction.
We begin by discussing resource polyphenism's possible impacts on speciation. To do so, it is useful to view speciation as a three-stage process (Freeman & Herron 2007): an initial step that begins when gene flow is disrupted between populations, such that they become genetically isolated from each other; a second step that arises when selection, drift and mutation act on isolated populations differentially, thereby causing them to diverge from one another; and a final step in which reproductive isolation evolves between populations, such that they either no longer interbreed, or, when they do, they fail to produce viable, fertile offspring. As we describe in detail below, the evolution of a resource polyphenism potentially facilitates each of these three stages.
First, the evolution of alternative resource-use morphs will frequently cause populations that differ in expression of such morphs to become physically separated and thereby genetically isolated from each other. Alternative morphs often differ in the locations and times that they seek their separate resources. Consider, for example, the situation in many Nearctic lakes, where conspecific fishes may occur as distinct, environmentally triggered benthic and limnetic morphs (figure 2b; see §2). These two morphs, although sympatric, typically forage in different locations within the same lake: the limnetic morph forages on zooplankton in the open water of the lake, whereas the benthic morph forages on invertebrates in the littoral zone of the lake (Robinson & Wilson 1994; Wimberger 1994; Skúlason et al. 1999; Robinson & Parsons 2002). Thus, once a resource polyphenism evolves, individuals from different populations that differ in their propensity to express each morph will not encounter each other as frequently as members of the same population. In this way, the evolution of a resource polyphenism promotes genetic isolation between populations, which is speciation's first stage.
Second, the evolution of a resource polyphenism also results in populations diverging from each other; i.e. speciation's second stage. In particular, once alternative morphs arise, natural selection promotes further divergence between them by favouring morph-specific traits that enhance each morph's ability to use its particular resource type and/or to survive in its distinctive habitat. For example, in stickleback fishes, which occur as sympatric benthic and limnetic morphs, the benthic morph is a larger, deeper bodied fish with fewer, shorter gill rakers. By contrast, the limnetic morph is a smaller, more fusiform fish with longer, more numerous gill rakers. These features appear to be adaptations for each morph's distinctive lifestyle (e.g. Schluter 1993; Walker 1997; Blake et al. 2005). In essence, once such alternative morphs arise, selection improves their functionality by favouring morph-specific traits that render each morph more effective at occupying its particular niche. This process could arise through genetic accommodation (West-Eberhard 2003) or through differential sorting of standing genetic variation (Rice & Pfennig 2007; Barrett & Schluter 2008). Whatever their causes, morph-specific adaptations characterize all resource polyphenisms. Such adaptations can contribute to the accumulation of genetic differences between populations that differ in their expression of resource polyphenism that, in turn, promotes further divergence. Indeed, there are numerous cases in which potentially sympatric populations appear to be diverging from one another genetically owing to differences in resource or habitat use (reviewed in Rundle & Nosil 2005). Thus, populations that differ in the expression of alternative resource-use morphs will tend to diverge from one another, thereby completing speciation's second stage.
Finally, the evolution of resource polyphenism can enhance reproductive isolation between populations, thereby completing the third stage of speciation. In particular, once populations that differ in the expression of alternative morphs begin to accumulate ecological and genetic differences, matings between such populations should produce offspring with low fitness. For example, in sticklebacks, matings between benthics with limnetics produce offspring that are intermediate in phenotype and therefore competitively inferior in either of the parental niches (Hatfield & Schluter 1999). Similarly, in spadefoot toads, matings between individuals from different populations that differ in expression of resource polyphenism produce unfit offspring, presumably because such offspring face a mismatch with their environment (Pfennig & Rice 2007). Generally, whenever offspring resulting from matings between populations are unfit, selection should favour assortative mating on population. This process of reinforcement will finalize speciation by promoting the evolution of complete reproductive isolation between populations that differ in morph expression (Servedio & Noor 2003; Coyne & Orr 2004).
There are no clear-cut examples from nature in which researchers have followed this entire process to completion, from initial genetic isolation to the evolution of reproductive isolation. Such examples are likely to be hard to come by, given how long it is thought to take for complete reproductive isolation to evolve (Coyne & Orr 1989). Nevertheless, laboratory experiments with fruitflies support the scenario for speciation outlined above (e.g. Rice & Hostert 1993; Higgie et al. 2000). Moreover, complete or partial reproductive isolation has been found in several natural systems that express resource polyphenism. For example, in sticklebacks, benthic and limnetic ecomorphs have repeatedly become reproductively isolated from one another in freshwater lakes along the coast of British Columbia (see Rundle & Schluter (2004) for a review of the evidence), and populations of spadefoot toads that differ in their expression of resource polyphenism similarly appear to be evolving reproductive isolation (Pfennig & Rice 2007; Rice & Pfennig in press). Furthermore, plasticity may have contributed to the divergence in both systems (in sticklebacks: Day et al. 1994; Wund et al. 2008; in spadefoot toads: Pfennig et al. 2006).
Ironically, it is also possible that there are no clear-cut examples from nature in which researchers have followed this entire process to completion, not because it happens too slowly to detect, but because it may happen too rapidly. There are numerous cases in which only one morph in a resource polyphenism has become developmentally canalized, or ‘fixed’ in a population (reviewed in West-Eberhard 1989, 2003). Once such fixation occurs in a population, it can effectively cause that population to become reproductively isolated from other, potentially sympatric populations that have not undergone similar fixation. Moreover, this process can unfold rapidly.
Fixation of one of a series of alternative phenotypes could come about through selection. In particular, if the environment changes such that one morph is no longer favoured, then selection should favour mechanisms that canalize development towards the alternative morph (e.g. Pfennig & Murphy 2000, 2002; Pfennig & Martin 2009). Furthermore, such selection should become increasingly effective in producing a better version of the newly favoured morph as it becomes expressed more frequently (West-Eberhard 1989). Selection could bring about these changes by acting on the underlying genes and/or developmental pathways that regulate expression of resource polyphenism (e.g. there may be changes in the threshold at which a phenotypic response to an environmental signal is triggered, in the level of hormones that mediate expression of alternative morphs, or in the critical period during which external cues must be detected to produce each morph).
Alternatively, fixation could arise through genetic drift. As one phenotype is expressed continuously in a population, and as the alternative phenotype is never expressed, alleles that regulate expression of this ‘hidden’ phenotype would not be exposed to selection, and thus are at risk of chance loss through drift (Masel et al. 2007). Such drift would be especially potent in small, isolated populations.
Regardless of how fixation arises, once populations diverge in morph expression, matings between them will generally result in offspring that perform poorly. Such poor performance could occur because matings between individuals from different populations that differ in morph expression would likely produce offspring that are poorly adapted to either population's particular environment (see above). Consequently, selection should favour assortative mating based on population and thereby finalize the speciation process by promoting the evolution of complete reproductive isolation.
Polyphenism should lead to especially rapid speciation because speciation's first two stages (isolation and divergence) occur simultaneously. Moreover, fixation should occur rapidly when the environment strongly influences the production of alternative morphs (West-Eberhard 1989, 2003). When alternative phenotypes are environmentally induced, a sudden change in the environment can immediately induce or select for a single alternative phenotype (see Pfennig & Murphy 2000). Although the initial ‘phenotypic’ fixation (in which only one phenotype is expressed) can be entirely non-genetic in nature, this fixation process is likely to promote rapid genetic changes. In particular, once one morph is expressed, selection should then favour those alleles or gene combinations that best stabilize, refine or extend the newly favoured morph's expression through genetic accommodation (for possible examples, see Ledón-Rettig et al. 2008; Wund et al. 2008). West-Eberhard (2003) provides numerous instances in which the differential fixation of alternative morphs in different populations may have led to rapid speciation.
The above discussion focused on how resource polyphenism increases species richness by promoting speciation. Resource polyphenism might also increase species richness indirectly by reducing the risk of extinction. Because polyphenic species can occupy more diverse habitats (see §4b and figures 4 and 5), they may be less restrictive in their habitat requirements and therefore less likely to experience chance extinction owing to habitat change or loss. Indeed, taxa with wider geographical ranges are generally less likely to go extinct (Jablonski 1986). Thus, by enabling a lineage to occupy a wider range of habitats, resource polyphenism may reduce the lineage's risk of extinction. By reducing the extinction risk of individual lineages in a particular clade, resource polyphenism should also tend to promote greater species richness in that clade, because reduced extinction (i) leads to more species in a clade, each of which provides additional opportunity for speciation, and (ii) gives the clade more time to diversify. Therefore, not only might resource polyphenism facilitate the formation of new species, it might also maintain existing species by reducing their risk of extinction.
Further research is needed to determine the degree to which resource polyphenism increases species richness by facilitating speciation as opposed to buffering existing lineages from extinction. Both routes, alone or in combination, could explain why clades in which resource polyphenism has evolved are more species rich. Regardless of how resource polyphenism increases species richness, our results reveal that resource polyphenism may play a key role in facilitating diversification and that species in which resource polyphenism has evolved may be predisposed to diversify.
Acknowledgements
D.W.P. devised the basic idea, both authors carried out the analyses, and D.W.P. wrote the manuscript. We thank K. Pfennig, P. Wainwright, C. Ledón-Rettig, R. Martin and two anonymous referees for comments on the manuscript and J. Burns, S. Price and J. Wiens for advice on the analyses.
This study was supported, in part, by grants from the US National Science Foundation (NSF) and the NSF's National Evolutionary Synthesis Center.
Appendix A
Taxa used in replicated sister-group comparisons.
| name of polyphenic taxon | name of sister (non-polyphenic) taxon | reference for the molecular phylogeny used to construct the replicated sister-group comparison | name(s) of polyphenic species | reference(s) for evidence of sympatric trophic phenotypes | reference(s) for evidence of plasticity in morph determination |
|---|---|---|---|---|---|
| Lepomis (12 species) | Micropterus (8 species) | Near et al. (2005) | Lepomis gibbosus | Robinson et al. (1993) | Robinson & Wilson (1996) |
| Lepomis macrochirus | Ehlinger & Wilson (1988) | Belk (1995) | |||
| Heroini (126 species) | Cichlasomatini (95 species) | Smith et al. (2008) | Amphilophus citrinellum | Meyer (1989) | Meyer (1990) |
| Herichthys minckleyi | Kornfield & Taylor (1983) | Trapani (2003) | |||
| Salmoninae and Coregoninae (184 species) | Thymallinae (11 species) | Crespi & Fulton (2004) and Koop et al. (2008) | Coregonus artedii | Turgeon & Bernatchez (2003) | Hile (1936) and Woodger (1976) |
| Coregonus clupeaformis | Fenderson (1964) | Bernatchez & Dodson (1990) | |||
| Oncorhynchus nerka | Wood & Foote (1996) | Foote et al. (1999) | |||
| Salvelinus alpinus | Skúlason et al. (1989) | Hindar & Jonsson (1993) | |||
| Salvelinus fontinalis | Proulx & Magnan (2004) | Proulx & Magnan (2004) | |||
| Spea (4 species) | Scaphiopus (3 species) | Wiens & Titus (1991) and García-Paris et al. (2003) | Spea bombifrons | Bragg (1965) | Pomeroy (1981) and Pfennig (1990) |
| Spea hammondii | J. Arendt (2001, personal communication) | J. Arendt (2001, personal communication) | |||
| Spea intermontana | Hall (1998) and J. Arendt (2001, personal communication) | J. Arendt (2001, personal communication) | |||
| Spea multiplicata | Bragg (1965) | Pomeroy (1981) and Pfennig (1990) | |||
| Ambystoma (27 species) | Dicamptodon (4 species) | Larson (1996) | Ambystoma annulatum | Nyman et al. (1993) | Nyman et al. (1993) |
| Ambystoma macrodactylum | Walls et al. (1993) | Walls et al. (1993) | |||
| Ambystoma tigrinum | Collins (1981) | Collins & Cheek (1983), Pfennig et al. (1991) and Hoffman & Pfennig (1999) |
Footnotes
One contribution of 12 to a Theme Issue ‘From polyphenism to complex metazoan life cycles’.
References
- AmphibiaWeb 2009Information on amphibian biology and conservation. Berkeley, CA. See http://amphibiaweb.org [Google Scholar]
- Bambach R. K.1985Classes and adaptive variety: the ecology of diversification in marine faunas through the Phanerozoic. In Phanerozoic diversity patterns: profiles in macroevolution (ed. Valentine J. W.), pp. 191–253 Princeton, NJ: Princeton University Press [Google Scholar]
- Barraclough T. G., Nee S.2001Phylogenetics and speciation. Trends Ecol. Evol. 16, 391–399 (doi:10.1016/S0169-5347(01)02161-9) [DOI] [PubMed] [Google Scholar]
- Barraclough T. G., Vogler A. P., Harvey P. H.1999Revealing the factors that promote speciation. In Evolution of biological diversity (eds Magurran A. E., May R. M.), pp. 202–219 Oxford, UK: Oxford University Press [Google Scholar]
- Barrett R. D. H., Schluter D.2008Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 (doi:10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- Belk M. C.1995Variation in growth and age at maturity in bluegill sunfish: genetic or environmental effects? J. Fish Biol. 47, 237–247 (doi:10.1111/j.1095-8649.1995.tb01891.x) [Google Scholar]
- Bernatchez L., Dodson J. J.1990Allopatric origin of sympatric populations of lake whitefish (Coregonus clupeaformis) as revealed by mitochondrial DNA restriction analysis. Evolution 44, 1263–1271 (doi:10.2307/2409287) [DOI] [PubMed] [Google Scholar]
- Blake R. W., Law T. C., Chan K. H. S., Li J. F. Z.2005Comparison of the prolonged swimming performances of closely related, morphologically distinct three-spined sticklebacks Gasterosteus spp. J. Fish Biol. 67, 834–848 (doi:10.1111/j.0022-1112.2005.00788.x) [Google Scholar]
- Bolnick D. I., Fitzpatrick B.2007Sympatric speciation: models and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 38, 459–487 (doi:10.1146/annurev.ecolsys.38.091206.095804) [Google Scholar]
- Bradshaw A. D.1965Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155 (doi:10.1016/S0065-2660(08)60048-6) [Google Scholar]
- Bragg A. N.1965Genomes of the night: the spadefoot toads Philadelphia, PA: University of Pennsylvania Press [Google Scholar]
- Calsbeek R., Smith T. B., Bardeleben C.2007Intraspecific variation in Anolis sagrei mirrors the adaptive radiation of Greater Antillean anoles. Biol. J. Linn. Soc. 90, 189–199 (doi:10.1111/j.1095-8312.2007.00700.x) [Google Scholar]
- Charnov E. L., Bull J. J.1977When is sex environmentally determined? Nature 266, 828–830 (doi:10.1038/266828a0) [DOI] [PubMed] [Google Scholar]
- Collins J. P.1981Distribution, habitats and life-history variation in the tiger salamander, Ambystoma tigrinum, in east-central and southeast Arizona. Copeia 1981, 666–675 (doi:10.2307/1444572) [Google Scholar]
- Collins J. P., Cheek J. E.1983Effect of food and density on development of typical and cannibalistic salamander larvae in Ambystoma tigrinum nebulosum. Am. Zool. 23, 77–84 [Google Scholar]
- Conant R., Collins J. T.1998A field guide to the reptiles and amphibians: eastern and central North America Boston, MA: Houghton Mifflin [Google Scholar]
- Coyne J. A., Orr H. A.1989Patterns of speciation in Drosophila. Evolution 43, 362–381 (doi:10.2307/2409213) [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation Sunderland, MA: Sinauer [Google Scholar]
- Crespi B. J., Fulton M. J.2004Molecular systematics of Salmonidae: combined nuclear data yields a robust phylogeny. Mol. Phylogenet. Evol. 31, 658–679 (doi:10.1016/j.ympev.2003.08.012) [DOI] [PubMed] [Google Scholar]
- Day T., Young K. A.2004Competitive and facilitative evolutionary diversification. BioScience 54, 101–109 (doi:10.1641/0006-3568(2004)054[0101:CAFED]2.0.CO;2) [Google Scholar]
- Day T., Pritchard J., Schluter D.1994A comparison of two sticklebacks. Evolution 48, 1723–1734 (doi:10.2307/2410260) [DOI] [PubMed] [Google Scholar]
- Dieckmann U., Doebeli M.1999On the origin of species by sympatric speciation. Nature 400, 354–357 (doi:10.1038/22521) [DOI] [PubMed] [Google Scholar]
- Dieckmann U., Doebeli M., Metz J. A. J., Tautz D. (eds) 2004Adaptive speciation Cambridge, UK: Cambridge University Press [Google Scholar]
- Ehlinger T. J., Wilson D. S.1988Complex foraging polymorphism in bluegill sunfish. Proc. Natl Acad. Sci. USA 85, 1878–1882 (doi:10.1073/pnas.85.6.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J.1981Skepticism toward Santa Rosalia, or why are there so few kinds of animals? Evolution 35, 124–138 (doi:10.2307/2407946) [DOI] [PubMed] [Google Scholar]
- Fenderson O. C.1964Evidence of subpopulations of lake whitefish, Coregonus clupeaformis, involving a dwarfed form. Trans. Am. Fisheries Soc. 93, 77–94 (doi:10.1577/1548-8659(1964)93[77:EOSOLW]2.0.CO;2) [Google Scholar]
- Foote C. J., Moore K., Stenberg K., Craig K. J., Wenburg J. K., Wood C. C.1999Genetic differentiation in gill raker number and length in sympatric anadromous and nonanadromous morphs of sockeye salmon, Oncorhynchus nerka. Environ. Biol. Fishes 54, 263–274 (doi:10.1023/A:1007548807233) [Google Scholar]
- Frankino W. A., Pfennig D. W.2001Condition-dependent expression of trophic polyphenism: effects of individual size and competitive ability. Evol. Ecol. Res. 3, 939–951 [Google Scholar]
- Freeman S., Herron J. C.2007Evolutionary analysis, 4th edn.Upper Saddle River, NJ: Pearson Education [Google Scholar]
- Futuyma D. J.2005Evolution Sunderland, MA: Sinauer [Google Scholar]
- García-Paris M., Buchholz D. R., Para-Olea G.2003Phylogenetic relationships of Pelobatidae re-examined using mtDNA. Mol. Phylogenet. Evol. 28, 12–23 (doi:10.1016/S1055-7903(03)00036-8) [DOI] [PubMed] [Google Scholar]
- Gilbert J. J.1973Induction and ecological significance of gigantism in the rotifer Asplancha sieboldi. Science 181, 63–66 (doi:10.1126/science.181.4094.63) [DOI] [PubMed] [Google Scholar]
- Gomez-Mestre I., Buchholz D. R.2006Developmental plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proc. Natl Acad. Sci. USA 103, 19 021–19 026 (doi:10.1073/pnas.0603562103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. R., Grant B. R.2008How and why species multiply: the radiation of Darwin's finches Princeton, NJ: Princeton University Press [Google Scholar]
- Greene E.1989A diet-induced developmental polymorphism in a caterpillar. Science 243, 643–646 (doi:10.1126/science.243.4891.643) [DOI] [PubMed] [Google Scholar]
- Hall J.1998Scaphiopus intermontanus Cat. Am. Amphib. Rept. 650, 1–17 [Google Scholar]
- Hatfield T., Schluter D.1999Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution 53, 866–873 (doi:10.2307/2640726) [DOI] [PubMed] [Google Scholar]
- Heard S. B., Hauser D. L.1995Key evolutionary innovations and their ecological mechanisms. Historical biology 10, 151–173 (doi:10.1080/10292389509380518) [Google Scholar]
- Hendry A. P.2009Ecological speciation! Or lack thereof? Can. J. Fish. Aquat. Sci. 66, 1383–1398 (doi:10.1139/F09-074) [Google Scholar]
- Hendry A. P., Grant P. R., Grant B. R., Ford H. A., Brewer M. J., Podos J.2006Possible human impacts on adaptive radiation: beak size bimodality in Darwin's finches. Proc. R. Soc. B 273, 1887–1894 (doi:10.1098/rspb.2006.3534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgie M., Chenoweth S., Blows M. W.2000Natural selection and the reinforcement of mate recognition. Science 290, 519–521 (doi:10.1126/science.290.5491.519) [DOI] [PubMed] [Google Scholar]
- Hile R.1936Summary of investigations on the morphometry of the cisco, Leucichthys artedi (Le Sueur), in the lakes of the Northeastern Highlands, Wisconsin. Pap. Mich. Acad. Sci. 21, 619–634 [Google Scholar]
- Hindar K., Jonsson B.1993Ecological polymorphism in Arctic charr. Biol. J. Linn. Soc. 48, 63–74 (doi:10.1111/j.1095-8312.1993.tb00877.x) [Google Scholar]
- Hoffman E. A., Pfennig D. W.1999Proximate causes of cannibalistic polyphenism in larval tiger salamanders. Ecology 80, 1076–1080 [Google Scholar]
- Jablonka E., Lamb M. J.1995Epigenetic inheritance and evolution: the Lamarkian dimension New York, NY: Oxford University Press [Google Scholar]
- Jablonski D.1986Background and mass extinctions: the alternation of macroevolutionary regimes. Science 231, 129–133 (doi:10.1126/science.231.4734.129) [DOI] [PubMed] [Google Scholar]
- Koop B. F., et al. 2008A salmonid EST genomic study: genes, duplications, phylogeny and microarrays. BMC Genom. 9, 545 (doi:10.1186/1471-2164-9-545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp M., Tollrian R.2003Trophic size polyphenism in Lembadion bullinum: costs and benefits of an inducible offense. Ecology 84, 641–651 (doi:10.1890/0012-9658(2003)084[0641:TSPILB]2.0.CO;2) [Google Scholar]
- Kornfield I., Taylor J. N.1983A new species of polymorphic fish, Cichlasoma minckleyi, from Cuatro Cienegas, Mexico (Teleostei: Cichlidae). Proc. Biol. Soc. Washington 96, 253–269 [Google Scholar]
- Larson A.1996Ambystomatidae. Mole salamanders. See http://tolweb.org/Ambystomatidae/15448/1996.01.01 in The Tree of Life Web Project, http://tolweb.org/ [Google Scholar]
- Ledón-Rettig C., Pfennig D. W., Nascone-Yoder N.2008Ancestral variation and the potential for genetic accommodation in larval amphibians: implications for the evolution of novel feeding strategies. Evol. Dev. 10, 316–325 [DOI] [PubMed] [Google Scholar]
- Liem K. F., Kaufman L. S.1984Intraspecific macroevolution: functional biology of the polymorphic cichlid species Cichlasoma minckleyi. In Evolution of fish species flocks (eds Echelle A. A., Kornfield I.), pp. 203–215 Orono, ME: University of Maine Press [Google Scholar]
- Lively C. M.1986Canalization versus developmental conversion in a spatially variable environment. Am. Nat. 128, 561–572 (doi:10.1086/284588) [Google Scholar]
- Losos J. B., Creer D. A., Glossip D., Goellner R., Hampton A., Roberts G., Haskell N., Taylor P., Ettling J.2000Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54, 301–305 [DOI] [PubMed] [Google Scholar]
- Maddison W. P.2000Testing character correlation using pairwise comparisons on a phylogeny. J. Theor. Biol. 202, 195–204 (doi:10.1006/jtbi.1999.1050) [DOI] [PubMed] [Google Scholar]
- Maddison W. P., Maddison D. R.2007Mesquite: a modular system for evolutionary analysis, version 2.0. See http://mesquiteproject.org/ [Google Scholar]
- Mallet J.2008Hybridization, ecological races and the nature of species: empirical evidence for the ease of speciation. Phil. Trans. R. Soc. B 363, 2971–2986 (doi:10.1098/rstb.2008.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret T. J., Collins J. P.1997Ecological origin of morphological diversity: a study of alternative trophic phenotypes in larval salamanders. Evolution 51, 898–905 (doi:10.2307/2411164) [DOI] [PubMed] [Google Scholar]
- Martin R. A., Pfennig D. W.2009Disruptive selection in natural populations: the roles of ecological specialization and resource competition. Am. Nat. 174, 268–281 (doi:10.1086/600090) [DOI] [PubMed] [Google Scholar]
- Martin R. A., Pfennig D. W.In press Field and experimental evidence that competition and ecological opportunity promote resource polymorphism. Biol. J. Linn. Soc. Lond [Google Scholar]
- Masel J., King O. D., Maughan H.2007The loss of adaptive plasticity during long periods of environmental stasis. Am. Nat. 169, 38–46 (doi:10.1086/510212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J.1966Sympatric speciation. Am. Nat. 104, 487–490 [Google Scholar]
- Mayr E.1963Animal species and evolution Cambridge, MA: Harvard University Press [Google Scholar]
- McCart P.1970Evidence for the existence of sibling species of pygmy whitefish (Prosopium coulteri) in three Alaskan lakes. In Biology of Coregonid fishes (eds Lindsey C. C., Woods C. S.), pp. 81–98 Winnipeg, MB: University of Manitoba Press [Google Scholar]
- Meyer A.1989Cost of morphological specialization: feeding performance of the two morphs in the trophically polymorphic cichlid fish, Cichlasoma citrinellum. Oecologia 80, 431–436 (doi:10.1007/BF00379047) [DOI] [PubMed] [Google Scholar]
- Meyer A.1990Ecological and evolutionary consequences of the trophic polymorphism in Cichlasoma citrinellum (Pisces: Cichlidae). Biol. J. Linn. Soc. 39, 279–299 (doi:10.1111/j.1095-8312.1990.tb00517.x) [Google Scholar]
- Meyer A.1993Trophic polymorphisms in cichlid fish: do they represent intermediate steps during sympatric speciation and explain their rapid adaptive radiation? In New trends in ichthyology (eds Schröder J.-H., Bauer J., Schartl M.), pp. 257–266 London, UK: GSF-Bericht, Blackwell [Google Scholar]
- Michimae H., Wakahara M.2002A tadpole-induced polyphenism in the salamander Hynobius retardatus. Evolution 56, 2029–2038 [DOI] [PubMed] [Google Scholar]
- Moran N. A.1992The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989 (doi:10.1086/285369) [Google Scholar]
- Near T. J., Bolnick D. I., Wainwright P. C.2005Fossil calibrations and molecular divergence time estimates in centrarchid fishes (Teleostei: Centrarchidae). Evolution 59, 1768–1782 [PubMed] [Google Scholar]
- Nijhout H. F.2003Development and evolution of adaptive polyphenisms. Evol. Dev. 5, 9–18 (doi:10.1046/j.1525-142X.2003.03003.x) [DOI] [PubMed] [Google Scholar]
- Niklas K. J., Tiffney B. H., Knoll A. H.1983Patterns in vascular land plant diversification. Nature 303, 614–616 (doi:10.1038/303614a0) [Google Scholar]
- Nyman S., Wilinson R. F., Hutcherson J. E.1993Cannibalism and size relations in a cohort of larval ringed salamanders (Ambystoma annulatum). J. Herpetol. 27, 78–84 (doi:10.2307/1564909) [Google Scholar]
- Padilla D. K.2001Food and environmental cues trigger an inducible offence. Evol. Ecol. Res. 3, 15–25 [Google Scholar]
- Pál C., Miklos I.1999Epigenetic inheritance, genetic assimilation and speciation. J. Theor. Biol. 200, 19–37 (doi:10.1006/jtbi.1999.0974) [DOI] [PubMed] [Google Scholar]
- Parsons K. J., Robinson B. W.2006Replicated evolution of integrated plastic responses during early adaptive divergence. Evolution 60, 801–813 [PubMed] [Google Scholar]
- Pfennig D. W.1990The adaptive significance of an environmentally-cued developmental switch in an anuran tadpole. Oecologia 85, 101–107 (doi:10.1007/BF00317349) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W.1992Polyphenism in spadefoot toad tadpoles as a locally-adjusted evolutionarily stable strategy. Evolution 46, 1408–1420 (doi:10.2307/2409946) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W.1999Cannibalistic tadpoles that pose the greatest threat to kin are most likely to discriminate kin. Proc. R. Soc. Lond. B 266, 57–61 (doi:10.1098/rspb.1999.0604) [Google Scholar]
- Pfennig D. W., Martin R. A.2009A maternal effect mediates rapid population divergence and character displacement in spadefoot toads. Evolution 63, 898–909 (doi:10.1111/j.1558-5646.2008.00544.x) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Murphy P. J.2000Character displacement in polyphenic tadpoles. Evolution 54, 1738–1749 [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Murphy P. J.2002How fluctuating competition and phenotypic plasticity mediate species divergence. Evolution 56, 1217–1228 [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Rice A. M.2007An experimental test of character displacement's role in promoting postmating isolation between conspecific populations in contrasting competitive environments. Evolution 61, 2433–2443 (doi:10.1111/j.1558-5646.2007.00190.x) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Loeb M. L. G., Collins J. P.1991Pathogens as a factor limiting the spread of cannibalism in tiger salamanders. Oecologia 88, 161–166 (doi:10.1007/BF00320806) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Rice A. M., Martin R. A.2006Ecological opportunity and phenotypic plasticity interact to promote character displacement and species coexistence. Ecology 87, 769–779 (doi:10.1890/05-0787) [DOI] [PubMed] [Google Scholar]
- Pomeroy L. V.1981Developmental polymorphism in the tadpoles of the spadefoot toad Scaphiopus multiplicatus. PhD thesis, University of California, Riverside, CA [Google Scholar]
- Price T. D., Qvarnström A., Erwin D. E.2003The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440 (doi:10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx R., Magnan P.2004Contribution of phenotypic plasticity and heredity to the trophic polymorphism of lacustrine brook charr (Salvelinus fontinalis M.). Evol. Ecol. Res. 6, 503–522 [Google Scholar]
- Ptashne M.1986A genetic switch Cambridge, UK: Cell Press and Blackwell [Google Scholar]
- Read A. F., Nee S.1995Inference from binary comparative data. J. Theor. Biol. 173, 99–108 (doi:10.1006/jtbi.1995.0047) [Google Scholar]
- Rice W. R., Hostert E. E.1993Laboratory experiments on speciation: what have we learned in 40 years? Evolution 47, 1637–1653 (doi:10.2307/2410209) [DOI] [PubMed] [Google Scholar]
- Rice A. M., Pfennig D. W.2007Character displacement: in situ evolution of novel phenotypes or sorting of pre-existing variation? J. Evol. Biol. 20, 448–459 (doi:10.1111/j.1420-9101.2006.01187.x) [DOI] [PubMed] [Google Scholar]
- Rice A. M., Pfennig D. W.In press Does character displacement initiate speciation? Evidence of reduced gene flow between populations experiencing divergent selection. J. Evol. Biol [DOI] [PubMed] [Google Scholar]
- Robinson B. W., Parsons K. J.2002Changing times, spaces, and faces: tests and implications of adaptive morphological plasticity in the fishes of northern postglacial lakes. Can. J. Fish. Aquat. Sci. 59, 1819–1833 (doi:10.1139/f02-144) [Google Scholar]
- Robinson B. W., Wilson D. S.1994Character release and displacement in fish: a neglected literature. Am. Nat. 144, 596–627 (doi:10.1086/285696) [Google Scholar]
- Robinson B. W., Wilson D. S.1996Genetic variation and phenotypic plasticity in a trophically polymorphic population of pumpkinseed sunfish (Lepomis gibbosus). Evol. Ecol. 10, 631–652 (doi:10.1007/BF01237711) [Google Scholar]
- Robinson B. W., Wilson D. S., Margosian A. S., Lotito P. T.1993Ecological and morphological differentiation of pumpkinseed sunfish in lakes without bluegill sunfish. Evol. Ecol. 7, 451–464 (doi:10.1007/BF01237641) [Google Scholar]
- Rockwell D.1998The nature of North America New York, NY: Berkeley [Google Scholar]
- Rosenzweig M. L.1978Competitive speciation. Biol. J. Linn. Soc. 10, 274–289 [Google Scholar]
- Rueffler C., Van Dooren T. J. M., Leimar O., Abrams P. A.2006Disruptive selection and then what? Trends Ecol. Evol. 21, 238–245 (doi:10.1016/j.tree.2006.03.003) [DOI] [PubMed] [Google Scholar]
- Rundle H. D., Nosil P.2005Ecological speciation. Ecol. Lett. 8, 336–352 (doi:10.1111/j.1461-0248.2004.00715.x) [Google Scholar]
- Rundle H. D., Schluter D.2004Natural selection and ecological speciation in sticklebacks. In Adaptive speciation (eds Dieckmann U., Doebeli M., Metz J. A. J., Tautz D.), pp. 192–209 Cambridge, UK: Cambirdge University Press [Google Scholar]
- Ryals P. E., Smith-Somerville H. E., Buhse H. E., Jr2002Phenotype switching in polymorphic Tetrahymena: a single-cell Jekyll and Hyde. Int. Rev. Cytol. 212, 209–238 (doi:10.1016/S0074-7696(01)12006-1) [DOI] [PubMed] [Google Scholar]
- Schlichting C. D.2004The role of phenotypic plasticity in diversification. In Phenotypic plasticity: functional and conceptual approaches (eds DeWitt T. J., Scheiner S. M.). New York, NY: Oxford University Press [Google Scholar]
- Schlichting C. D., Pigliucci M.1998Phenotypic evolution: a reaction norm perspective Sunderland, MA: Sinauer [Google Scholar]
- Schluter D.1993Adaptive radiation in sticklebacks: size, shape, and habitat use efficiency. Ecology 74, 699–709 (doi:10.2307/1940797) [Google Scholar]
- Schluter D.2000The ecology of adaptive radiation Oxford, UK: Oxford University Press [Google Scholar]
- Servedio M. R., Noor M. A. F.2003The role of reinforcement in speciation: theory and data. Annu. Rev. Ecol. Evol. Syst. 34, 339–364 (doi:10.1146/annurev.ecolsys.34.011802.132412) [Google Scholar]
- Simpson G. G.1944Tempo and mode in evolution New York, NY: Columbia University Press [Google Scholar]
- Simpson G. G.1953The major features of evolution New York, NY: Columbia University Press [Google Scholar]
- Skúlason S., Noakes D., Snorrason S. S.1989Ontogeny of trophic morphology in four sympatric morphs of arctic charr Salvelinus alpinus in Thingvallavatn, Iceland. Biol. J. Linn. Soc. 38, 281–301 (doi:10.1111/j.1095-8312.1989.tb01579.x) [Google Scholar]
- Skúlason S., Snorrason S. S., Jónsson B.1999Sympatric morphs, populations and speciation in freshwater fish with emphasis on arctic charr. In Evolution of biological diversity (eds Magurran A. E., May R. M.), pp. 70–92 Oxford, UK: Oxford University Press [Google Scholar]
- Smith T. B., Skúlason S.1996Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 27, 111–133 (doi:10.1146/annurev.ecolsys.27.1.111) [Google Scholar]
- Smith W. L., Chakrabarty P., Sparks J. S.2008Phylogeny, taxonomy, and evolution of Neotropical cichlids (Teleostei: Cichlidae: Cichlinae). Cladistics 24, 625–641 [Google Scholar]
- Stauffer J. R., Gray E. V.2004Phenotypic plasticity: its role in trophic radiation and explosive speciation in cichlids (Teleostei: Cichlidae). Anim. Biol. 54, 137–158 (doi:10.1163/1570756041445191) [Google Scholar]
- Stebbins R. C.2003A field guide to western reptiles and amphibians Boston, MA: Houghton Mifflin [Google Scholar]
- Trapani J.2003Morphological variability in the Cuatro Cienegas cichlid, Cichlasoma minckleyi. J. Fish Biol. 62, 276–298 (doi:10.1046/j.1095-8649.2003.00006.x) [Google Scholar]
- Turgeon J., Bernatchez L.2003Reticulate evolution and phenotypic diversity in North American ciscoes, Coregonus ssp. [sic] (Teleostei: Salmonidae): implications for the conservation of an evolutionary legacy. Conserv. Genet. 4, 67–81 (doi:10.1023/A:1021860910719) [Google Scholar]
- Waddington C. H.1953Genetic assimilation of an acquired character. Evolution 7, 118–126 (doi:10.2307/2405747) [Google Scholar]
- Walker J. A.1997Ecological morphology of lacustrine threespine stickleback Gasterosteus aculeatus L. (Gasterosteidae) body shape. Biol. J. Linn. Soc. 61, 3–50 [Google Scholar]
- Walls S. C., Belanger S. S., Blaustein A. R.1993Morphological variation in a larval salamander: dietary induction of plasticity in head shape. Oecologia 96, 162–168 (doi:10.1007/BF00317728) [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J.1989Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278 (doi:10.1146/annurev.es.20.110189.001341) [Google Scholar]
- West-Eberhard M. J.2003Developmental plasticity and evolution Oxford, UK: Oxford University Press [Google Scholar]
- West-Eberhard M. J.2005Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102, 6543–6549 (doi:10.1073/pnas.0501844102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens J. J., Titus T. A.1991A phylogenetic analysis of Spea (Anura: Pelobatidae). Herpetologica 47, 21–28 [Google Scholar]
- Wilson D. S.1989The diversification of single gene pools by density- and frequency-dependent selection. In Speciation and its consequences (eds Otte D., Endler J. A.), pp. 366–383 Sunderland, MA: Sinauer [Google Scholar]
- Wilson D. S., Turelli M.1986Stable underdominance and the evolutionary invasion of empty niches. Am. Nat. 127, 835–850 (doi:10.1086/284528) [Google Scholar]
- Wimberger P. H.1994Trophic polymorphisms, plasticity, and speciation in vertebrates. In Theory and application in fish feeding ecology (eds Stouder D. J., Fresh K. L., Feller R. J.), pp. 19–43 Columbia, SC: University of South Carolina Press [Google Scholar]
- Windig J. J., De Kovel C. G. F., De Jong G.2004Genetics and mechanics of plasticity. In Phenotypic plasticity (eds DeWitt T. J., Scheiner S. M.), pp. 31–49 Oxford, UK: Oxford University Press [Google Scholar]
- Wood C. C., Foote C. J.1996Evidence for sympatric genetic divergence of anadromous and nonanadromous morphs of sockeye salmon (Oncorhynchus nerka). Evolution 50, 1265–1279 (doi:10.2307/2410667) [DOI] [PubMed] [Google Scholar]
- Woodger C. D.1976Morphological variations as induced by environment in coregonids. Environ. Biol. Fishes 1, 101–105 (doi:10.1007/BF00761735) [Google Scholar]
- Wund M. A., Baker J. A., Clancy B., Golub J. L., Foster S. A.2008A test of the ‘flexible stem’ model of evolution: ancestral plasticity, genetic accommodation, and morphological divergence in the threespine stickleback radiation. Am. Nat. 172, 449–462 (doi:10.1086/590966) [DOI] [PubMed] [Google Scholar]