Abstract
Comparative genomics of representative basal metazoans leaves little doubt that the most recent common ancestor to all modern metazoans was morphogenetically complex. Here, we support this interpretation by demonstrating that the demosponge Amphimedon queenslandica has a biphasic pelagobenthic life cycle resembling that present in a wide range of bilaterians and anthozoan cnidarians. The A. queenslandica life cycle includes a compulsory planktonic larval phase that can end only once the larva develops competence to respond to benthic signals that induce settlement and metamorphosis. The temporal onset of competence varies between individuals as revealed by idiosyncratic responses to inductive cues. Thus, the biphasic life cycle with a dispersing larval phase of variable length appears to be a metazoan synapomorphy and may be viewed as an ancestral polyphenic trait. Larvae of a particular age that are subjected to an inductive cue either maintain the larval form or metamorphose into the post-larval/juvenile form. Variance in the development of competence dictates that only a subset of a larval cohort will settle and undergo metamorphosis at a given time, which in turn leads to variation in dispersal distance and in location of settlement. Population divergence and allopatric speciation are likely outcomes of this conserved developmental polyphenic trait.
Keywords: Amphimedon queenslandica, dispersal, larval competence, metamorphosis, pelagobenthic, Porifera
1. Introduction
Today's oceans teem with a huge diversity of microscopic animals, many of which represent the short and transient larval phase in the life cycles of larger and more recognizable benthic invertebrates. This pelagobenthic biphasic life cycle is the most widely distributed of all metazoan life cycles, existing in at least some species of most modern phyla. It is characterized by having a morphologically distinct microscopic planktonic larval form that settles and metamorphoses into a benthic juvenile that matures into a reproductive adult. Despite the diminutive nature of these zooplankters, their development and morphology have figured heavily in considerations of the evolution of the animal kingdom, and thus they have been a point of focus for zoologists and evolutionary biologists alike. Indeed, extrapolations from the comparison of extant larval forms have formed the foundation for a number of theories on metazoan evolution (reviewed in Valentine 2004; Minelli 2009).
Here, we explore the specific importance of larval settlement and metamorphosis in the evolution of metazoan life cycles. Among extant pelagobenthic species, intraspecific variation in response to benthic cues that induce settlement and metamorphosis is very widespread. Typically, at a given time post-fertilization, individuals within a cohort of larvae that are subjected to an inductive environmental cue either maintain their larval form (do not respond) or metamorphose into the benthic post-larval/juvenile form (do respond). This variation can be viewed as a polyphenism. Variation in settlement response has the potential to impact significantly on the dispersal capacity of a cohort because responsive individuals may settle nearer their source, while unresponsive plankters may continue to disperse, even to a distance that restricts future gene flow. Ultimately, this variation in time to settlement and metamorphosis may fuel population differentiation and speciation.
We posit that metamorphic polyphenism may have been critical in early metazoan evolution (§6). To support this position, we reconstruct key developmental and life-cycle features of the last common ancestor (LCA) to all living metazoans. This we do by combining new results about larval development and metamorphosis in the demosponge Amphimedon queenslandica (§3) with insights into the development of the metazoan LCA formulated through comparisons of recently sequenced basal metazoan genomes (§§4 and 5). First, we establish a context for consideration of these LCA traits by briefly reviewing the key features of the pelagobenthic life cycle based on observations of extant bilaterians (§2).
2. Initiation of metamorphosis in extant bilaterians: developmental plasticity
The capacity of metazoan larvae to disperse and successfully recruit into a breeding population depends on both pre- and post-settlement processes. In a wide range of bilaterians, it has been shown that pre-settlement dispersal is contingent upon (i) the precise timing of larval release or spawning, (ii) the morphological properties (e.g. size, shape and density) of the larva, (iii) larval swimming behaviour and ability, (iv) the minimal length of time in the plankton to develop the ability to respond to specific or general exogenous cues that induce settlement and metamorphosis, and (v) the distribution of benthic cues that induce settlement and metamorphosis (Sutherland 1990; Todd 1990; Rodriguez et al. 1993; Underwood & Keough 2001; Hadfield et al. 2001; Pechenik 2006). Settlement typically occurs when a larva which is developmentally competent to respond to environmental signals and initiate metamorphosis comes in contact with an inductive cue that is associated with an appropriate substratum (Pawlik 1992; Degnan & Morse 1995; Hadfield et al. 2001). Thus, both the extent of larval dispersal and the success of the critical ecological transition from the pelagial to the benthos are contingent upon the developmental state of the larva when it is delivered by oceanic processes to a potentially inductive environment.
The morphological, sensorial and behavioural characteristics of the larvae are generated by genomically encoded ontogenetic programmes operational during embryogenesis and larval development. For some species, the developmental capacity to settle and metamorphose occurs within hours of fertilization, while other species require a significantly longer period before competence develops (e.g. Hadfield & Strathmann 1996; Degnan et al. 1997; Todd et al. 1998; Jackson et al. 2005; Heyland & Moroz 2006; Williams et al. 2008). Typically, direct-developing and non-feeding lecithotrophic larvae have markedly shorter pre-competent larval development periods than do feeding planktotrophic larvae (Strathmann 1974). Further impacting on dispersal capacity, direct-developing species tend to brood their embryos internally, while planktotroph development typically occurs externally. These differences are reflected often, but not always, in the genetic structure of adult populations, with planktotrophs tending to show less population genetic structure indicative of increased gene flow (e.g. Arndt & Smith 1998; Ayre & Hughes 2000; Kyle & Boulding 2000; Breton et al. 2003; Imron et al. 2007).
The factors that regulate the acquisition of competence have been difficult to identify. Studies in the vetigastropod Haliotis rufescens suggest that competence develops when a threshold concentration of chemosensory receptors accumulates in the larva (Trapido-Rosenthal & Morse 1986a,b), and transcriptional analysis in H. asinina reveals extensive and diverse gene activity associated with the endogenous attainment of competence (Williams et al. 2009). In the ascidian Herdmania curvata, the expression of Hemps, a gene encoding an epidermal growth-factor-like signalling protein, is correlated with the development of competence and has been shown to directly control settlement and metamorphosis (Eri et al. 1999; Woods et al. 2004).
Laboratory-based observations of a wide range of marine invertebrate larvae have revealed substantial intraspecific variation in the age at which competence develops (e.g. Hadfield 1977; Pechenik & Heyman 1987; Degnan et al. 1997; Pechenik & Qian 1998; Jackson et al. 2005; Williams et al. 2008). At a given age post-fertilization, individual larvae within a cohort do not respond to an inductive metamorphic cue in a stereotypic manner, suggesting that there is individual variation in the rate of development of metamorphic competence. A logical consequence of this natural variation is that a single cohort of larvae can disperse over a wide range of distances, which in turn impacts on the ecology and evolution of the species (Strathmann 1974; Pechenik & Heyman 1987; Raimondi & Keough 1990; Pechenik & Gee 1993; Hadfield & Strathmann 1996; Pechenik & Qian 1998). By conducting selective breeding experiments, Hadfield (1984) demonstrated that even after 27 generations of intense selection for the trait of early development of competence in the tropical nudibranch Phestilla sibogae, no correlation between generation and time to acquisition of competence could be statistically detected. This suggests that competence in this species is either influenced epigenetically or that there are specific genetic mechanisms in place to ensure that this variation is maintained from generation to generation. The consistent observation, across protostome and deuterostome clades, of variation in time to acquiring competence supports the notion that there exists a deeply homologous mechanism to maintain genetic variance at the controlling loci.
In addition to extensive intraspecific variation in the temporal onset of metamorphic competence, there can be differences in preference to particular benthic settlement cues. The specificity and distribution of these external inductive cues is crucial in determining spatial and temporal patterns of marine invertebrate population structure and evolution (Rodriguez et al. 1993; Underwood & Keough 2001). Intraspecific variation in response to inductive cues must in turn play a crucial role in determining the capacity for species range shifting, local adaptation and population differentiation (Toonen & Pawlik 2001). This is illustrated by the recent analysis of the response of H. asinina larvae to a range of coralline algae, which are known to occupy a wide range of habitats (Williams et al. 2008). The differential responses of individuals and family lines of H. asinina larvae to different species of coralline algae further enable the segregation of subgroups to different benthic locations (Williams & Degnan 2009).
3. Larval development, competence and settlement in the haplosclerid demosponge a. queenslandica
Sponges are one of the oldest, if not the oldest, of the contemporary phyletic metazoan lineages. Small fossil sponges have been reported from the Doushantuo Formation in Guizhou Province, China, some 580–600 Ma (Li et al. 1998), and demosponge fossil steroids date back at least 100 Myr before the Cambrian explosion (Love et al. 2009). Despite their basal position within the animal kingdom, and the apparent simplicity of their body plan, sponges have a strong affinity with their more complex metazoan relatives, in terms of both their genome and their life cycle (Adell & Müller 2005; Larroux et al. 2006, 2007, 2008; Nichols et al. 2006; Adamska et al. 2007a,b; Sakarya et al. 2007; Simionato et al. 2007; Fahey et al. 2008; Gauthier & Degnan 2008; Gazave et al. 2008; Grimson et al. 2008; Richards et al. 2008; Lapébie et al. 2009). The sponge life cycle progresses through embryonic, planktonic larval, benthic juvenile and mature adult phases, just as in marine representatives of most phyla (Leys 2004; Leys & Ereskovsky 2006; Maldonado 2006).
Amphimedon queenslandica is a viviparous demosponge that releases ciliated parenchymella larvae from internal brood chambers (Degnan et al. 2009). These larvae comprise a large number of different cell types that are organized into three discrete layers—an outer epithelial layer, a middle subepithelial cell layer and an inner cell mass—and are patterned along the larval anterior–posterior swimming axis (Leys & Degnan 2001; Degnan et al. 2005, 2009; Adamska et al. 2007a). Like many other parenchymella larvae, A. queenslandica larvae have a photosensory system at their posterior end that consists of a set of ciliated sensory and pigment cells organized into a ring. Although these cells do not show a neuronally coordinated behaviour, they all do respond to light in a stereotypic manner, such that the resultant effect is a rudder-like structure that directs movement of the larvae away from areas of higher light (Leys & Degnan 2001; Leys et al. 2002). This photosensory system appears to become non-functional 12–24 h after emergence in most individuals, indicating that it is ontogenetically regulated (Leys & Degnan 2001).
The surface of A. queenslandica larvae also appears to include both chemosensory and mechanosensory cells that contribute to the detection of an appropriate settlement location (Leys & Degnan 2001, 2002). In particular, larval globular cells (originally annotated as mucous cells) and flask cells are interspersed among the columnar epithelial cells, and have morphological (Leys & Degnan 2001) and molecular (Sakarya et al. 2007; Richards et al. 2008) characteristics strongly suggestive of a role in sensing the environment.
(a). Amphimedon queenslandica larvae are not competent to settle and metamorphose upon release from the brood chamber
We have shown previously that A. queenslandica larvae differentially respond to an undefined cue associated with pieces of coral rubble containing coralline algae from the same habitat as the adult (i.e. inner reef flat) (Jackson et al. 2002). Using a laboratory-based assay system, with larvae spawned from adults directly collected from the wild, here we sought to determine whether the larva's ability to respond to this inductive cue has an ontogenetic basis. Specifically, we experimentally determined whether or not larvae require further development after emerging from the adult to acquire competence to settle and initiate metamorphosis. To do this, we placed larvae of different ages (i.e. hours post-emergence (hpe)) in sterile wells containing 0.2 µm filtered sea water (FSW), with and without small shards of crustose coralline algae (CCA) rubble. The proportion of larvae that had settled and were metamorphosing was assessed either 4 or 24 h later (see figure 1 for details on the methods).
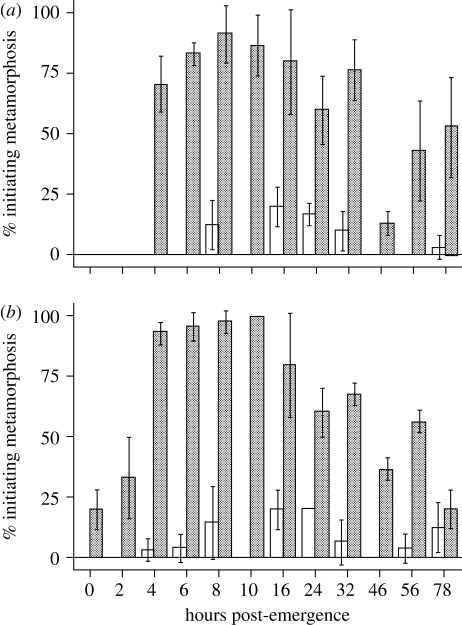
Figure 1.
Acquisition of competence in A. queenslandica larvae. Larvae were either introduced to inductive CCA rubble (stippled bars) or placed in FSW (white bars) at different times after emerging from the adult sponge (x-axis) and the percentage initiating metamorphosis was scored after (a) 4 h and (b) 24 h. Error bars are one standard deviation of the mean. Times post-emergence represent the minimal time emerged over a 2 h window (e.g. 3 h = 3–5 hpe). Methods: A. queenslandica larvae were collected over a period of 2 h from wild adults obtained from the field an hour before initiation of the experiment, and maintained in ambient sea water as described in Leys et al. (2008). All settlement assays were performed at 24°C in six-well 35 mm diameter sterile polycarbonate tissue culture dishes, with 10 ml of FSW per well. For induction experiments, six sets (replicates) of 10 larvae were incubated continuously in wells containing either 10 ml FSW or 10 ml FSW with CCA shards covering approximately 25% of the bottom of the well. CCA shards were chipped off the surfaces of coral rubble pieces collected from the same habitat as A. queenslandica just prior to experimentation, washed and placed in the wells for immediate use. We assayed different aged swimming larvae, spanning 0–78 hpe from the adult. Experiments used larvae that were collected over a 2 h period. For example, larvae termed 0 hpe are from a pool of individuals that together represent 0–2 hpe. After the addition of larvae to both control wells and CCA rubble-containing wells, dishes were incubated in the dark for either 4 or 24 h. Separate cohorts of larvae were used to score rates of metamorphosis at each of these times. At the conclusion of the experiment, each well was scanned to record the number of larvae that had initiated metamorphosis. This was defined by the larval anterior being clearly attached to the substratum, the primary larval axis (anterior–posterior axis) being compressed and the larval pigment ring being resorbed (Leys & Degnan 2002).
Larvae reared in FSW alone displayed very low rates of metamorphosis, regardless of age or length of time reared in these artificial conditions (figure 1). By contrast, the presence of the CCA rubble induced high rates of metamorphosis, with a majority of larvae older than 4–6 hpe initiating metamorphosis within 4 h of coming into contact with the inducer (figure 1a). Strikingly, there was not a single larva younger than 4 hpe that showed signs of initiating metamorphosis when the experiment was scored just 4 h later. This indicates that, under these experimental conditions, A. queenslandica larvae required at least 4 h of further development outside of the adult before they acquire the ability to metamorphose. That is, these larvae do not attain competence until at least 4 h after emergence.
Once competent, most, but not all, larvae initiated metamorphosis within the first 4 h of coming into contact with the inductive cue associated with the CCA rubble, although the percentage metamorphosing generally increased after longer (24 h) exposure to CCA rubble (figure 1). Some newly emerged larvae (i.e. 0–2 and 2–4 hpe) began metamorphosing when they were exposed to the CCA rubble for 24 h. However, rates were clearly greater in older larvae, with nearly all 4–6 to 10–12 hpe initiating metamorphosis within 24 h (figure 1b). Indeed, the percentage metamorphosis in 0–2 and 2–4 hpe after 24 h was still substantially lower than in the 4–6 to 32–34 hpe larvae after just 4 h of exposure to the CCA rubble (figure 1). These data indicate that exposure to CCA rubble prior to the acquisition of competence (approx. 4–6 hpe) has the effect of inhibiting larvae from developing competence.
(b). Amphimedon queenslandica larvae do not only swim to darker areas or to the bottom
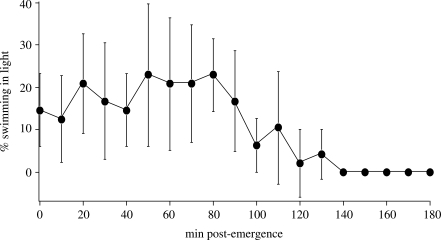
Previous studies have shown that A. queenslandica larvae are negatively phototactic immediately upon emerging from the adult, although they lose this response between 12 and 24 hpe (Leys & Degnan 2001; Leys et al. 2002). However, we have observed anecdotally on numerous occasions that newly emerged larvae swim towards both the dark bottom and the lighter top of the water column. To assess whether newly emerged larvae are strictly negatively phototactic, we observed the swimming behaviour of larvae—collected over a 1 h period—in a stagnant cylinder of FSW, in which we gave larvae the choice of swimming in the bottom shaded half or the top half exposed to natural midday sunlight. We found that for the first 90 min post-emergence (mpe), there were always 10–20% of the larvae swimming in the upper bright-light portion of the water column (figure 2). From 110 to 130 mpe, this proportion reduced, but still there were always 2–10% of larvae that remained out of the shaded area. After 140 mpe, we no longer observed any larvae in the bright upper portion of the water column, indicating that all larvae were concentrating near the shaded bottom. Although this experiment does not discriminate between geotactic or phototactic abilities of A. queenslandica, it does reveal that over the first 2 h of emerging from the adult, some larvae swim from the dark bottom up into the water column. Regardless of the underlying cellular basis to this behaviour, the upward swimming of A. queenslandica larvae should increase dispersal range.
Figure 2.
Swimming behaviour of A. queenslandica larvae after emerging from the adult. Larvae were given the choice of swimming in the top half of a cylinder exposed to direct sunlight or the shaded bottom half. The percentage of larvae swimming in the light is shown. Error bars are 1 s.d. of the mean. Times post-emergence represent the minimal time emerged over a 60 min window (e.g. 10 min = 10–70 mpe). Methods: Newly emerged larvae were collected for 1 h, washed and immediately transferred into 500 ml glass graduated cylinders filled with 500 ml of FSW that were placed in midday sunlight. The lower half of each cylinder (up to the 250 ml mark) was covered with an inner layer of matt white paper and an outer layer of aluminium foil, to simulate a low-light environment. Twenty larvae were placed in each cylinder and there were 12 replicates. The number of larvae visible in the top (exposed to light) half of the cylinder was scored every 10 min until the experiment ended at 180 mpe.
(c). The A. queenslandica biphasic life cycle
Sponges are known to reproduce both sexually and asexually, but there is limited understanding of how potential recruits colonize the benthos and how recruitment influences population structure. Sexual reproduction in poriferans is generally part of a pelagobenthic life cycle, which includes a miniscule mobile larva that settles and metamorphoses into a juvenile form, which in turn grows and matures into a reproductive adult (reviewed in Maldonado 2006). Morphologically, the larval form differs markedly within and between the different classes of sponges (reviewed in Leys & Ereskovsky 2006; Maldonado 2006), yet most appear to function in dispersal and site selection for settlement.
As described above, A. queenslandica larvae require at least 4 h in the plankton before they are competent to respond to an inductive cue associated with CCA, and subsequently settle and initiate metamorphosis (figures 1 and 3). Larvae that come in contact with this CCA-associated cue before developing competence appear to be desensitized to CCA exposure, with only a low percentage of these larvae initiating metamorphosis even after being exposed to the cue for 24 h. Gastropod and ascidian larvae exposed to natural and artificial inducers before developing competence also become desensitized (Hadfield 1978; Trapido-Rosenthal & Morse 1986a; Avila et al. 1996; Degnan et al. 1997). The low settlement and metamorphosis rates of pre-competent marine larvae exposed to an inducer is compatible with the internalization of surface receptors of sensory cells, which prevents the propagation of an inductive morphogenetic signal (Trapido-Rosenthal & Morse 1986a; Woodman 2009). Regardless of the mechanism, A. queenslandica larvae that come in contact with the CCA-associated cue within the first few hours of emerging from its mother are less likely to recruit locally than individuals that do not.
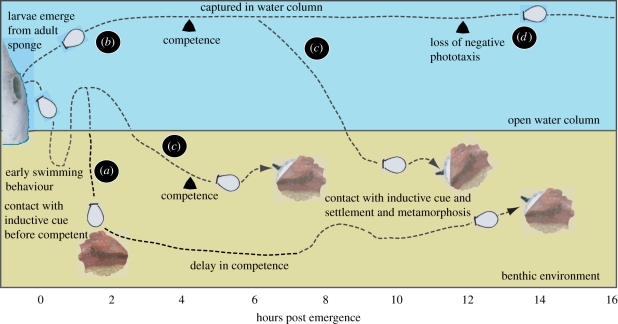
Figure 3.
Possible fates of A. queenslandica larvae after emerging from the adult. Adults live in still, shallow reef flat environments consisting of stretches of fine grain sand and patchy stands of decaying dead coral boulders that provide shady habitats for coralline algae, sponges and a wide range of microbes, animals and algae; these habitats are depicted as red algae patches in this figure. (a,c) For approximately 2 h, larvae move between the water column and bottom, (b) although some newly emerged larvae may get caught in currents and remain in the water column. (a) Some larvae may come in contact with inductive cues prior to developing competence. These will not settle at that time and need further development beyond the normal 4 h period to become competent to settle and metamorphose (depicted as juvenile sponge on red algal patch). (c) After about 4 h in the water column, some larvae are competent to settle and metamorphose as long as they have not come in prior contact with the inductive cue. (d) Larvae that are trapped in the water column lose the ability to sense light after approximately 12 h. Although these older larvae are still able to settle and metamorphose, they may be less likely to swim into a suitable low-light location.
There are a number of cell types on the A. queenslandica larval surface that may be involved in sensing the external environment, and coordinating settlement and metamorphosis (Jackson et al. 2002; Leys & Degnan 2002). Candidate larval chemo- or mechanosensory cells include (i) large cuboidal cells that are located only at the anterior end, which is also the site of attachment at settlement, (ii) flask cells, which are intercalated in the epithelial columnar cells and are restricted to the anterior third of the larva, and (iii) the globular cells, which are also intercalated in the epithelial columnar cells but are found throughout the larva except at the anterior-most end where the cuboidal cells are located. Of these, there is mounting evidence that the globular cells are probably playing a sensory role and contributing to the regulation of metamorphosis. First, genes encoding post-synaptic structural proteins and neurogenic circuit signalling proteins and transcription factors are expressed in these cells, suggesting an evolutionary link with eumetazoan neurons (Sakarya et al. 2007; Richards et al. 2008). Second, nitric oxide synthase (NOS) is also expressed in these cells. Induction of metamorphosis by CCA is inhibited when larvae are cultured in the presence of a competitive inhibitor of NOS, l-nitroarginine methyl ester (Kranz & Degnan unpublished data). We have often observed A. queenslandica larvae ‘probing’ the settlement surface by lying sideways such that the globular cells are in contact with the surface, and spinning rapidly and repeatedly on their anterior–posterior axis. This behaviour is frequently observed prior to attaching with the anterior end. We conclude that, as is the case in other metazoan larvae, A. queenslandica larvae are populated with sensory cells that allow them to respond to exogenous abiotic and biotic signals in a manner that ensures settlement occurs in habitats that allow for effective recruitment and eventual reproduction.
4. Gastrulation, modularity and the evolution of the pelagobenthic life cycle
Phylogenomic analyses of the deepest branches of the metazoan tree have yet to achieve a consensus on the order of divergence (e.g. Putnam et al. 2007; Dunn et al. 2008; Srivastava et al. 2008; Philippe et al. 2009; Schierwater et al. 2009). While resolving this polytomy will certainly allow us to more confidently assign particular events in early metazoan evolution to specific branches, at this stage we can still infer some general and important aspects of the LCA to all modern Metazoa. The recent sequencing of representative basal metazoan genomes (Putnam et al. 2007; Srivastava et al. 2008) has convincingly shown that the LCA to all modern metazoans was morphogenetically complex and possessed essentially all the gene families that populate gene-regulatory networks underpinning the development of complex bilaterians. Analysis of the choanoflagellate Monosiga brevicollis genome (King et al. 2008) reveals that many of these transcription factor and signalling pathway gene families are innovations that emerged in stem metazoans, after diverging from the holozoan LCA but prior to cladogenesis of extant animal taxa (Larroux et al. 2006, 2008; Adamska et al. 2007a). The developmental expression of many transcription factors and signalling pathway genes during sponge, ctenophore and cnidarian embryogenesis is consistent with these gene families having a regulatory role in the development of the first metazoans (e.g. Adamska et al. 2007a,b; Matus et al. 2007; Mazza et al. 2007; Yamada et al. 2007; Fahey et al. 2008; Gauthier & Degnan 2008; Jager et al. 2008; Pang & Martindale 2008; Rentzsch et al. 2008). As we have proposed previously (Degnan & Degnan 2006), this shared-developmental ancestry reflects the morphogenetic and, potentially, the morphological complexity of the metazoan LCA.
In Degnan & Degnan (2006), we note, as many others also have, that sexual reproduction via the fusion of meiotically derived eggs and sperm predates metazoan cladogenesis. In all multi-cellular animals, fertilization is followed by cleavage and gastrulation to generate a modular, multi-layered embryo (Leys 2004). In proto-metazoans, this embryo can itself be considered as the precursor to a biphasic life cycle wherein the embryo represents one phase and the adult another phase (i.e. at some point in its ontogeny, the embryo separates from the adult). Under this scenario, the first biphasic life cycles evolved from one in which the embryo develops directly into the adult but does not necessarily yet have morphological adaptations for a pelagic existence.
Once adult and embryo are separated, selection will act differentially on these two phases, given their morphological and ecological differences. If the adult is benthic—a point of debate, but one which we support (Degnan & Degnan 2006)—then the release of embryos can also create habitat differences (in addition to size differences), with the embryo spending a period up in the water column. Because these original embryos consist of multiple layers, as a consequence of gastrulation, they have the capacity to develop a wide range of morphologies and functions. The multiple cell layers of the gastrula create a simple modular organism in which the different layers operate in a semiautonomous manner. The superimposition of axial polarity and cell patterning mechanisms on this embryo allows for further modularity and evolvability, localizing specific cell types into contiguous tissues. Given there is evidence for axial polarity in sponges (Degnan et al. 2005; Adamska et al. 2007a), it can be inferred that this is a feature of the development of the metazoan LCA.
The progressive increase in overall cellular complexity during development, with an expansion in the number of cell types and the localization of these into different cell layers and territories, allows new modules, such as localized patterns of ciliation, to evolve in the later embryo (Degnan & Degnan 2006). These novelties, intercalated into the direct life cycle, would lead to what is recognized as a larval form that includes localized sensory systems that allow for selection of a suitable settlement habitat, such as the aneural photosensors present in extant sponges (Maldonado 2006). Natural selection on these systems is particularly strong, as any individual who settles away from congeners is not likely to contribute to future generations. The existence of sophisticated sensory systems in all extant metazoan larvae is compatible with the metazoan LCA having a pelagobenthic life cycle with a larva with a well-developed sensory system.
The nature of metazoan development, with its ontogenetic increase in complexity and modularity, should lead to the highest diversity of phenotypes at the latest stages of development, as observed in extant animals. Among evolutionary developmental processes, the heterochronic shift of embryonic, larval, juvenile and adult developmental programmes relative to each other contributes to the diversification of ontologies and phenotypes (e.g. Wray & Lowe 2000; Sly et al. 2003; Byrne 2006).
5. Plasticity in the acquisition of competence: a conserved metazoan trait?
In this section, we restrict our analysis to the metazoan LCA, recognizing the caveats associated with the polytomy at the base of the metazoan tree. Regardless of the order of ancient metazoan lineages—sponges, ctenophores, placozoans, cnidarians and bilaterians—it is now clear that the metazoan LCA was morphogenetically complex. The consistent expression of metazoan-specific transcription factors and signalling pathway genes in different cells and at different times in the development of extant sponges (e.g. Adamska et al. 2007a; Fahey et al. 2008; Gauthier & Degnan 2008), ctenophores (e.g. Yamada et al. 2007; Jager et al. 2008; Pang & Martindale 2008) and cnidarians (e.g. Matus et al. 2007; Mazza et al. 2007; Rentzsch et al. 2008) indicates that the cis-regulatory systems for these gene-regulatory network components also existed in the LCA, enabling expression, and presumably function, in multiple life-cycle contexts. Given the remarkable similarity in gene content, gene structure and genome organization between complex bilaterians and basal metazoans (Putnam et al. 2007; Larroux et al. 2008; Srivastava et al. 2008), the existence of gene-expression pleiotropy in protometazoans and crown metazoans hardly seems surprising, even if the extent of this pleiotropy may differ in these ancient lineages (Fahey et al. 2008).
The ancestral modular nature of gene cis-regulatory systems allows for the co-option of regulatory genes into new developmental contexts and the exaptation of existing networks into new roles. Thus, it appears that the metazoan LCA genome harboured the necessary regulatory information to specify and pattern multiple life-cycle forms under natural selection. Based on these observations and the points raised in §4, the metazoan LCA probably had a biphasic life cycle, which evolved from a life cycle where embryonic, larval and adult stages were blurred. A pelagobenthic life cycle in the metazoan LCA is the most parsimonious based on comparison of extant taxa, although a holopelagic life cycle could have been ancestral (see Degnan & Degnan 2006 for further discussion). Regardless, the modular nature of cis-regulatory systems enabled gene pleiotropy, which underpinned morphological innovation within the developing, modular body plan.
How much of the metazoan LCA life cycle has been conserved in modern taxa is unknown, particularly with respect to metamorphosis. There is currently little evidence for phylogenetically consistent expression of a specific gene or gene family at metamorphosis, although genes related to stress response, immunity and apoptosis (Davidson & Swalla 2002; Woods et al. 2004; Heyland & Moroz 2006; Williams et al. 2009) and to calcium ion flux (e.g. Amador-Cano et al. 2006) are associated with metamorphosis in a handful of taxa studied to date. Further, conserved signalling pathways have been implicated in regulating metamorphosis in particular species and groups (e.g. Eri et al. 1999; Bishop & Brandhorst 2003; Bishop et al. 2008; Rentzsch et al. 2008). Nonetheless, this lack of explicit and widespread conservation has led some authors to propose that metamorphosis is a convergent trait (Hadfield 2000; Hadfield et al. 2001). Although there are clear cases of metamorphosis being derived (e.g. in chordates and arthropods), the pelagobenthic life cycle appears to be homologous in other phyla, with conserved traits being evident in the less-evolvable earlier stages. The link between the cnidarian planula larva and bilaterians is evident (Gröger & Schmid 2001; Hejnol & Martindale 2008; Rentzsch et al. 2008; Marlow et al. 2009), and morphological and gene-expression data from sponge larvae suggest a deep metazoan conservation (reviewed in Maldonado 2006; see also Adamska et al. 2007a,b; Sakarya et al. 2007; Richards et al. 2008). If the bulk of the modern pelagobenthic life cycles evolved from the one that existed prior to metazoan cladogenesis, then some common traits in extant larvae (e.g. ciliation, patterned sensory cell types and systems) may be homologous.
As we present here, another common feature of modern larvae is a high level of variance between individuals in terms of timing of competence. This developmental variance can be observed in a wide range of bilaterians (e.g. Hadfield 1984; Degnan et al. 1997), cnidarians (e.g. Morse & Morse 1991; Rentzsch et al. 2008) and sponges (e.g. Ettinger-Epstein et al. 2008; this study). Thus, an ancient and conserved feature of the metazoan genome is the maintenance of developmental rate variance through the generational preservation of polymorphisms in loci controlling the acquisition of competence and thus the timing of metamorphosis; there is no reason to exclude the possibility that key polymorphic genes are expressed early in embryonic development or even during oogenesis.
If variance in the timing of the acquisition of metamorphic competence is fuelled by variation in genes expressed in larvae, there are two likely ways in which this variation can be manifested: in coding sequences or in regulatory sequences. The former results in polymorphic factors that have different affinities for exogenous external cues or intrinsic signalling partners, and the latter results in genes with variable expression levels. Currently, there is stronger evidence that variation in the level of gene expression may be the underlying agent in the disparity in age to competence within a species (e.g. Williams & Degnan 2009; Williams et al. 2009). Consistent with this assertion is the observation that a wide range of metazoan larvae, including molluscs, polychaetes, ascidians and sponges (Hadfield 1984; Trapido-Rosenthal & Morse 1986a; Degnan et al. 1997; this study), habituate to an inductive cue when exposed to it prior to developing competence. The removal of critical chemosensory receptors from larval sensory cell surfaces prior to the onset of competence (and deployment of the requisite signalling pathways) may underlie the habituation of larvae (Trapido-Rosenthal & Morse 1986a). The early removal and possible degradation of these chemosensory receptors (Woodman 2009) prior to competency may then extend the period of development to obtain the threshold concentration required to elicit a response to an inductive cue. These observations, along with the high degree of variance in larval response to cues, are compatible with the acquisition of competence being directly related to gene-expression levels; certainly, there is evidence that substantial transcriptional activity is associated with attainment of competency in the abalone H. asinina (Williams et al. 2009). Candidate chemosensory receptors that may be involved in the acquisition of metamorphic competence may be in the rhodopsin-like family of G-protein-coupled receptors (GPCRs) (Trapido-Rosenthal & Morse 1986a; Raible et al. 2006), which are known to play a role in chemoreception in a wide array of animals (e.g. Glusman et al. 2001; Raible et al. 2006) and evolve rapidly to yield species-specific repertoires (Nei et al. 2008). The A. queenslandica genome encodes over 100 rhodopsin-like GPCR genes that are organized in the genome in a similar manner to bilaterians (B. Degnan et al. 2009, unpublished data). This raises the possibility that the development of competence can be regulated by the expression of GPCRs in metazoans with conserved pelagobenthic life cycles and thus is an ancestral feature of crown metazoans.
6. Initiation of metamorphosis in ancient metazoans: a polyphenic trait?
Intraspecific variation in the onset of metamorphic competence can be viewed as a means to increase dispersal potential and a form of bet-hedging, allowing conspecific larvae to settle in a variety of habitats that have inductive benthic cues. As illustrated in a number of haliotid gastropods, not only do individuals vary in the age they become competent to respond to a particular benthic coralline alga, but they also vary in their responsiveness to different species of coralline algae at a particular age (Williams et al. 2008). These observations, combined with the differential responsiveness of family lines to different algae (Williams et al. 2008), support the notion that there is a genetic component to this plasticity. In modern taxa, this variance provides a means for a cohort of larvae produced from a particular spawning event to expand its dispersal, with successful recruitment contingent upon larval behaviour and density, micro- and macroscale ocean currents and the distribution of benthic inductive cues. The intrinsic variation in larval development and external variation in environmental conditions can lead to population segregation and divergence, which in turn has the potential to even lead to speciation.
Elsewhere (Degnan & Degnan 2006), we reiterate the proposition raised by Jägersten (1972) and others that the pelagobenthic life cycle is an ancestral feature of the Metazoa. Here, we expand our description of the LCA life cycle to include a larval phase that is variable in length, with individuals developing the competence to settle and metamorphose at different ages. This essentially means that the mechanisms operating to facilitate population differentiation and speciation in modern oceans were present at the origin of crown metazoans. Plasticity in the timing of competence can be viewed as a polyphenic trait. Larvae of a particular age either (i) respond to an environmental signal, and settle and metamorphose into the juvenile/adult, dramatically changing their body plan and ecology, (ii) are refractory to the signal, remaining as pre-competent larvae, or (iii) habituate to the signal so as to have a protracted pre-competence period. Thus, the variance in the timing of the onset of metamorphic competence dictates that only a subset of the population will settle and undergo metamorphosis at a given time.
The emergence of heritable variance of metamorphic competence requires a system that maintains polymorphisms at a critical locus or loci in a manner that is akin to that used to maintain individual variation at histocompatibility and immunity loci, as described in chordates, molluscs and sponges (e.g. Fernàndez-Busquets & Burger 1997; Zhang et al. 2004; De Tomaso et al. 2005). Based on the habituation of modern pre-competent marine larvae to inductive cues (Hadfield 1984; Trapido-Rosenthal & Morse 1986a; Degnan et al. 1997; this study), we propose that the polymorphisms in loci underlying metamorphic competence in the metazoan LCA resided in the cis-regulatory regions responsible for controlling the expression of specific genes. While it is unclear how natural selection maintains this particular polymorphic region of the genome, the variance in metamorphic competence in a wide range of extant metazoans argues for its existence in their last shared ancestor.
Acknowledgements
This research was supported by grants for the Australian Research Council to S.M.D. and B.M.D.
Footnotes
One contribution of 12 to a Theme Issue ‘From polyphenism to complex metazoan life cycles’.
REFERENCES
- Adamska M., Degnan S. M., Green K. M., Adamski M., Craigie A., Larroux C., Degnan B. M.2007aWNT and TGF-β expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning. PLoS One 2, e1031 (doi:10.1371/journal.pone.0001031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamska M., Matus D. Q., Adamski M., Green K. M., Rokhsar D. S., Martindale M. Q., Degnan B. M.2007bThe evolutionary origin of hedgehog proteins. Curr. Biol. 17, R836–R837 (doi:10.1016/j.cub.2007.08.010) [DOI] [PubMed] [Google Scholar]
- Adell T., Müller W. E.2005Expression pattern of the Brachyury and Tbx2 homologues from the sponge Suberites domuncula. Biol. Cell 97, 641–650 (doi:10.1042/BC20040135) [DOI] [PubMed] [Google Scholar]
- Amador-Cano G., Carpizo-Ituarte E., Cristino-Jorge D.2006Role of protein kinase C, G-protein coupled receptors, and calcium flux during metamorphosis of the sea urchin Strongylocentrotus purpuratus. Biol. Bull. 210, 121–131 (doi:10.2307/4134601) [DOI] [PubMed] [Google Scholar]
- Arndt A., Smith M. J.1998Genetic diversity and population structure in two species of sea cucumber: differing patterns according to mode of development. Mol. Ecol. 7, 1053–1064 (doi:10.1046/j.1365-294x.1998.00429.x) [Google Scholar]
- Avila C., Tamse C. T., Kuzirian A. M.1996Induction of metamorphosis in Hermissenda crassicornis larvae (Mollusca: Nudibranchia) by GABA, choline and serotonin. Invert. Reprod. Dev. 29, 127–141 [Google Scholar]
- Ayre D. J., Hughes T. P.2000Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54, 1590–1605 [DOI] [PubMed] [Google Scholar]
- Bishop C. D., Brandhorst B. P.2003On nitric oxide signaling, metamorphosis, and the evolution of biphasic life cycles. Evol. Dev. 5, 542–550 (doi:10.1046/j.1525-142X.2003.03059.x) [DOI] [PubMed] [Google Scholar]
- Bishop C. D., Pires A., Norby S. W., Boudko D., Moroz L. L., Hadfield M. G.2008Analysis of nitric oxide-cyclic guanosine monophosphate signaling during metamorphosis of the nudibranch Phestilla sibogae Bergh (Gastropoda: Opisthobranchia). Evol. Dev. 10, 288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S., Dufresne F., Desrosiers G., Blier P. U.2003Population structure of two northern hemisphere polychaetes, Neanthes virens and Hediste diversicolor (Nereididae), with different life-history traits. Mar. Biol. 142, 707–715 [Google Scholar]
- Byrne M.2006Life history diversity and evolution in the Asterinidae. Integr. Comp. Biol. 46, 243–254 (doi:10.1093/icb/icj033) [DOI] [PubMed] [Google Scholar]
- Davidson B., Swalla B. J.2002A molecular analysis of ascidian metamorphosis reveals activation of an innate immune response. Development 129, 4739–4751 [DOI] [PubMed] [Google Scholar]
- Degnan B. M., Morse D. E.1995Developmental and morphogenetic gene regulation in Haliotis rufescens larvae at metamorphosis. Am. Zool. 35, 391–398 [Google Scholar]
- Degnan B. M., Souter D., Degnan S. M., Long S. C.1997Induction of metamorphosis in larvae of the ascidian Herdmania momus with potassium ions requires attainment of competence and an anterior signalling center. Dev. Genes Evol. 206, 370–376 (doi:10.1007/s004270050066) [DOI] [PubMed] [Google Scholar]
- Degnan B. M., Leys S. P., Larroux C.2005Sponge development and antiquity of animal pattern formation. Integr. Comp. Biol. 45, 335–341 (doi:10.1093/icb/45.2.335) [DOI] [PubMed] [Google Scholar]
- Degnan B. M., et al. 2009The demosponge Amphimedon queenslandica: reconstructing the ancestral metazoan genome and deciphering the origin of animal multicellularity. In Emerging model organisms: a laboratory manual, vol. 1, pp. 139–166 New York, NY: Cold Spring Harbor Laboratory Press; [DOI] [PubMed] [Google Scholar]
- Degnan S. M., Degnan B. M.2006The origin of the pelagobenthic metazoan life cycle: what's sex got to do with it? Integr. Comp. Biol. 46, 683–690 (doi:10.1093/icb/icl028) [DOI] [PubMed] [Google Scholar]
- De Tomaso A. W., Nyholm S. V., Palmeri K. J., Ishizuka K. J., Ludington W. B., Mitchel K., Weissman I. L.2005Isolation and characterization of a protochordate histocompatibility locus. Nature 438, 454–459 (doi:10.1038/nature04150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C. W., et al. 2008Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 (doi:10.1038/nature06614) [DOI] [PubMed] [Google Scholar]
- Eri R., Arnold J. M., Hinman V. F., Green K. M., Jones M. K., Degnan B. M., Lavin M. F.1999Hemps, a novel EGF-like protein, plays a central role in ascidian metamorphosis. Development 126, 5809–5818 [DOI] [PubMed] [Google Scholar]
- Ettinger-Epstein P., Whalan S., Battershill C. N., de Nys R.2008A hierarchy of settlement cues influences larval behaviour in a coral reef sponge. Mar. Ecol. Prog. Ser. 365, 103–113 (doi:10.3354/meps07503) [Google Scholar]
- Fahey B., Larroux C., Woodcroft B., Degnan B. M.2008Does the high gene density in the sponge NK homeobox gene cluster reflect limited regulatory capacity? Biol. Bull. 214, 205–217 [DOI] [PubMed] [Google Scholar]
- Fernàndez-Busquets X., Burger M. M.1997The main protein of the aggregation factor responsible for species-specific cell adhesion in the marine sponge Microciona prolifera is highly polymorphic. J. Biol. Chem. 272, 27 839–27 847 (doi:10.1074/jbc.272.44.27839) [DOI] [PubMed] [Google Scholar]
- Gauthier M., Degnan B. M.2008The transcription factor NF-κB in the sponge Amphimedon queenslandica: insights into the evolutionary origin of the Rel homology domain. Dev. Genes Evol. 218, 23–32 (doi:10.1007/s00427-007-0197-5) [DOI] [PubMed] [Google Scholar]
- Gazave E., Lapébie P., Renard E., Bézac C., Boury-Esnault N., Vacelet J., Pérez T., Manuel M., Borchiellini C.2008NK homeobox genes with choanocyte-specific expression in homoscleromorph sponges. Dev. Genes Evol. 218, 479–489 (doi:10.1007/s00427-008-0242-z) [DOI] [PubMed] [Google Scholar]
- Glusman G., Yanai I., Rubin I., Lancet D.2001The complete human olfactory subgenome. Genome Res. 11, 685–702 (doi:10.1101/gr.171001) [DOI] [PubMed] [Google Scholar]
- Grimson A., Srivastava M., Fahey B., Woodcroft B. J., Chiang H. R., King N., Degnan B. M., Rokhsar D. S., Bartel D. P.2008Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature 455, 1193–1197 (doi:10.1038/nature07415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröger H., Schmid V.2001Larval development in Cnidaria: a connection to Bilateria? Genesis 29, 110–114 (doi:10.1002/gene.1013) [DOI] [PubMed] [Google Scholar]
- Hadfield M. G.1977Chemical interactions in larval settling of a marine gastropod. In Marine natural products chemistry (eds Faulkner D. J., Fenical W. H.), pp. 403–413 San Diego, CA: Plenum Press [Google Scholar]
- Hadfield M. G.1978Metamorphosis in marine molluscan larvae: an analysis of stimulus and response. In Settlement and metamorphosis of marine invertebrate larvae (eds Chia F. S., Rice M. E.), pp. 165–175 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- Hadfield M. G.1984Settlement requirements of molluscan larvae: new data on chemical and genetic roles. Aquaculture 39, 283–298 (doi:10.1016/0044-8486(84)90272-2) [Google Scholar]
- Hadfield M. G.2000Why and how marine-invertebrate larvae metamorphose so fast. Semin. Cell Dev. Biol. 11, 437–443 (doi:10.1006/scdb.2000.0197) [DOI] [PubMed] [Google Scholar]
- Hadfield M. G., Strathmann M. F.1996Variability, flexibility and plasticity in life histories of marine invertebrates. Oceanol. Acta 19, 323–334 [Google Scholar]
- Hadfield M. G., Carpizo-Ituarte E. J., del Carmen K., Nedved B. T.2001Metamorphic competence, a major adaptive convergence in marine invertebrate larvae. Am. Zool. 41, 1123–1131 (doi:10.1668/0003-1569(2001)041[1123:MCAMAC]2.0.CO;2) [Google Scholar]
- Hejnol A., Martindale M. Q.2008Acoel development supports a simple planula-like urbilaterian. Phil. Trans. R. Soc. B 363, 1493–1501 (doi:10.1098/rstb.2007.2239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyland A., Moroz L. L.2006Signalling mechanisms underlying metamorphic transitions in animals. Integr. Comp. Biol. 46, 743–759 (doi:10.1093/icb/icl023) [DOI] [PubMed] [Google Scholar]
- Imron, Jefferey B., Hale, Degnan P., Degnan S. M.2007Pleistocene isolation and recent gene flow in Haliotis asinina, an Indo-Pacific vetigastropod with limited dispersal capacity and specific settlement requirements. Mol. Ecol. 16, 289–304 (doi:10.1111/j.1365-294X.2006.03141.x) [DOI] [PubMed] [Google Scholar]
- Jackson D., Leys S. P., Hinman V. F., Woods R., Lavin M. F., Degnan B. M.2002Ecological regulation of development: induction of marine invertebrate metamorphosis. Int. J. Dev. Biol. 46, 679–686 [PubMed] [Google Scholar]
- Jackson D. J., Ellemor N., Degnan B. M.2005Correlating gene expression with larval competence and the effect of larval age and parentage on rates of metamorphosis in the tropical abalone. Haliotis asinina. Mar. Biol. 147, 681–697 (doi:10.1007/s00227-005-1603-z) [Google Scholar]
- Jager M., Quéinnec E., Chiori R., Le Guyader H., Manuel M.2008Insights into the early evolution of SOX genes from expression analyses in a ctenophore. J. Exp. Zool. (Mol. Dev. Evol.) 310B, 650–667 (doi:10.1002/jez.b.21244) [DOI] [PubMed] [Google Scholar]
- Jägersten G.1972Evolution of the metazoan life cycle: a comprehensive theory New York, NY: Academic Press [Google Scholar]
- King N., et al. 2008The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451, 783–788 (doi:10.1038/nature06617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle C. J., Boulding E. G.2000Comparative population genetic structure of marine gastropods (Littorina spp.) with and without pelagic larval dispersal. Mar. Biol. 137, 835–845 (doi:10.1007/s002270000412) [Google Scholar]
- Lapébie P., Gazave E., Ereskovsky A., Derelle R., Bézac C., Renard E., Houliston E., Borchiellini C.2009WNT/beta-catenin signalling and epithelial patterning in the homoscleromorph sponge Oscarella. PLoS One 4, e5823 (doi:10.1371/journal.pone.0005823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroux C., Fahey B., Liubicich D., Hinman V. F., Gongora M., Green K. M., Wörheide G., Leys S. P., Degnan B. M.2006Developmental expression of transcription factor genes in a demosponge: insights into the origin of metazoan multicellularity. Evol. Dev. 8, 150–173 (doi:10.1111/j.1525-142X.2006.00086.x) [DOI] [PubMed] [Google Scholar]
- Larroux C., Fahey B., Degnan S. M., Adamski M., Rokhsar D. S., Degnan B. M.2007NK homeobox gene cluster predates the origin of Hox genes. Curr. Biol. 17, 706–710 (doi:10.1016/j.cub.2007.03.008) [DOI] [PubMed] [Google Scholar]
- Larroux C., Luke G. N., Koopman P., Rokhsar D. S., Shimeld S. M., Degnan B. M.2008Genesis and expansion of metazoan transcription factor gene families and classes. Mol. Biol. Evol. 25, 980–996 (doi:10.1093/molbev/msn047) [DOI] [PubMed] [Google Scholar]
- Leys S. P.2004Gastrulation in sponges. In Gastrulation: from cells to embryo (ed. Stern C. D.), pp. 23–32 New York, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Leys S. P., Degnan B. M.2001The cytological basis of photoresponsive behavior in a sponge larva. Biol. Bull. 201, 323–338 (doi:10.2307/1543611) [DOI] [PubMed] [Google Scholar]
- Leys S. P., Degnan B. M.2002Embryogenesis and metamorphosis in a haplosclerid demosponge: gastrulation and transdifferentiation of larval ciliated cells to choanocytes. Invert. Biol. 121, 171–189 [Google Scholar]
- Leys S. P., Ereskovsky A. E.2006Embryogenesis and larval differentiation in sponges. Can. J. Zool. 84, 262–287 (doi:10.1139/z05-170) [Google Scholar]
- Leys S. P., Cronin T. W., Degnan B. M., Marshall J. N.2002Spectral sensitivity in a sponge larva. J. Comp. Physiol. A 188, 199–202 (doi:10.1007/s00359-002-0293-y) [DOI] [PubMed] [Google Scholar]
- Li C., Chen J., Hua T.1998Precambrian sponges with cellular structures. Science 279, 879–882 (doi:10.1126/science.279.5352.879) [DOI] [PubMed] [Google Scholar]
- Love G. D., et al. 2009Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457, 718–721 (doi:10.1038/nature07673) [DOI] [PubMed] [Google Scholar]
- Maldonado M.2006Ecology of the sponge larva. Can. J. Zool. 84, 175–194 (doi:10.1139/z05-177) [Google Scholar]
- Marlow H. Q., Srivastava M., Matus D. Q., Rokhsar D., Martindale M. Q.2009Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev. Neurobiol. 69, 235–254 (doi:10.1002/dneu.20698) [DOI] [PubMed] [Google Scholar]
- Matus D. Q., Pang K., Daly M., Martindale M. Q.2007Expression of Pax gene family members in the anthozoan cnidarian, Nematostella vectensis. Evol. Dev. 9, 25–38 [DOI] [PubMed] [Google Scholar]
- Mazza M. E., Pang K., Martindale M. Q., Finnerty J. R.2007Genomic organization, gene structure, and developmental expression of three clustered otx genes in the sea anemone Nematostella vectensis. J. Exp. Zool. (Mol. Dev. Evol.) 308B, 494–506 [DOI] [PubMed] [Google Scholar]
- Minelli A.2009Perspectives in animal phylogeny and evolution Oxford, UK: Oxford University Press [Google Scholar]
- Morse D. E., Morse A. N. C.1991Enzymatic characterization of the morphogen recognized by Agaricia humilis (scleractinian coral) larvae. Biol. Bull. 181, 104–122 (doi:10.2307/1542493) [DOI] [PubMed] [Google Scholar]
- Nei M., Niimura Y., Nozawa M.2008The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat. Rev. Genet. 9, 951–963 (doi:10.1038/nrg2480) [DOI] [PubMed] [Google Scholar]
- Nichols S. A., Dirks W., Pearse J. S., King N.2006Early evolution of animal cell signaling and adhesion genes. Proc. Natl Acad. Sci. USA 103, 12 451–12 456 (doi:10.1073/pnas.0604065103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang K., Martindale M. Q.2008Developmental expression of homeobox genes in the ctenophore Mnemiopsis leidyi. Dev. Genes Evol. 218, 307–319 (doi:10.1007/s00427-008-0222-3) [DOI] [PubMed] [Google Scholar]
- Pawlik J. R.1992Chemical ecology of the settlement of benthic marine invertebrates. Oceanogr. Mar. Biol. 30, 273–335 [Google Scholar]
- Pechenik J. A.2006Larval experience and latent effects—metamorphosis is not a new beginning. Integr. Comp. Biol. 46, 323–333 (doi:10.1093/icb/icj028) [DOI] [PubMed] [Google Scholar]
- Pechenik J. A., Gee C. C.1993Onset of metamorphic competence in larvae of the gastropod Crepidula fornicata (L.), judged by a natural and an artificial cue. J. Exp. Mar. Biol. Ecol. 167, 59–72 (doi:10.1016/0022-0981(93)90184-P) [Google Scholar]
- Pechenik J. A., Heyman W. D.1987Using KCl to determine size at competence for larvae of the marine gastropod Crepidula fornicata (L.). J. Exp. Mar. Biol. Ecol. 112, 27–38 (doi:10.1016/S0022-0981(87)80012-6) [Google Scholar]
- Pechenik J. A., Qian P. Y.1998Onset and maintenance of metamorphic competence in the marine polychaete Hydroides elegans Haswell in response to three chemical cues. J. Exp. Mar. Biol. Ecol. 226, 51–74 (doi:10.1016/S0022-0981(97)00237-2) [Google Scholar]
- Philippe H., et al. 2009Phylogenomics revives traditional views on deep animal relationships. Curr. Biol. 19, 706–712 (doi:10.1016/j.cub.2009.02.052) [DOI] [PubMed] [Google Scholar]
- Putnam N. H., et al. 2007Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 (doi:10.1126/science.1139158) [DOI] [PubMed] [Google Scholar]
- Raible F., Tessmar-Raible K., Arboleda E., Kaller T., Bork P., Arendt D., Arnone M. I.2006Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 300, 461–475 (doi:10.1016/j.ydbio.2006.08.070) [DOI] [PubMed] [Google Scholar]
- Raimondi P. T., Keough M. J.1990Behavioural variability in marine larvae. Aust. J. Ecol. 15, 427–437 (doi:10.1111/j.1442-9993.1990.tb01468.x) [Google Scholar]
- Rentzsch F., Fritzenwanker J. H., Scholz C. B., Technau U.2008FGF signalling controls formation of the apical sensory organ in the cnidarian Nematostella vectensis. Development 135, 1761–1769 (doi:10.1242/dev.020784) [DOI] [PubMed] [Google Scholar]
- Richards G. S., Simionato E., Perron M., Adamska M., Vervoort M., Degnan B. M.2008Sponge genes provide new insight into the evolutionary origin of the neurogenic circuit. Curr. Biol. 18, 1156–1161 (doi:10.1016/j.cub.2008.06.074) [DOI] [PubMed] [Google Scholar]
- Rodriguez S. R., Ojeda F. P., Inestrosa N. C.1993Settlement of benthic marine invertebrates. Mar. Ecol. Prog. Ser. 97, 193–207 (doi:10.3354/meps097193) [Google Scholar]
- Sakarya O., et al. 2007A post-synaptic scaffold at the origin of the animal kingdom. PLoS One 2, e506 (doi:10.1371/journal.pone.0000506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierwater B., Eitel M., Jakob W., Osigus H. J., Hadrys H., Dellaporta S. L., Kolokotronis S. O., Desalle R.2009Concatenated analysis sheds light on early metazoan evolution and fuels a modern ‘urmetazoon’ hypothesis. PLoS Biol. 7, e20 (doi:10.1371/journal.pbio.1000020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionato E., Ledent V., Richards G., Thomas-Chollier M., Kerner P., Coornaert D., Degnan B. M., Vervoort M.2007Origin and diversification of the basic helix-loop-helix gene family in metazoans: insights from comparative genomics. BMC Evol. Biol. 7, 33 (doi:10.1186/1471-2148-7-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly B. J., Snoke S. S., Raff R. A.2003Who came first—larvae or adults? Origins of bilaterian metazoan larvae. Int. J. Dev. Biol. 47, 623–632 [PubMed] [Google Scholar]
- Srivastava M., et al. 2008The Trichoplax genome and the nature of placozoans. Nature 454, 955–960 (doi:10.1038/nature07191) [DOI] [PubMed] [Google Scholar]
- Strathmann R.1974The spread of sibling larvae of sedentary marine invertebrates. Am. Nat. 108, 29–44 (doi:10.1086/282883) [Google Scholar]
- Sutherland J. P.1990Recruitment regulates demographic variation in a tropical intertidal barnacle. Ecology 71, 955–972 (doi:10.2307/1937365) [Google Scholar]
- Todd C. D.1990Larval supply and recruitment of benthic invertebrates: do larvae always disperse as much as we believe? Hydrobiologica 376, 1–21 (doi:10.1023/A:1017007527490) [Google Scholar]
- Todd C. D., Lambert W. J., Thorpe J. P.1998The genetic structure of intertidal populations of two species of nudibranch molluscs with planktotrophic and pelagic lecithotrophic larval stages: are pelagic larvae ‘for’ dispersal? J. Exp. Mar. Biol. Ecol. 228, 1–28 (doi:10.1016/S0022-0981(98)00005-7) [Google Scholar]
- Toonen R. J., Pawlik J. R.2001Foundations of gregariousness: a dispersal polymorphism among the planktonic larvae of a marine invertebrate. Evolution 55, 2439–2454 [DOI] [PubMed] [Google Scholar]
- Trapido-Rosenthal H. G., Morse D. E.1986aAvailability of chemosensory receptors is down-regulated by habituation of larvae to a morphogenetic signal. Proc. Natl Acad. Sci. USA 83, 7658–7662 (doi:10.1073/pnas.83.20.7658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapido-Rosenthal H. G., Morse D. E.1986bRegulation of receptor-mediated settlement and metamorphosis in larvae of a gastropod mollusc (Haliotis rufescens). Bull. Mar. Sci. 39, 383–392 [Google Scholar]
- Underwood A. J., Keough M. J.2001Supply-side ecology: the nature and consequences of variations in recruitment of intertidal organisms. In Marine community ecology (eds Bertness M. D., Gaines S. D., Hay M. E.), pp. 183–200 Sunderland, Maine: Sinauer Associates, Inc [Google Scholar]
- Valentine J. W.2004On the origin of phyla Chicago, IL: The University of Chicago Press [Google Scholar]
- Williams E. A., Degnan S. M.2009Carry-over effect of larval settlement cue on postlarval gene expression in the marine gastropod Haliotis asinina. Mol. Ecol. 18, 4434–4449 (doi:10.1111/j.1365-294X.2009.04371.x) [DOI] [PubMed] [Google Scholar]
- Williams E. A., Craigie A., Yeates A., Degnan S. M.2008Articulated coralline algae of the genus Amphiroa are highly effective natural inducers of settlement in the tropical abalone Haliotis asinina. Biol. Bull. 215, 98–107 [DOI] [PubMed] [Google Scholar]
- Williams E. A., Degnan B. M., Gunter H., Jackson D. J., Woodcroft B. J., Degnan S. M.2009Widespread transcriptional changes pre-empt the critical pelagic-benthic transition in the vetigastropod Haliotis asinina. Mol. Ecol. 18, 1006–1025 (doi:10.1111/j.1365-294X.2008.04078.x) [DOI] [PubMed] [Google Scholar]
- Woodman P.2009ESCRT proteins, endosome organization and mitogenic receptor down-regulation. Biochem. Soc. Trans. 37, 146–150 (doi:10.1042/BST0370146) [DOI] [PubMed] [Google Scholar]
- Woods R. G., Roper K. E., Gauthier M., Bebell L. M., Sung K., Degnan B. M., Lavin M. F.2004Gene expression during early ascidian metamorphosis requires signalling by Hemps, an EGF-like protein. Development 131, 2921–2933 (doi:10.1242/dev.01120) [DOI] [PubMed] [Google Scholar]
- Wray G. A., Lowe C. J.2000Developmental regulatory genes and echinoderm evolution. Syst. Biol. 49, 28–51 (doi:10.1080/10635150050207375) [DOI] [PubMed] [Google Scholar]
- Yamada A., Pang K., Martindale M. Q., Tochinai S.2007Surprisingly complex T-box gene complement in diploblastic metazoans. Evol. Dev. 9, 220–230 [DOI] [PubMed] [Google Scholar]
- Zhang S. M., Adema C. M., Kepler T. B., Loker E. S.2004Diversification of Ig superfamily genes in an invertebrate. Science 305, 251–254 (doi:10.1126/science.1088069) [DOI] [PubMed] [Google Scholar]