Abstract
It is proposed here that a biphasic life cycle with partial dedifferentiation of intermediate juvenile or larval stages represents the mainstream developmental mode of metazoans. Developmental plasticity of differentiated cells is considered the essential characteristic of indirect development, rather than the exclusive development of the adult from ‘set-aside’ cells. Many differentiated larval cells of indirect developers resume proliferation, partially dedifferentiate and contribute to adult tissues. Transcriptional pluripotency of differentiated states has premetazoan origins and seems to be facilitated by histone variant H2A.Z. Developmental plasticity of differentiated states also facilitates the evolution of polyphenism. Uncertainty remains about whether the most recent common ancestor of protostomes and deuterostomes was a direct or an indirect developer, and how the feeding larvae of bilaterians are related to non-feeding larvae of sponges and cnidarians. Feeding ciliated larvae of bilaterians form their primary gut opening by invagination, which seems related to invagination in cnidarians. Formation of the secondary gut opening proceeds by protostomy or deuterostomy, and gene usage suggests serial homology of the mouth and anus. Indirect developers do not use the Hox vector to build their ciliated larvae, but the Hox vector is associated with the construction of the reproductive portion of the animal during feeding-dependent posterior growth. It is further proposed that the original function of the Hox cluster was in gonad formation rather than in anteroposterior diversification.
Keywords: annelid, H2A.Z, lophotrochozoan, multipotency, posterior growth zone, terminal addition
1. Regulatory evolution of indirect development
(a). Evolutionary-developmental continuity between direct and indirect development
Development seems more remarkable when it proceeds throughout an intermediate larval stage that has little resemblance to the adult. In indirect development, the embryonic phase generates an intermediate larva, and the post-embryonic phase generates an adult by substantial transformation and replacement of larval tissues. In some cases, the larval stage has feeding ability, and post-embryonic construction of the macroscopic adult is dependent on nourishment obtained by the larva. The shift from the larval to adult body plan is mediated by gradual growth that often culminates with a dramatic metamorphosis and a radical change of life mode. Nevertheless, the prompt metamorphosis often entails more of a mechanical rearrangement than a constructive developmental process, and major elements of the adult body plan are gradually transformed or formed de novo during larval stages. The boundary between larval and adult phases is less sharp than metamorphosis would suggest. Accordingly, the term ‘larva’ is ambiguous (Minelli 2009). In contrast, the embryos of directly developing organisms generate at once a juvenile already possessing the complete adult body plan, which then grows and acquires sexual maturity. Amniotes and cephalopods exemplify outmost direct development. In contrast, lophotrochozoan and deuterostome marine invertebrates with feeding ciliated larvae are extreme representatives of indirect development (figure 1). Planktotrophic ciliated larvae of hemichordates, echinoderms, polychaetes, nemerteans and other marine invertebrates are endowed with the minimal organs required for nourishing the slow development of their macroscopic adults (Pechenik 2004). Between these two extremes, life cycles encompass a broad range of developmental modes (Reitzel et al. 2006; Allen & Pernet 2007; Minelli 2009). Indirect development, understood as a biphasic developmental mode, is not confined to marine invertebrates (Brusca & Brusca 2003). Metamorphosis in amphibians and some fish species is accompanied by substantial, although peripheral, body transformations over a mainly conserved core body plan, and holometabolous insects dramatically transform and/or replace extensively the tissues of the larva.
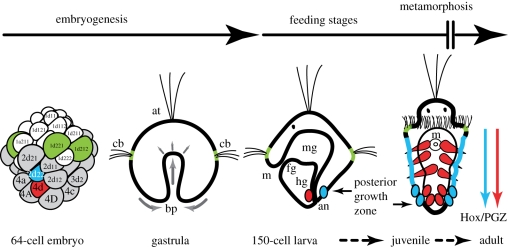
Figure 1.
Indirect development of the annelid Hydroides elegans. Embryogenesis promptly forms a microscopic feeding trochophore larva. Nourishment ensures the growth and transformation of larval tissues. The trochoblasts are the first larval cells that differentiate (green), and will be discarded during metamorphosis. Differentiated endodermal, ectodermal and mesodermal cells of the larva (black) are ‘recycled’ and contribute to adult transformation. Multipotent adult precursors in the posterior growth zone (PGZ), mesodermal 4d (red) and ectodermal 2d22 (blue), form the segmented portion of the worm, which largely corresponds to the reproductive side of the animal. an, anus; at, apical tuft; bp, blastopore; cb, ciliary band; fg, foregut; hg, hindgut; mg, midgut.
(b). The unresolved origin of indirect development
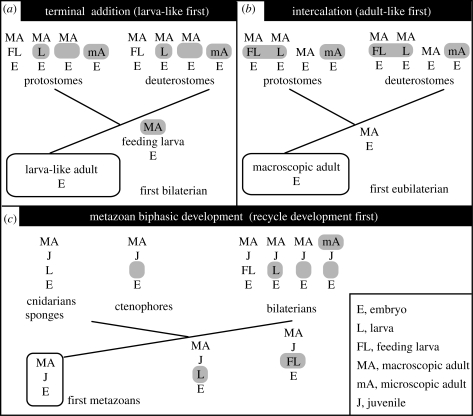
The origin and evolutionary significance of indirect development by means of feeding ciliated larvae of marine invertebrates remain among the major enigmas of animal evolution (Jägersten 1972; Nielsen & Nørrevang 1985; Peterson et al. 1997; Valentine & Collins 2000; Martindale 2005; Raff 2008; Minelli 2009). The protostome–deuterostome ancestor (PDA) may have been a direct or an indirect developer. The adult stages of bilaterians are generally considered homologous, at least at a very deep level, but we do not know if the feeding ciliated larvae of indirectly developing protostomes and deuterostomes represent convergent adaptations or derive from a larva already present in the life cycle of the PDA. The ciliated larval stages of protostomes and deuterostomes are similarly endowed with a longitudinally tripartite gut, an apical sensory organ and a locomotory ciliary band that also serves for food particle capture. These similarities are differently explained by the terminal addition and the intercalation models. The terminal addition, or ‘larva-like first’, scenarios propose that early bilaterians, long before the PDA, were simple, small and generally similar to the larval stage of indirect developers (figure 2a). The macroscopic stage of indirect developers then evolved by terminal addition of a complex phase to the life cycle; terminal addition here has an ontogenetic-evolutionary sense rather than an anatomical sense. In this scenario, the PDA was already an indirect developer with a complex adult and a simple larva (Nielsen & Nørrevang 1985; Davidson et al. 1995; Peterson et al. 1997), and the similarity among bilaterian larvae would be due to common origin. In contrast, the intercalation or ‘adult-like first’ scenarios propose that the PDA was a direct developer similar to the adult phase of indirect developers and that the larval stages convergently evolved along various protostome and deuterostome lineages by intercalation during early development (figure 2b) (Valentine & Collins 2000; Sly et al. 2003; Dunn et al. 2007). Despite their contrasting views, in both scenarios direct development is the starting developmental mode from which indirect development evolved. The intercalation scenario proposes that the original direct developer was complex (adult-like), whereas the terminal addition model proposes that the original direct developer was simple (larva-like). Both scenarios also generally limit the discussion to the origin of the bilaterian larva to the exclusion of the cnidarian and sponge larvae, and consider embryonic and post-embryonic development as largely independent evolutionary-developmental entities.
Figure 2.
Outline of the different scenarios for the evolution of indirect development in bilaterians. Evolutionary novelties highlighted in grey. (a) The terminal addition model proposes that the first bilaterians were similar to the larval stage of current indirect developers; the macroscopic adult represents a secondary addendum to the final phase of the life cycle sometime before the bilaterian radiation. (b) The intercalation model proposes a relatively complex protostome/deuterostome ancestor and that the larval stages of protostomes and deuterostomes represent convergent adaptations intercalated during early development. (c) The metazoan biphasic development model proposed here suggests that a differentiated stage at the end of embryogenesis represents the basal condition for metazoans. Adult development proceeds by partial dedifferentiation and subsequent proliferation. In direct developers, the intermediate differentiated stage is similar to the adult; the differentiated stage is a juvenile. The evolution of distinct specializations of the intermediate differentiated stage generates a larval stage.
A third possibility is that indirect development represents the mainstream developmental mode of metazoans. We could dub this model as ‘biphasic development first’. Ciliated larvae would have evolved as specializations for enhanced dispersal and substrate selection (figure 2c). Perhaps, swimming larvae originated early during metazoan evolution in association with sessile adults, which would explain why both motile ctenophores and placozoans are direct developers. Feeding variants of indirectly developing bilaterians could have evolved by shifting gastrulation to early embryogenesis (Rieger 1994; Arenas-Mena 2008), thereby transforming the originally non-feeding larva that was similar to those of extant cnidarians and sponges (Degnan & Degnan 2006). The feeding variants could have evolved once prior to the PDA or independently in protostomes and deuterostomes (Jägersten 1972; Arenas-Mena 2008).
(c). Insights and uncertainties of metazoan phylogenies and secondary body simplifications
The phylogenetic distribution of direct and indirect development is scattered, with a multitude of closely related species having feeding and non-feeding larval variants. The polarity of change from indirect to direct development is well documented in echinoderms (McEdward & Miner 2001) and has been particularly well studied in the sister echinoid species Heliocidaris tuberculata and Heliocidaris erythrogramma (Raff 2008). This evolutionary transition generally proceeds by losing feeding-related adaptations of the larva, gaining yolkier eggs and actively shifting adult developmental programmes to early embryonic stages (Wray 1996; Wilson et al. 2005; Raff 2008). The evolutionary trend towards secondary loss of larval stages confirms that losing a structure and shifting developmental programmes is more feasible than evolving a new developmental stage. The evolution of indirect development from direct development is possible, as illustrated by the evolution of holometabolous insects from hemimetabolous insects (Suzuki et al. 2008); however the insect transition occurred gradually during a long period of time and does not fully represent the reverse counterexample of the sister echinoid species.
Reconstruction of the PDA life cycle is obscured by uncertainties about phylogenetic relationships and secondary evolution among lineages leading to extant representatives. It is generally considered that the last common ancestor of echinoderms was an indirect developer (Nakano et al. 2003). The larval stage of hemichordates is similar to the larval stage of echinoderms, and it is unknown if they represent homologous or convergent stages. The phylogeny of lophotrochozoans remains unresolved, probably reflecting a true radiation (Giribet 2008). The extant sister group of protostomes and deuterostomes remains uncertain. It has been proposed that acoel flatworms are the sister group of eubilaterians (a new group that includes all the other bilaterians, i.e. protostomes and deuterostomes) (Baguñà & Riutort 2004; Sempere et al. 2007). Acoels are relatively simple, and it has been suggested that they may represent the extant version of the microbilaterian planuloid from which the stem eubilaterians evolved (Hejnol & Martindale 2008b), that is, the crawling version of the larva-like ancestor (figure 2a). The acoelomate mesodermal organization of acoels corresponds to the intermediate grade of organization of the classical coelomate bilaterian scheme (Hyman 1940) and would conform to the assumption of incremental complexity along the lineage leading to eubilaterians, as previously discussed (Deutsch 2008). However, the basal position of acoels has been challenged. New analysis using phylogenomic methods suggests that acoels do not ally with planarians, but remain within the lophotrochozoans (Giribet et al. 2007; Brinkmann & Philippe 2008; Dunn et al. 2008). Even if acoels are confirmed as the sister group of eubilaterians, uncertainties would remain about the developmental mode and complexity of the most recent common ancestor of acoels and eubilaterians. Acoels appear generally simpler than most bilaterians, and perhaps even cnidarians. All acoelomates and pseudocoelomates have been demoted from their basal position and are currently thought to have evolved from more complex forms (Deutsch 2008), perhaps by losing the complex phase of their life cycle, by generalized simplification of a direct life cycle or both. Because acoels may have secondarily evolved a simplified body plan and because they may not be basal bilaterians, the relevance of acoels to reconstructing the evolutionary transformations leading to the PDA remains uncertain.
The relationships between major animal groups, sponges, ctenophores, cnidarians and bilaterians are also controversial (Philippe et al. 2005; Giribet et al. 2007; Schierwater et al. 2009). Phylogenetic uncertainty does not preclude some evolutionary inferences. Cnidarians and sponges also have their own version of indirect development through non-feeding larval stages (figure 2c). The repeated convergent evolution of larval stages across metazoans cannot be completely discarded, but the broad distribution of indirect development among extant representatives of the major animal groups favours the hypothesis of a last common ancestor of bilaterians, cnidarians and sponges with some sort of biphasic development and transitional non-feeding ciliated larva (figure 2c) (Rieger 1994; Degnan & Degnan 2006; Arenas-Mena 2008). The question may be narrowed to whether there was a feeding larva version of indirect development in the PDA. The characterization of the regulatory entities that control larval and adult development should provide new insights to help resolve this enigma.
(d). Evolutionary dynamics of gene regulatory networks and the homology/convergence of ciliated larvae
Current comparative evolutionary studies of gene regulatory networks (GRNs) reveal that core regulatory circuits responsible for the development of homologous characters are resilient to evolutionary change (Hinman et al. 2003a; Davidson & Erwin 2006; Olson 2006; Wagner 2007). Early regulatory circuits set the early draft of the structure that is about to be formed, and differentiation gene batteries that manifest the distinct identities of cells are wired peripherally to these earlier and internal regulatory states. Early core regulatory circuits are evolutionarily conserved (among organisms that generally maintain the structure, of course), whereas later peripheral regulatory circuits are evolutionarily variable (Davidson & Erwin 2006; Wagner 2007). It follows that the core circuits are conserved among organisms that belong to a higher-order phyletic group and the peripheral regulatory modifications correspond with lower-level phyletic groups and are more prone to adaptive change. Therefore, core regulatory networks are macroregulatory both in a developmental and an evolutionary sense. Among bilaterians, the most conserved and deeper regulatory entities should correspond to developmental characters already present in the PDA.
In principle, regulatory gene usage in larva-specific organs could provide a shortcut by which to test whether metazoan ciliated larvae represent homologous or convergent adaptations; but, are there any larva-specific organs in the first place? The apical tuft is a group of sensory cells, and the ciliary band is a ciliated belt for propulsion and feeding (figure 1). Both the apical tuft and ciliary bands are present in protostome and deuterostome larvae (Nielsen 2005b; Henry et al. 2007) and are, in principle, good candidates for larva-specific organs already present in the larvae of an indirectly developing PDA. However, these are very simple organs with a few cell types and the assessment of their direct homology is bound to be difficult (Arenas-Mena 2008). For example, the apical tufts of protostome (Arenas-Mena 2008) and deuterostome (Tagawa et al. 2000) larvae share expression of the transcription factor T-brain, which is also expressed in the olfactory organs of vertebrates (Ryan et al. 1998; Mione et al. 2001). Thus, apical tufts may have evolved by a process of simplification; that is, they may derive from adult sensory organs stripped of morphological complexity down to the cell-type level. Or conversely, the apical tufts may be homologous and represent the evolutionary precursors of adult chemosensory epitheliums. The evolutionary transformation towards adult organs could have been obtained by a process of intercalation of morphological complexity within original cell-type regulatory circuits, as previously proposed (Gehring & Ikeo 1999). For example, the transcription factor distal-less is involved in the specification of varied appendages across bilaterians, although its role in their last common ancestor almost certainly related to sensory cell-type specification functions and subsequent morphogenetic roles independently evolved in diverging lineages (Mittmann & Scholtz 2001). Similarly, the eyes of vertebrates, cephalopods and insects are convergent as eyes despite their almost certain independent evolution from ancestral photoreceptors (Pichaud & Desplan 2002; Donner & Maas 2004). In short, it does not seem possible to establish whether the adult or the larval organ is the precursor of the other based solely on the expression of differentiation regulators. Thus, similarity between the cell-type regulatory circuits that control the formation of very simple larval organs does not necessarily imply their homology; that is, they may not derive from a sensory organ present in the larva of the last common ancestor of protostomes and deuterostomes.
The regulatory machinery for ciliary band formation may be evolutionarily informative, perhaps within the limitations expected for relatively simple cell-type specification networks. There are many ciliated cells in adults, but there is no immediate adult equivalent for the ciliary band of protostome and deuterostome larvae. The net of serotonergic neurons that is associated with the ciliary band may have adult counterparts (Hay-Schmidt 2000), but probably only at the cell-type level as similarly discussed for the apical tuft. The prototroch is the primary ciliary band of spiralians (Nielsen 2005b). In support of ancestral regulatory entities, cis-regulatory sequences of a gastropod alpha-tubulin gene drive trochoblast expression in the primary ciliary band of various spiralians (Damen et al. 1997). Secondary ciliary bands have different lineage origins among spiralians (Arenas-Mena et al. 2007b; Henry et al. 2007), although different lineages do not preclude deeper regulatory homology.
(e). The macroregulatory frame of the bilaterian larvae
Core macroregulatory networks provide a better estimation for homology or convergence of the larval stage than do cell-type specification GRNs, but are there informative GRNs for the formation of the very simple larval stages? Perhaps yes. Informative macroregulatory networks should be those that control the relative positions of larval organs and broad developmental processes, such as gastrulation by invagination or regionalization of the different areas of the larva (i.e. dorsal versus ventral, etc.). These macroregulatory networks should remain similar between protostome and deuterostome larvae if the PDA was an indirect developer and should be substantially different if the protostome and deuterostome larvae represent convergent adaptations. Nevertheless, even if the core macroregulatory frames remain similar, the downstream connections with differentiation targets may have substantially changed or remained identical along descending evolutionary lineages. Thus, contrary to previous reports (Dunn et al. 2007; Love et al. 2008), the expression of differentiation genes provides weak evidence to trace deep larval homologies. For example, Otx is expressed in a remarkably similar location along the equator of a polychaete larva (Arenas-Mena & Wong 2007) and a gastropod larva (Nederbragt et al. 2002), but some of the blastomeres that express Otx become neurons in the former and trochoblasts in the later. Otx may belong to a core macroregulatory frame that sets a distinct transcriptional state along the equator of the larva, but its downstream connections with differentiation targets may have partially changed along the annelid and mollusc lineages. Similarly, generally conserved spatial deployment of various transcription factors is also found in the apical area of protostome and deuterostome larvae, although their downstream differentiation targets largely diverge (Dunn et al. 2007). The notion of a macroregulatory frame for the construction of the larval stage is also supported by the transcription factor Tbx2/3, which is expressed during embryogenesis in all three germ layers that occupy the dorsal side of the indirectly developing polychaete Hydroides elegans (Arenas-Mena & Wong submitted) and the indirectly developing sea urchin Lytechinus variegatus, where morphogenetic roles in all three germ layers have been demonstrated (Gross et al. 2003). A general dorsal specification role of Tbx2/3 has not been identified in bilaterians with direct development; this suggests that Tbx2/3 may have had dorsal identity specification functions in the larval stage of an indirectly developing PDA. Thus, homologous core regulatory frames could be wired to different peripheral targets that control different states of the same character, such as the respective dorsal side of protostome and deuterostome larvae or the particular cell types at the equator of mollusc and annelid larvae. Comparative topology of the macroregulatory frames for larval development should reflect phylogenetic branching events at different levels of the regulatory hierarchy, but spatial gene expression will reveal little about GRN structure. So, there is no shortcut in sight. We should decipher the macroregulatory structure for larval development in several protostomes and deuterostomes with indirect development in order to test the existence of evolutionarily conserved core regulatory circuits already present in the PDA.
(f). Gene regulatory networks for gastrulation by invagination among indirect developers
So far, the endomesoderm specification network of sea urchins is the most thoroughly characterized network among indirectly developing metazoans (Davidson et al. 2002), and therefore is an excellent starting point for broad comparative analysis. The core endoderm portion of the network is highly conserved between sea urchins and starfish (Hinman et al. 2003a). The echinoderm endomesoderm network controls endomesoderm identity and gastrulation. The structure of the network for germ layer specification should be conserved independent of the original developmental mode. Indeed, all metazoans use Gata4/6 and several other transcription factors for the specification of their endomesoderm (Technau & Scholz 2003). In contrast, the network for gastrulation should change between the direct and indirect developmental modes. This is clearly illustrated in polychaetes. Gastrulation by invagination is associated with indirect development in polychaetes (Anderson 1966; Arenas-Mena & Wong 2007, submitted), whereas directly developing polychaetes (Boyle & Seaver 2008) or polychaetes with non-feeding larva (Arendt et al. 2001) have epibolic gastrulation. Invagination involves complex morphogenesis and formation of functional epithelial endoderm, whereas epiboly consists of the overlay by ectoderm of passive and large yolky cells that do not even form an endoderm epithelium (Anderson 1966; Arendt 2004; Arenas-Mena & Wong submitted). Gastrulation must require the dynamic and coordinated cross-regulatory interactions of various transcription factors that set in motion the morphogenetic events for gut formation. A dedicated network sets the temporal succession of transcription factor gene expression during sea urchin gastrulation (Smith et al. 2008). Thus, temporally dynamic GRNs that control gastrulation by invagination should inform about larval origins.
Characterization of the endoderm-gastrulation network in an indirectly developing protostome with gastrulation by invagination such as H. elegans provides an excellent homology test for the developmental mode of the PDA. Several transcription factors with dynamic expression associated with gastrulation by invagination have been identified in H. elegans (Arenas-Mena 2006, 2008; Arenas-Mena & Wong 2007, submitted). In particular, dynamic expression of the transcription factor-encoding gene brachyury is associated with morphogenetic roles during gastrulation by invagination (Arenas-Mena & Wong submitted). The early endodermal expression of brachyury in H. elegans is similar to its expression in indirectly developing deuterostomes (Tagawa et al. 1998; Gross & McClay 2001) and cnidarians (Fritzenwanker et al. 2004) that also have gastrulation by invagination, but early endoderm expression of brachyury has not been detected in lophotrochozoans with epibolic gastrulation (Arendt et al. 2001; Lartillot et al. 2002), which suggests that gastrulation by invagination and its associated indirect development may represent the ancestral conditions from which epibolic gastrulation derived in directly developing polychaetes.
Gastrulation by invagination is considered the ancestral gastrulation mode of protostomes and deuterostomes (Arendt 2004) and perhaps also cnidarians (Byrum & Martindale 2004). Gut formation precedes the evolution of indirect development by means of feeding ciliated larvae, because cnidarians have a gut but non-feeding larvae (figure 2c). Therefore, if the feeding larvae of protostomes and deuterostomes evolved by shifting ancient gastrulation by invagination to early embryogenesis, as previously proposed (Arenas-Mena 2008), then conserved core GRNs should be observed between cnidarians and bilaterians that gastrulate by invagination (figure 3). In support of this expectation, the transcription factors brachyury and FoxA form an evolutionarily conserved synexpression group at the blastopore of protostomes, deuterostomes and cnidarians (Fritzenwanker et al. 2004; Arenas-Mena & Wong submitted). If gastrulation by invagination of indirectly developing bilaterians evolved from the peculiar gastrulation mode of acoels, in which the blastopore does not even correspond to the mouth and there is no endoderm but a gastric syncytium (Hejnol & Martindale 2008a), then the regulatory machinery of indirectly developing bilaterians should be quite dissimilar to that of cnidarians with gastrulation by invagination (figure 3).
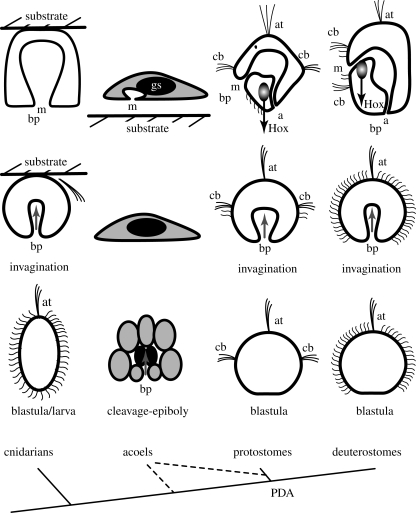
Figure 3.
Similar gastrulation by invagination of indirectly developing bilaterians and cnidarians may proceed by ancestral developmental programmes. If this is the case, then the core GRNs for gastrulation by invagination should be conserved between protostomes, deuterostomes and cnidarians. If, on the contrary, gastrulation by invagination in protostomes and deuterostomes secondarily evolved from gastrulation in acoels, then substantial variation of core GRNs is expected. In acoels, ingression of endoderm precursors proceeds during cleavage, the blastopore does not correspond to the mouth and there is no endodermal epithelium, only a gut syncytium (gs). For comparative purposes, the cnidarian larva settles to a floating substrate. Regulatory gene expression suggests the correspondence of the dorsal side and the blastopore of indirectly developing protostomes and deuterostomes. The protostome blastopore forms the mouth and the deuterostome blastopore forms the anus. Illuminated ovals represent adult precursors that generate the posterior portions of the respective adults, the associated black arrows represent the ‘Hox vectors’ and posterior growth zones (PGZs). Grey arrows represent gastrulation in the different groups. ap, adult precursor; at, apical tuft; bp, blastopore; cb, ciliary band; gs, gut syncytium; m, mouth; mg, midgut.
(g). Equivalence of the secondary gut openings of protostome and deuterostome feeding larvae and the absence of dorsoventral or anteroposterior inversion
The equivalence of the dorsal side of protostome and deuterostome larvae is marked by the expression of Tbx2/3 (Arenas-Mena & Wong submitted) and provides directionality that suggests the equivalence of the primary archenteron opening, i.e. the blastopore, which corresponds to the protostome mouth and the deuterostome anus (figure 3). The ectoderm area where the prospective secondary gut opening will form also expresses FoxA and brachyury in an indirectly developing polychaete with protostomous gastrulation (Arenas-Mena 2006; Arenas-Mena & Wong submitted) and in an indirectly developing sea urchin with deuterostomous gastrulation (Oliveri et al. 2006). The anticipated expression of FoxA and brachyury suggests that the affinity of ectoderm and endoderm is locally enhanced by the expression of ‘endoderm’ genes in the ectoderm. Therefore, it remains possible that not only the primary but also the secondary gut openings of protostomes and deuterostomes are homologous (Grobben 1908; Arenas-Mena & Wong submitted). Alternatively, the secondary gut opening may represent a convergent adaptation that unavoidably recruited FoxA and Brachyury for the independent evolution of a two-end gut in protostomes and deuterostomes. The evolution of protostomy and deuterostomy would not necessarily require reversing gut polarity, because the original two-end gut could have been largely devoid of polarity in their common ancestor. For example, cnidarians have a blind gut and ctenophores just a small anal opening. The equivalence of the blastopore with the deuterostome anus and the protostome mouth does not require a reversal of the anteroposterior axis, because the adult anteroposterior organization is driven by the Hox vector that points in the same direction, i.e. away from the mouth in indirectly developing protostomes and deuterostomes (figure 3).
The protostomy–deuterostomy scenario is in contrast with the amphistomy hypothesis for the origin of a two-ended gut (Arendt & Nübler-Jung 1997). Amphistomy is a gastrulation mode in which the blastopore elongates and merges along the midline, leaving an open mouth and anus at the extremities. Prototypic amphistomy is associated with epibolic gastrulation in more directly developing polychaetes that do not form a feeding trochophore (Anderson 1966). It has been proposed that amphistomy is the ancestral bilaterian condition from which deuterostomy and protostomy evolved by emphasizing blastopore closure from either the oral side or the anal side (Arendt & Nübler-Jung 1997). In this model, polychaetes with non-feeding larvae are considered extant representatives of the ancestral gastrulation mode from which vertebrate gastrulation evolved (Arendt & Nübler-Jung 1997), although no extant deuterostome has amphistomous gastrulation. A common expectation of both the amphistomy and protostomy–deuterostomy scenarios is that oral sensory functions originally associated with a one-ended gut would have been lost from the anal side of the two-ended gut. In both scenarios, at least part of the original GRNs for blastopore formation would be used at both ends of the gut. Nevertheless, GRN topologies should reflect the evolutionary relationships among protostomy, deuterostomy and amphistomy.
2. Developmental plasticity and metazoan origins
(a). Larva-to-juvenile transformation with multipotent and recycled cells
The conventional assumption of development as a one-way process does not fully conform to the reality of indirect development. In indirect development, the embryo promptly transitions to fully differentiated states of the larva, while a few exclusively adult precursor cells, such as those in the posterior growth zone (PGZ) of many bilaterians, remain multipotent and undifferentiated. Some terminally differentiated cells, such as those of the sensory apical tuft and the ciliary band, are exclusively larval, do not contribute to the adult, and undergo apoptosis during adult transformation (Yuan et al. 2008). Few modern lineage analyses precisely trace larval contributions to the adult. Nevertheless, current observations strongly suggest that not all adult structures derive from undifferentiated precursors, and only a fraction of terminally differentiated larval cells are discarded during metamorphosis (figure 4). In many indirect developers, a large fraction of differentiated larval cells contribute to adult fates; therefore, these cells must reactivate proliferation and/or shift to distinct transcriptional states. For example, in indirectly developing polychaetes there seems to be developmental continuity of the larval gut into the adult gut, large portions of ectoderm are not discarded during metamorphosis and the adult brain seems to form by transformation of larval ectoderm (Anderson 1966; Nielsen 2005a; Arenas-Mena & Wong 2007; Arenas-Mena et al. 2007a), which almost certainly involves transdifferentiation, that is, partial dedifferentiation and redifferentiation to new fates. Transdifferentiation of larval ciliated cells to adult choanocytes has been demonstrated in the metamorphosis of a haplosclerid demosponge (Leys & Degnan 2002), which adds evidence in support of deep metazoan origins of transdifferentiation and biphasic development.
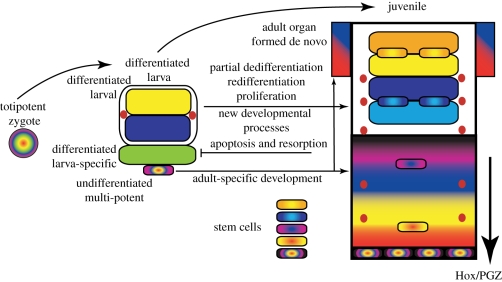
Figure 4.
General outline of the larva-to-adult transformation in indirect development. The multi-potent zygote generates a differentiated larva during embryogenesis. Larval-specific tissues (green) are discarded during metamorphosis. Some differentiated larval organs resume proliferation and contribute to adult transformation (yellow, blue and red). Yellow and blue tissues partially dedifferentiate and redifferentiate into related fates (light blue and orange). Substantial regions of the adult derive from multipotent precursors that do not contribute to differentiated larval fates (multi-coloured cells). Maintenance and homeostasis of adult organs is accomplished by a combination of stem cells and the same mechanisms of dedifferentiation–proliferation that constructed the adult.
Differentiation is not always terminal. Precise methods that estimate proliferation of differentiated cells demonstrate that pancreatic beta-cells are formed by self-duplication rather than by stem-cell differentiation (Dor et al. 2004). Similar methods should be applied to investigate juvenile development and adult tissue homeostasis to fully evaluate the developmental potential of differentiated cells. Two cells flanking the midgut of larval H. elegans express a trypsinogen orthologue (a digestive protease precursor), and they seem to proliferate without losing their differentiated state during adult transformation (results not shown). In the fruitfly, differentiated tracheal cells of the larva become proliferative and form the adult tracheal system; in addition, these cells also form adult-specific air sacs (Guha et al. 2008; Nakayama-Ishimura et al. 2009). In the silkworm, differentiated cells of the larval antennae contribute to the adult antennae, and imaginal discs derive from ectodermal differentiated cells (Svácha 1992). Similarly, differentiated cells of the larval legs contribute to the adult legs of the tobacco hornworm (Tanaka & Truman 2005). Therefore, differentiated cells maintain or regain developmental potency and then acquire new fates and proliferate during adult transformation.
The dramatic transformations during metamorphosis have inspired the prevailing idea of almost total independence of larval and adult phases. Metamorphosis is often very rapid and involves mechanical and developmental transformations and destruction and resorption of tissues as well as stimulation of new growth. The acquisition of competence for metamorphosis is gradual and requires the concerted expression of multiple genes, as illustrated in the ass's-ear abalone Haliotis asinina (Williams et al. 2009). The transformation involves environmental induction and neural transduction (Hadfield et al. 2000; Seipp et al. 2007; Nakayama-Ishimura et al. 2009) and results in an abrupt transition of lifestyle: in the case of H. elegans, the transition goes from free-swimming to a sessile stage encaged in a tube (Carpizo-Ituarte & Hadfield 2003); in the case of the purple sea urchin Strongylocentrotus purpuratus, metamorphosis involves unfolding of the pentameral rudiment, engulfing of bilateral structures and a shift from pelagic suspension feeding to benthic biofilm feeding (Arenas-Mena et al. 2000; Nakayama-Ishimura et al. 2009); in nemerteans, metamorphosis is also very dramatic with extensive replacement of larval tissues (Maslakova et al. 2004). Multiple constructive and destructive developmental processes are triggered during ascidian metamorphosis (Nakayama-Ishimura et al. 2009). Metamorphosis takes place in a few hours. Nevertheless, adult transformation is a slow developmental process that starts during larval stages and continues after metamorphosis. In the larvae of H. elegans at least several segments are formed by terminal growth during one week of feeding before metamorphosis (Carpizo-Ituarte & Hadfield 2003; Seaver & Kaneshige 2006), and in S. purpuratus the pentameral rudiment grows for about six weeks on the left side of the larva (Arenas-Mena et al. 2000).
The ‘adult-first’ (Valentine & Collins 2000; Sly et al. 2003; Dunn et al. 2007) and ‘larva-first’ (Nielsen & Nørrevang 1985; Davidson et al. 1995; Peterson et al. 1997) scenarios for the origin of indirect development emphasize the revolutionary transformations of metamorphosis and consider larval and adult phases largely independent developmental entities that required the evolution of ‘set-aside’ cells (Peterson et al. 1997; Sly et al. 2003). Set-aside cells were originally defined as those that do not contribute to larval fates and remain multipotent for the generation of adult structures (Pehrson & Cohen 1986). In sea urchins, these would generally correspond to coelomic precursors, in long germ band insects to imaginal discs and in indirectly developing polychaetes to the multi-potent precursors in the PGZ (figure 1). Proliferation of these cells depends on feeding and contributes to the main length of the adult, but they are not the exclusive source of adult tissues and do not organize the first outline of the body plan. Larvae have dorsal and ventral aspects, left and right sides, a gut with mouth and anus, sensory and excretory organs, etc. Many of these structures and the general organization of the larva are ‘recycled’ during adult transformation (figure 1). Thus, indirect development is less discontinuous than proposed by the ‘larva-first’ or ‘adult-first’ hypotheses and probably conforms to more gradual developmental and evolutionary transitions (Arenas-Mena 2007).
Extensive developmental continuity between larval and adult stages implies that there will be few larva- or adult-specific macroregulatory entities, except those for exclusive larval adaptations and those for adult organs that are completely feeding-dependent, such as gonad formation. In addition, developmental programmes for eyes, nephridiums, chemoreceptors, digestive glands etc. may have evolved in one phase and then been redeployed forward or backward to the other phase. Therefore, substantial overlap of core macroregulatory entities is expected between direct and indirect developers for the organization of their respective body plans. Nevertheless, if the PDA was an indirect developer, we should expect that the topology of major regulatory architectures that set the larva body plan should reflect their direct origin from ancestral core networks.
(b). Transcriptional potency in embryos, stem cells and differentiated cells
During early development, blastomeres are capable of expressing many fates if exposed to the appropriate inductive cues, but this ability is gradually lost during subsequent development. The evolution of differentiated cell types is favoured by the stabilization of alternative gene expression states. One way to achieve differentiation stability is by irreversibly burying all the non-transcribed genes in transcription-factor inaccessible heterochromatin. Interestingly, the ancient histone variant H2A.Z is associated with transcriptional regulatory DNA in yeast (Albert et al. 2007) and metazoans (Jin & Felsenfeld 2007; Mavrich et al. 2008), where it helps in maintaining an open chromatin state across the genome (Meneghini et al. 2003). The contrast between undifferentiated adult precursors and differentiated larval cells in the trochophore of H. elegans and the larva of a sea urchin was key to revealing the association of H2A.Z with undifferentiated and multipotent cells during metazoan development (Arenas-Mena 2007; Arenas-Mena et al. 2007a). In multipotent cells, H2A.Z should facilitate the accessibility of transcription factors to their DNA binding sites (Arenas-Mena et al. 2007a). Conversely, the invariable decline of H2A.Z expression during differentiation should allow the spread of compact heterochromatin, lessening the transcriptional potential of cells, reducing transcriptional noise and stabilizing the differentiated transcriptional states (Arenas-Mena 2007). In the case of the trochophore larva of H. elegans, primary trochoblasts are the first cells that lose H2A.Z expression and the first ones to differentiate (Arenas-Mena et al. 2007a). The sea urchin ciliary band is a differentiated structure, but in contrast it continues to transform and proliferate during larval arm elongation and maintains the expression of H2A.Z during such continuous remodelling (Arenas-Mena et al. 2007a). Recent in vitro studies of stem cells (Creyghton et al. 2008) further support my original hypothesis of H2A.Z as a key regulator of transcriptional multipotency during metazoan development.
Heterochromatin decondensation may allow transdifferentiation. Interestingly, the expression of H2A.Z is reactivated in differentiated cells as they engage in developmental processes related to adult transformation of larval tissues, suggesting that these cells partially reverse to a state of transcriptional pluripotency (Arenas-Mena et al. 2007a). For example, reactivation of H2A.Z expression in differentiated cells is concomitant with the activation of developmental programmes for adult transformation, such as the reactivation H2A.Z and Otx in animal cap cells likely fated to contribute to adult brains (Arenas-Mena 2007; Arenas-Mena & Wong 2007). The association of H2A.Z with cancer further supports a dedifferentiation role (Hua et al. 2008). These results further suggest that indirect development may represent a less dramatic transition. Indeed, the redeployment of H2A.Z provides a mechanistic path that allows the evolutionary-developmental continuity of early embryogenesis to postembryonic development with an intermediate differentiated stage in between (Arenas-Mena et al. 2007a).
Developmental plasticity also facilitates the evolution of alternative life cycles. Polyphenism is the manifestation of more than one phenotype by the same genome (see accompanying articles in this issue). Development evolves in response to previous ecological interactions and is influenced by existing environmental conditions (Gilbert & Epel 2009; Minelli 2009). Transcriptional flexibility during physiological adaptations and during development must be facilitated by overlapping epigenetic mechanisms, which probably include cis-regulatory DNA enriched in H2A.Z nucleosomes. Thus, the evolution of alternative developmental routes for distinct environmental conditions may share similarities with the evolution of physiological adaptability. Distinct developmental outcomes may originate as exclusive alternatives of a life cycle when they involve irreversible terminal differentiation. In other cases, transient developmental adaptations could evolve into alternative life cycles by irreversibly burying cis-regulatory DNA into compact heterochromatin, perhaps after depletion of H2A.Z and any other transcriptional multipotency promoting factors (Arenas-Mena 2007; Arenas-Mena et al. 2007a).
(c). Developmental recycling of differentiated cells and the evolution of indirect development
Recycling of differentiated cells by reacquisition of proliferative potential and/or pluripotency is the distinctive characteristic of indirect development (figure 4), rather than the exclusive formation of the adult from ‘set-aside’ cells and the complete demolition of the larvae during metamorphosis. Indirect developers destabilize the transcriptional states of the differentiated larva and reacquire new developmental potential. This seems in contrast to the one-way developmental pathway of direct developers, but, as discussed above, proliferation of differentiated cells and transdifferentiation may be more common in the juvenile and adult phases of development in metazoans than is currently appreciated.
Developmental flexibility has ancient origins that paradoxically predate multicellularity. Differential gene expression in space in metazoans almost certainly evolved from differential gene expression in time already present in unicellular organisms (Kirk 1999; Arenas-Mena 2007; Mikhailov et al. 2009). The functions of H2A.Z in unicellular organisms and indirect developers suggest that transcriptional regulatory mechanisms that allow the transition from one differentiated state to another in unicellular organisms are the precursors of those that allow and stabilize differential gene expression in space in multicellular organisms (Arenas-Mena 2007). In other words, the global epigenetic mechanisms that facilitate transcriptional adaptation to the environment that are already present in unicellular organisms apparently overlap with those that facilitate transcriptional shifts during development. Comparative molecular, anatomical and ecological evidence among opisthokonts (a monophyletic group that includes metazoans, fungi and unicellular allies) further supports such a scenario (Mikhailov et al. 2009) and the hypothesis of a very early origin of biphasic development in metazoan evolution (Arenas-Mena 2007).
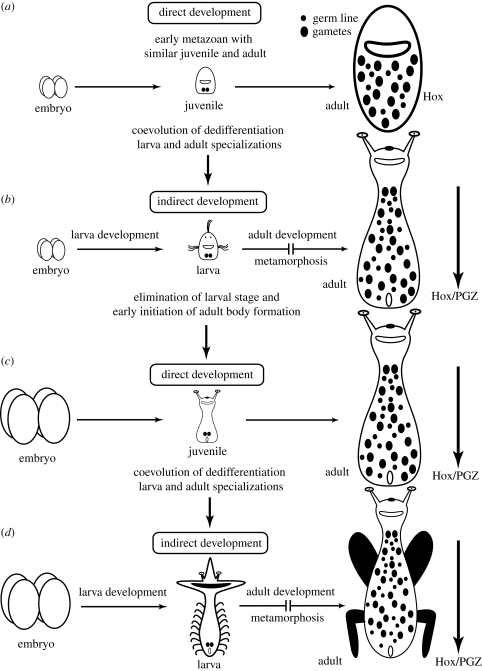
Developmental potential of differentiated cells allows the gradual evolution of indirect development. In a direct developer, juvenile-to-adult development proceeds by successively growing and remodelling a differentiated stage. Indirect development could evolve by introducing developmental novelties during the earliest differentiated stage of the life cycle, i.e. just after embryogenesis (figure 5). The process will eventually result in a very distinct early-differentiated stage, the larva, with little resemblance to the macroscopic adult. This may have been the route for the origin of ciliated larvae in early metazoans (figure 5a,b). This also seems to have been the general route for the evolution of indirect development in hemimetabolous insects (figure 5c,d), which eventually resorted to extensive use of imaginal cells in the more derived long-germband clades (Svácha 1992; Tanaka & Truman 2005; Guha et al. 2008; Nakayama-Ishimura et al. 2009). Thus, the introduction of ‘set-aside’ imaginal discs seems to be a secondary shortcut to the original route of partial dedifferentiation and subsequent restoration of developmental potential, i.e. transdifferentiation. Apparently, the developmental limitations of dedifferentiation could be circumvented with stem cells and imaginal cells, but only after the new adult developmental programmes gradually evolved.
Figure 5.
Evolution of indirect development. (a) Early metazoans with similar juvenile and adult stages. Adult development proceeds by general growth of differentiated juvenile cells and multipotent cells that are precursors of reproduction-related organs. (b) Indirect development evolves by specializing the juvenile stage to the point of being distinct from the adult (larval stage). Adult specializations also evolve during the second developmental phase, further enhancing differences between larva and adult. (c) Evolution of indirect development by increasing the resources of the egg, which allows elimination of early feeding specializations (elimination of the larval stage). (d) ‘Insect variant’ for evolution of indirect development also proceeds by specializing the juvenile stage and the adult stage.
The alternative evolutionary intercalation of an intermediate differentiated stage during the embryogenesis of a complex direct developer is mechanistically possible but unlikely, because it requires the simultaneous evolution of multiple developmental and transdifferentiation programmes. For example, the evolution of a complex pattern of transient H2A.Z silencing and reactivation would have to evolve in coordination with developmental and differentiation programmes for the eventual construction of an integrated larval stage with no actual precursor. This major coevolutionary constraint may explain why we find only the recent evolution of larval variants within the context of a biphasic life cycle (Haszprunar et al. 1995; McHugh & Rouse 1998; Reitzel et al. 2006), but no cases of recent evolution of indirect development from a life cycle that completely lacks a transiently differentiated stage.
The evolution of irreversible early differentiation would result in direct development of minute body organizations without a macroscopic phase. Indeed, the evolution of minute, simple animals from relatively complex ones has occurred repeatedly (Deutsch 2008). The acquisition of dedifferentiation without a subsequent developmental programme has no adaptive value and may result in cancer. Thus, the only option for the evolution of indirect development is gradual departure from a state of concerted differentiation and its reversibility, that is, a juvenile transforming into an adult.
(d). The larval adult outline and post-embryonic formation of reproductive organs under the control of the Hox cluster
In the context of indirect development, the anterior side of the animal corresponds to the larva and the posterior side is generated by terminal growth during feeding stages (figure 1). Terminal (or subterminal) growth by addition of successively more posterior structures that are diversified by a Hox code is considered the ancestral developmental mode of bilaterians, according to molecular, embryological and palaeontological evidence (Jacobs et al. 2005). Formation of the feeding larva of an indirectly developing sea urchin proceeds during embryogenesis without Hox cluster control (Arenas-Mena et al. 1998, 2000), although the cluster is later used in the mesoderm of the developing adult, where it abides by the spatial colinearity rule (Arenas-Mena et al. 2000). The generality of Hox cluster exclusion from embryogenesis in indirect developers was confirmed in two polychaetes with non-feeding trochophores (Irvine & Martindale 2000; Peterson et al. 2000; Kulakova et al. 2007), and Hox expression was first detected in the trochophore of a gastropod in association with adult central nervous system (CNS) formation (Hinman et al. 2003b). Thus, the embryonic functions that set the larval organization of indirect developers are independent of PGZ and Hox regulatory functions. Similarly, the anterior-most part of all bilaterians remains Hox-free territory (Hughes & Kaufman 2002; Reichert 2005; Aronowicz & Lowe 2006), whereas the posteriorly elongated body is organized by the regulatory functions of the Hox cluster (McGinnis & Krumlauf 1992). The transcription factors Caudal/Cdx, Evx/Even-skipped and Sal/Spalt are associated with the PGZ of protostomes and deuterostomes (Ferrier et al. 2001; Rosa et al. 2005; Frobius & Seaver 2006; Arenas-Mena & Wong submitted). Unlike the expression of Hox genes, expression of Caudal/Cdx, Evx/Even-skipped, and Sal/Spalt does not directly correlate with anteroposterior identities. Hox gene expression is consolidated in the new domains that are generated by posterior growth, and their early expression is controlled by PGZ transcription factors. Caudal/Cdx activates (Bel-Vialar et al. 2002) and Sal/Spalt (Copf et al. 2006) represses the early expression of Hox genes in protostomes and deuterostomes. Thus, transient regulatory events in the multipotent PGZ result in long-lasting expression of Hox genes that later implement regional specification states (Bel-Vialar et al. 2002; Copf et al. 2006).
In general, the secondarily formed posterior portion of the body is largely dedicated to the expansion of reproductive organs. This is more readily appreciated in animals with a relatively uniform anteroposterior axis such as annelids, onychophorans, millipedes, sipunculans, nemerteans or hemichordates. It is therefore possible that the developmental programmes for posterior growth and anteroposterior patterning originated in association with the development of reproductive functions. The ancestral role proposed may be obscured in animals with strong anteroposterior diversification such as insects and vertebrates, but it should be dominant in those with little diversification along the anteroposterior axis or with divergent body plans. Paradoxically, the highly derived body plan of echinoderms may offer some clues about the basal functions of the Hox cluster. In the case of echinoderms, adult formation does not proceed by anteroposterior growth. Instead, the pentameral rudiment of the adult engulfs during metamorphosis the internal bilateral structures, including the posterior coelomic mesodermal cavities (left and right somatocoels) where the Hox cluster is colinearly expressed (Arenas-Mena et al. 2000). The pentameral adult could be considered a hypertrophied feeding apparatus that overrides the bilateral organization (Arenas-Mena et al. 2000). Thus, the pentameral echinoderm rays belong to the anterior portion of the animal, and, despite their centrifugal growth, they are not patterned by the Hox vector (Arenas-Mena et al. 1998, 2000). In sea urchins, the Hox vector in the posterior coelom follows the curved gut underneath, thus it points in a curved anteroposterior direction and is perpendicular to the oral–aboral axis, the larval equivalent of the dorsoventral axis of the adult (Arenas-Mena & Wong submitted). Thus, the echinoderm Hox vector has an orientation that is similar to its orientation in other bilaterians. In the sea urchin coelom, the Hox code does not correspond to any immediate morphological diversification, although the typical spatial colinearity is maintained (Arenas-Mena et al. 2000). Gonads in echinoderms derive from the posterior coeloms, which perhaps not surprisingly express the full repertoire of germ-line markers (Juliano et al. 2006). Thus in sea urchins the Hox vector may have more to do with the construction of reproductive organs than with regional diversification.
The adult body plan of cnidarians is also radial and there is no obvious equivalent of the PGZ of bilaterians (Martindale et al. 2002). Interestingly, Cdx and Hox genes have nested patterns of expression in the anthozoan Nematostella vectensis along the directive axis (Ryan et al. 2007). The directive axis passes through the plane of bilateral symmetry and is perpendicular to the primary apical–blastoporal axis that goes from the apical tuft to the blastopore (figure 3) (Martindale et al. 2002). The directive axis intersects the siphonoglyph, a ciliated stripe of cells, and the expression domain of various genes that do not have radial distribution but instead have bilateral distribution (Martindale et al. 2002). Gametogenesis in N. vectensis takes place within the mesenteries and temporally correlates with the expression of germ-line-specific genes (Extavour et al. 2005). Mesenteries are endodermal folds elongated along the primary axis. The first two mesenteries form bilaterally at the siphonoglyph side of the directive axis and the remaining six are formed subsequently. In agreement with a potential role in gonad formation, the nested expression of the Hox and Cdx genes anthox6a, anthox7, anthox8a, anthox8b, anthox1a and NVHD065 (Cdx) correlates spatially with mesentery precursors (Ryan et al. 2007). In addition, the sequence of mesentery formation and their involvement in gametogenesis also correlates with the spatial expression of Hox genes along the directive axis (Extavour et al. 2005; Ryan et al. 2007). Interestingly, Cdx and posterior group Hox genes are also coexpressed in the gonads of the hydrozoan Clytia hemisphaerica (Chiori et al. 2009). Perhaps the main Hox vector of bilaterians corresponds with the Hox vector of the directive axis of N. vectensis and relates in both groups primarily to gonad formation. If this is the case, some core Cdx–Hox regulatory circuits should remain similar between bilaterians and cnidarians.
It has been proposed that the Hox code along the primary axis of N. vectensis is equivalent to the anteroposterior Hox code of bilaterians (Finnerty et al. 2004; Ryan et al. 2007). However, nested expression of the Hox and ParaHox genes along the secondary axis is more reminiscent of the expression in bilaterians than the restricted expression along the primary axis (Ryan et al. 2007). Also, the expression of Otx is associated with neural specifications and with the blastopore in many bilaterians (Arenas-Mena & Wong 2007), and it is therefore not the best anchor for the anterior side of the animal. The primary apical–blastoporal axis seems homologous between cnidarians and bilaterians (Wikramanayake et al. 2003), but does not need to be parallel to the Hox vector (Arenas-Mena & Wong submitted). Actually, it has been proposed that the restricted expression of basal ‘Pre’-Hox-related lineages (Gbx, HlxB, Rough, Eve, Mox) along the primary axis evolved before the Hox genes and, therefore, Hox- and ParaHox-nested expression along the secondary axis represents a secondary innovation within the group (Ryan et al. 2007).
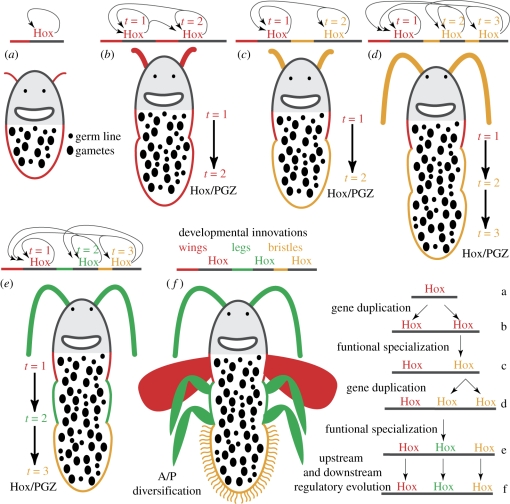
The hypothesis proposed implies that different Hox genes control the formation of virtually identical gonads in different places (figure 6), which is counter to the conventional association of the Hox code with diversification. Hox gene duplications probably released many conflicting restraints on the evolution of multifunctionality (Ganfornina & Sanchez 1999; Massingham et al. 2001) of their protein products and spatial expression (figure 6b,c). The existence of anterior, middle and posterior Hox groups suggests early protein divergence, but protein specialization may relate to functions in the distinct expression domains outside the vectorial domain (figure 6a). For example, in cnidarians, protein specializations may relate to the various functions associated with their scattered expression outside the gonads (Ryan et al. 2007; Chiori et al. 2009). Thus, paradoxically, the original function of the Hox vector may have been to control the formation of the same structure elsewhere rather than establishing differences between structures, akin to a firefighter's ladder or a freight train, with identical steps or boxcars. But why not just a single Hox gene redeployed multiple times? Perhaps largely irreversible epigenetic silencing mechanisms that control Hox gene expression (Soshnikova & Duboule 2009) would be circumvented with multiple copies, or a finite number of gonads are better regulated with multiple Hox copies. Whatever the reason, the investment in gonads is a vital process that should be under precise developmental control that may happen to be suitable for the evolution of subsequent regulatory recruitments.
Figure 6.
Hypothesis for the ancestral association of the Hox cluster with the reproductive side of the animal. (a) In early metazoans, Hox gene precursors had multiple developmental roles, which probably included gonad formation, and, in our illustration, sensory epithelium development. (b) Hox gene dosage after duplication would result in developmental changes in the structures formed; in this case, enhanced gonad and sensory organ development. Autoregulatory interactions become intergenic interactions. Temporal colinearity may concomitantly result as a byproduct of distance-dependent chromatin regulatory functions previously in place or after the asymmetric loss of intergenic regulatory interactions. (c) The duplicates undergo subfunctionalization of the original coding and regulatory multi-functionality; that is, the new genes independently adopt specializations within their original functions. (d,e) Additional tandem duplication and subsequent coding and regulatory subfunctionalization. (e,f) Once in place, recruitment of vectorial Hox cluster gene expression to anteroposterior diversification is possible. Legs, wings and sensory bristles can be differentially deployed along the anteroposterior axis. In (c–e), protein evolution is driven by functional specializations outside the colinear domain of expression.
(e). Indirect development and the genomic organization of the Hox cluster
The Hox cluster and its expression have been characterized in various direct developers (Lemons & McGinnis 2006) but in only one indirectly developing representative with feeding ciliated larva (Arenas-Mena et al. 1998, 2000; Cameron et al. 2006). This biased sample reveals a Hox cluster more ‘unorganized’ than was assumed (Duboule 2007). It has been proposed that the ancestral Hox cluster was unorganized and remained unorganized through evolution, or evolved either towards atomization or towards cluster consolidation in different lineages (Duboule 2007). Cluster consolidation in different clades would be a concomitant to the evolution of convergent regulatory mechanisms for the temporal control of colinear expression (Duboule 2007). Despite the revolutionary body plan of echinoderms, the echinoderm Hox cluster is not completely unorganized (Cameron et al. 2006): clustered organization and corresponding spatial colinear expression were found for Hox6, Hox7, Hox8, Hox9/10, Hox11/13a and Hox11/13b (Arenas-Mena et al. 2000). Two genes, Hox7 (Angerer et al. 1989) and Hox11/13b (Arenas-Mena et al. 2006), are expressed during embryogenesis, but it is misleading to claim that early embryonic expression domains contravene temporal colinearity in the post-embryonic coeloms (Monteiro & Ferrier 2006) anymore than it could be said that expression of Hox9 genes in the mammary gland of mice (Chen & Capecchi 1999) contravenes the colinearity of axial expression. Characterization of Hox cluster organization and expression of indirectly developing protostome and deuterostome representatives with more conventional body plans will also be informative.
Cluster organization correlates with temporal colinearity, and animals that develop their body at once are prone to have unorganized or atomized clusters (Duboule 2007). Feeding-dependent terminal and gradual growth of indirect developers should therefore correlate with well-organized clusters. If the PDA was an indirect developer, then the Hox clusters of indirect developers with feeding larvae should remain highly organized and should maintain the original regulatory constraints that prevent cluster atomization. If, on the contrary, the PDA had an unorganized cluster, then distinct cluster consolidation pathways (Duboule 2007) among indirect developers may have evolved. Thus, evidence of an indirectly developing PDA should manifest not only in conserved GRNs for the construction of the larvae, but also for the construction of the adults. We do not know if the spatial colinearity in the coelom of the sea urchin corresponds with temporal colinearity, but because the coelom's growth proceeds during a slow feeding-phase and seems to proceed towards the posterior, we would expect temporal colinearity rather than the contrary (Duboule 2007).
Finally, indirect development maintains temporal separation of the different modules of metazoan development, which should prevent their regulatory entanglement. This could be the case for the Hox vector, the apical–blastoporal axis, dorsal specification and probably adult CNS formation. On the contrary, the compact nature of direct development should promote some extent of entanglement among GRNs of distinct developmental processes. Thus, if indirect development is ancestral for bilaterians, then enhanced GRN conservation is expected among homologous developmental modules.
Acknowledgements
I would like to thank the insightful suggestions provided by Alessandro Minelli, Giuseppe Fusco and an anonymous reviewer during the elaboration of this manuscript. Research in our laboratory has been supported by NIH-NCRR grant 1R21RR024 195.
Footnotes
One contribution of 12 to a Theme Issue ‘From polyphenism to complex metazoan life cycles’.
References
- Albert I., Mavrich T. N., Tomsho L. P., Qi J., Zanton S. J., Schuster S. C., Pugh B. F.2007Translational and rotational settings of H2A.Z nucleosomes across the Saccharomyces cerevisiae genome. Nature 446, 572–576 (doi:10.1038/nature05632) [DOI] [PubMed] [Google Scholar]
- Allen J. D., Pernet B.2007Intermediate modes of larval development: bridging the gap between planktotrophy and lecithotrophy. Evol. Dev. 9, 643–653 [DOI] [PubMed] [Google Scholar]
- Anderson D. T.1966The comparative embryology of the Polychaeta. Acta Zool. (Stockh.) 47, 1–42 [Google Scholar]
- Angerer L., Dolecki G. J., Gagnon M. L., Lum R., Wang G., Yang Q., Humphreys T., Angerer R. C.1989Progressively restricted expression of a homeo box gene within the aboral ectoderm of developing sea urchin embryos. Genes Dev. 3, 370–383 (doi:10.1101/gad.3.3.370) [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C.2006Embryonic expression of HeFoxA1 and HeFoxA2 in an indirectly developing polychaete. Dev. Genes Evol. 216, 727–736 (doi:10.1007/s00427-006-0099-y) [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C.2007Developmental transcriptional-competence model for a histone variant and a unicellular origin scenario for transcriptional-multipotency mechanisms. Evol. Dev. 9, 208–211 [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C.2008The transcription factors HeBlimp and HeT-Brain of an indirectly developing polychaete suggest ancestral endodermal, gastrulation, and sensory cell-type specification roles. J. Exp. Zool. (Mol. Dev. Evol.) 310B, 567–576 [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C., Wong Y.2007HeOtx expression in an indirectly developing polychaete correlates with gastrulation by invagination. Dev. Genes Evol. 217, 373–384 (doi:10.1007/s00427-007-0150-7) [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C., Wong Y.Submitted The bilaterian ciliated larvae and the origin of the deuterostome mouth and the protostome anus. [Google Scholar]
- Arenas-Mena C., Martinez P., Cameron R. A., Davidson E. H.1998Expression of the Hox gene complex in the indirect development of a sea urchin. Proc. Natl Acad. Sci. USA 95, 13 062–13 067 (doi:10.1073/pnas.95.22.13062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Mena C., Cameron A. R., Davidson E. H.2000Spatial expression of Hox cluster genes in the ontogeny of a sea urchin. Development 127, 4631–4643 [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C., Cameron R. A., Davidson E. H.2006Hindgut specification and cell-adhesion functions of spHox11/13b in the endoderm of the sea urchin embryo. Dev. Growth Differ. 48, 463–472 (doi:10.1111/j.1440-169X.2006.00883.x) [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C., Suk-Ying Wong K., Arandi Foroshani N. R.2007aHistone H2A.Z expression in two indirectly developing marine invertebrates correlates with undifferentiated and multipotent cells. Evol. Dev. 9, 231–243 [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C., Wong K. S.-Y., Arandi-Foroshani N.2007bCiliary band gene expression patterns in the embryo and trochophore larva of an indirectly developing polychaete. Gene Expr. Patterns 7, 544–549 [DOI] [PubMed] [Google Scholar]
- Arendt D.2004Comparative aspects of gastrulation. In Gastrulation: from cells to embryo (ed. Stern C. D.). New York, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Arendt D., Nübler-Jung K.1997Dorsal or ventral: similarities in fate maps and gastrulation patterns in annelids, arthropods and chordates. Mech. Dev. 61, 7–21 (doi:10.1016/S0925-4773(96)00620-X) [DOI] [PubMed] [Google Scholar]
- Arendt D., Technau U., Wittbrodt J.2001Evolution of the bilaterian larval foregut. Nature 409, 81–85 (doi:10.1038/35051075) [DOI] [PubMed] [Google Scholar]
- Aronowicz J., Lowe C. J.2006Hox gene expression in the hemichordate Saccoglossus kowalevskii and the evolution of deuterostome nervous systems. Integr. Comp. Biol. 46, 890–901 (doi:10.1093/icb/icl045) [DOI] [PubMed] [Google Scholar]
- Baguñà J., Riutort M.2004The dawn of bilaterian animals: the case of acoelomorph flatworms. BioEssays 26, 1046–1057 (doi:10.1002/bies.20113) [DOI] [PubMed] [Google Scholar]
- Bel-Vialar S., Itasaki N., Krumlauf R.2002Initiating Hox gene expression: in the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development 129, 5103–5115 [DOI] [PubMed] [Google Scholar]
- Boyle M. J., Seaver E. C.2008Developmental expression of foxA and gata genes during gut formation in the polychaete annelid, Capitella sp. I. Evol. Dev. 10, 89–105 [DOI] [PubMed] [Google Scholar]
- Brinkmann H., Philippe H.2008Animal phylogeny and large-scale sequencing: progress and pitfalls. J. Syst. Evol. 46, 274–286 [Google Scholar]
- Brusca R. C., Brusca G. J.2003Invertebrates Sunderland, MA: Sinauer Associates [Google Scholar]
- Byrum C. A., Martindale M. Q.2004Gastrulation in cnidaria and ctenophora. In Gastrulation: from cells to embryo (ed. Stern C.), New York, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Cameron R. A., et al. 2006Unusual gene order and organization of the sea urchin Hox cluster. J. Exp. Zool. (Mol. Dev. Evol.) 306B, 45–58 [DOI] [PubMed] [Google Scholar]
- Carpizo-Ituarte E. J., Hadfield M. G.2003Transcription and translation inhibitors permit metamorphosis up to radiole formation in the serpulid polychaete Hydroides elegans Haswell. Biol. Bull. 204, 114–125 (doi:10.2307/1543547) [DOI] [PubMed] [Google Scholar]
- Chen F., Capecchi M. R.1999Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc. Natl Acad. Sci. USA 96, 541–546 (doi:10.1073/pnas.96.2.541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiori R., Jager M., Denker E., Wincker P., Da Silva C., Le Guyader H., Manuel M., Queinnec E.2009Are Hox genes ancestrally involved in axial patterning? Evidence from the hydrozoan Clytia hemisphaerica (Cnidaria). PLoS One 4, e4231.(doi:10.1371/journal.pone.0004231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copf T., Rabet N., Averof M.2006Knockdown of spalt function by RNAi causes de-repression of Hox genes and homeotic transformations in the crustacean Artemia franciscana. Dev. Biol. 298, 87–94 (doi:10.1016/j.ydbio.2006.07.024) [DOI] [PubMed] [Google Scholar]
- Creyghton M. P., Markoulaki S., Levine S. S., Hanna J., Lodato M. A., Sha K., Young R. A., Jaenisch R., Boyer L. A.2008H2AZ is enriched at polycomb complex target genes in ES cells and is necessary for lineage commitment. Cell 135, 649–661 (doi:10.1016/j.cell.2008.09.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen W. G. M., Klerkx A., van Loon A. E.1997Cell-specific gene regulation in early molluscan development. Invertebr. Reprod. Dev. 31, 1–9 [Google Scholar]
- Davidson E. H., Erwin D. H.2006Gene regulatory networks and the evolution of animal body plans. Science 311, 796–800 (doi:10.1126/science.1113832) [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Peterson K. J., Cameron R. A.1995Origin of bilaterian body plans—evolution of developmental regulatory mechanisms. Science 270, 1319–1325 (doi:10.1126/science.270.5240.1319) [DOI] [PubMed] [Google Scholar]
- Davidson E., et al. 2002A genomic regulatory network for development. Science 295, 1669–1678 (doi:10.1126/science.1069883) [DOI] [PubMed] [Google Scholar]
- Degnan S. M., Degnan B. M.2006The origin of the pelagobenthic metazoan life cycle: what's sex got to do with it? Integr. Comp. Biol. 46, 683–690 (doi:10.1093/icb/icl028) [DOI] [PubMed] [Google Scholar]
- Deutsch J. S.2008Do acoels climb up the ‘Scale of Beings’? Evol. Dev. 10, 135–140 [DOI] [PubMed] [Google Scholar]
- Donner A. L., Maas R. L.2004Conservation and non-conservation of genetic pathways in eye specification. Int. J. Dev. Biol. 48, 743–753 (doi:10.1387/ijdb.041877ad) [DOI] [PubMed] [Google Scholar]
- Dor Y., Brown J., Martinez O. I., Melton D. A.2004Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (doi:10.1038/nature02520) [DOI] [PubMed] [Google Scholar]
- Duboule D.2007The rise and fall of Hox gene clusters. Development 134, 2549–2560 (doi:10.1242/dev.001065) [DOI] [PubMed] [Google Scholar]
- Dunn E. F., Moy V. N., Angerer L. M., Angerer R. C., Morris R. L., Peterson K. J.2007Molecular paleoecology: using gene regulatory analysis to address the origins of complex life cycles in the late Precambrian. Evol. Dev. 9, 10–24 [DOI] [PubMed] [Google Scholar]
- Dunn C. W., et al. 2008Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 (doi:10.1038/nature06614) [DOI] [PubMed] [Google Scholar]
- Extavour C. G., Pang K., Matus D. Q., Martindale M. Q.2005vasa and nanos expression patterns in a sea anemone and the evolution of bilaterian germ cell specification mechanisms. Evol. Dev. 7, 201–215 (doi:10.1111/j.1525-142X.2005.05023.x) [DOI] [PubMed] [Google Scholar]
- Ferrier D. E. K., Minguillón C., Cebrian C., Garcia-Fernàndez J.2001Amphioxus Evx genes: implications for the evolution of the midbrain–hindbrain boundary and the chordate tailbud. Dev. Biol. 237, 270–281 (doi:10.1006/dbio.2001.0375) [DOI] [PubMed] [Google Scholar]
- Finnerty J. R., Pang K., Burton P., Paulson D., Martindale M. Q.2004Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science 304, 1335–1337 (doi:10.1126/science.1091946) [DOI] [PubMed] [Google Scholar]
- Fritzenwanker J. H., Saina M., Technau U.2004Analysis of forkhead and snail expression reveals epithelial–mesenchymal transitions during embryonic and larval development of Nematostella vectensis. Dev. Biol. 275, 389–402 (doi:10.1016/j.ydbio.2004.08.014) [DOI] [PubMed] [Google Scholar]
- Frobius A. C., Seaver E. C.2006ParaHox gene expression in the polychaete annelid Capitella sp. I. Dev. Genes Evol. 216, 81–88 (doi:10.1007/s00427-005-0049-0) [DOI] [PubMed] [Google Scholar]
- Ganfornina M. D., Sanchez D.1999Generation of evolutionary novelty by functional shift. Bioessays 21, 432–439 (doi:10.1002/(SICI)1521-1878(199905)21:5<432::AID-BIES10>3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Ikeo K.1999Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 15, 371–377 (doi:10.1016/S0168-9525(99)01776-X) [DOI] [PubMed] [Google Scholar]
- Gilbert S. F., Epel D.2009Ecological developmental biology. Integrating epigenetics, medicine and evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- Giribet G.2008Assembling the lophotrochozoan (=spiralian) tree of life. Phil. Trans. R. Soc. B 363, 1513–1522 (doi:10.1098/rstb.2007.2241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giribet G., Dunn C. W., Edgecombe G. D., Rouse G. W.2007A modern look at the animal tree of life. Zootaxa 1668, 61–79 [Google Scholar]
- Grobben K.1908Die systematische Einteilung des Tierreichs. Verh. Zool. Bot. Ges. Wien 58, 491–511 [Google Scholar]
- Gross J. M., McClay D. R.2001The role of Brachyury (T) during gastrulation movements in the sea urchin Lytechinus variegatus. Dev. Biol. 239, 132–147 (doi:10.1006/dbio.2001.0426) [DOI] [PubMed] [Google Scholar]
- Gross J. M., Peterson R. E., Wu S. Y., McClay D. R.2003LvTbx2/3: a T-box family transcription factor involved in formation of the oral/aboral axis of the sea urchin embryo. Development 130, 1989–1999 (doi:10.1242/dev.00409) [DOI] [PubMed] [Google Scholar]
- Guha A., Lin L., Kornberg T. B.2008Organ renewal and cell divisions by differentiated cells in Drosophila. Proc. Natl Acad. Sci. USA 105, 10 832–10 836 (doi:10.1073/pnas.0805111105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield M., Meleshkevitch E., Boudko D.2000The apical sensory organ of a gastropod veliger is a receptor for settlement cues. Biol. Bull. 198, 67–76 (doi:10.2307/1542804) [DOI] [PubMed] [Google Scholar]
- Haszprunar G., Vonsalviniplawen L., Rieger R. M.1995Larval planktotrophy—a primitive trait in the Bilateria. Acta Zool. 76, 141–154 [Google Scholar]
- Hay-Schmidt A.2000The evolution of the serotonergic nervous system. Proc. R. Soc. Lond. B 267, 1071–1079 (doi:10.1098/rspb.2000.1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejnol A., Martindale M. Q.2008aAcoel development indicates the independent evolution of the bilaterian mouth and anus. Nature 456, 382–386 (doi:10.1038/nature07309) [DOI] [PubMed] [Google Scholar]
- Hejnol A., Martindale M. Q.2008bAcoel development supports a simple planula-like urbilaterian. Phil. Trans. R. Soc. B 363, 1493–1501 (doi:10.1098/rstb.2007.2239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. Q., Hejnol A., Perry K. J., Martindale M. Q.2007Homology of ciliary bands in spiralian trochophores. Integr. Comp. Biol. 47, 865–871 (doi:10.1093/icb/icm035) [DOI] [PubMed] [Google Scholar]
- Hinman V. F., Nguyen A. T., Cameron R. A., Davidson E. H.2003aDevelopmental gene regulatory network architecture across 500 million years of echinoderm evolution. Proc. Natl Acad. Sci. USA 100, 13 356–13 361 (doi:10.1073/pnas.2235868100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman V. F., O'Brien E. K., Richards G. S., Degnan B. M.2003bExpression of anterior Hox genes during larval development of the gastropod, Haliotis asinina. Evol. Dev. 5, 508–521 (doi:10.1046/j.1525-142X.2003.03056.x) [DOI] [PubMed] [Google Scholar]
- Hua S., et al. 2008Genomic analysis of estrogen cascade reveals histone variant H2A.Z associated with breast cancer progression. Mol. Syst. Biol. 4, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. L., Kaufman T. C.2002Hox genes and the evolution of the arthropod body plan. Evol. Dev. 4, 459–499 (doi:10.1046/j.1525-142X.2002.02034.x) [DOI] [PubMed] [Google Scholar]
- Hyman L. H.1940The invertebrates: Protozoa through Ctenophora New York, NY: McGraw-Hill [Google Scholar]
- Irvine S. Q., Martindale M. Q.2000Expression patterns of anterior Hox genes in the polychaete Chaetopterus: correlation with morphological boundaries. Dev. Biol. 217, 333–351 (doi:10.1006/dbio.1999.9541) [DOI] [PubMed] [Google Scholar]
- Jacobs D. K., Hughes N. C., Fitz-Gibbon S. T., Winchell C. J.2005Terminal addition, the Cambrian radiation and the Phanerozoic evolution of bilaterian form. Evol. Dev. 7, 498–514 (doi:10.1111/j.1525-142X.2005.05055.x) [DOI] [PubMed] [Google Scholar]
- Jägersten G.1972Evolution of the metazoan life cycle: a comprehensive theory London, UK: Academic Press [Google Scholar]
- Jin C., Felsenfeld G.2007Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 21, 1519–1529 (doi:10.1101/gad.1547707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C. E., Voronina E., Stack C., Aldrich M., Cameron A. R., Wessel G. M.2006Germ line determinants are not localized early in sea urchin development, but do accumulate in the small micromere lineage. Dev. Biol. 300, 406–415 (doi:10.1016/j.ydbio.2006.07.035) [DOI] [PubMed] [Google Scholar]
- Kirk D. L.1999Evolution of multicellularity in the volvocine algae. Curr. Opin. Plant Biol. 2, 496–501 (doi:10.1016/S1369-5266(99)00019-9) [DOI] [PubMed] [Google Scholar]
- Kulakova M., et al. 2007Hox gene expression in larval development of the polychaetes Nereis virens and Platynereis dumerilii (Annelida, Lophotrochozoa). Dev. Genes Evol. 217, 39–54 (doi:10.1007/s00427-006-0119-y) [DOI] [PubMed] [Google Scholar]
- Lartillot N., Le Gouar M., Adoutte A.2002Expression patterns of fork head and goosecoid homologues in the mollusc Patella vulgata supports the ancestry of the anterior mesendoderm across Bilateria. Dev. Genes Evol. 212, 551–561 (doi:10.1007/s00427-002-0274-8) [DOI] [PubMed] [Google Scholar]
- Lemons D., McGinnis W.2006Genomic evolution of Hox gene clusters. Science 313, 1918–1922 (doi:10.1126/science.1132040) [DOI] [PubMed] [Google Scholar]
- Leys S. P., Degnan B. M.2002Embryogenesis and metamorphosis in a haplosclerid demosponge: gastrulation and transdifferentiation of larval ciliated cells to choanocytes. Invertebr. Biol. 121, 171–189 [Google Scholar]
- Love A. C., Lee A. E., Andrews M. E., Raff R. A.2008Co-option and dissociation in larval origins and evolution: the sea urchin larval gut. Evol. Dev. 10, 74–88 [DOI] [PubMed] [Google Scholar]
- Martindale M. Q.2005The evolution of metazoan axial properties. Nat. Rev. Genet. 6, 917–927 (doi:10.1038/nrg1725) [DOI] [PubMed] [Google Scholar]
- Martindale M. Q., Finnerty J. R., Henry J. Q.2002The Radiata and the evolutionary origins of the bilaterian body plan. Mol. Phylogenet. Evol. 24, 358–365 (doi:10.1016/S1055-7903(02)00208-7) [DOI] [PubMed] [Google Scholar]
- Maslakova S., Martindale M. Q., Norenburg J.2004Vestigial prototroch in a basal nemertean, Carinoma tremaphoros (Nemertea; Palaeonemertea). Evol. Dev. 6, 219–226 (doi:10.1111/j.1525-142X.2004.04027.x) [DOI] [PubMed] [Google Scholar]
- Massingham T., Davies L. J., Lio P.2001Analysing gene function after duplication. Bioessays 23, 873–876 (doi:10.1002/bies.1128) [DOI] [PubMed] [Google Scholar]
- Mavrich T. N., et al. 2008Nucleosome organization in the Drosophila genome. Nature 453, 358–362 (doi:10.1038/nature06929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEdward L. R., Miner B. G.2001Larval and life-cycle patterns in echinoderms. Can. J. Zool. 79, 1125–1170 (doi:10.1139/cjz-79-7-1125) [Google Scholar]
- McGinnis W., Krumlauf R.1992Homeobox genes and axial patterning. Cell 68, 283–302 (doi:10.1016/0092-8674(92)90471-N) [DOI] [PubMed] [Google Scholar]
- McHugh D., Rouse G. W.1998Life history evolution of marine invertebrates: new views from phylogenetic systematics. Trends Ecol. Evol. 13, 182–186 (doi:10.1016/S0169-5347(97)01285-8) [DOI] [PubMed] [Google Scholar]
- Meneghini M. D., Wu M., Madhani H. D.2003Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112, 725–736 (doi:10.1016/S0092-8674(03)00123-5) [DOI] [PubMed] [Google Scholar]
- Mikhailov K. V., et al. 2009The origin of metazoa: a transition from temporal to spatial cell differentiation. BioEssays 31, 758–768 (doi:10.1002/bies.200800214) [DOI] [PubMed] [Google Scholar]
- Minelli A.2009Perspectives in animal phylogeny and evolution New York, NY: Oxford University Press [Google Scholar]
- Mione M., Shanmugalingam S., Kimelman D., Grifin K.2001Overlapping expression of zebrafish T-Brain-1 and eomesodermin during forebrain development. Mech. Dev. 100, 93–97 (doi:10.1016/S0925-4773(00)00501-3) [DOI] [PubMed] [Google Scholar]
- Mittmann B., Scholtz G.2001Distal-less expression in embryos of Limulus polyplremus (Chelicerata, Xiphosura) and Lepisma saccharina (Insecta, Zygentoma) suggests a role in the development of mechanoreceptors, chemoreceptors, and the CNS. Dev. Genes Evol. 211, 232–243 (doi:10.1007/s004270100150) [DOI] [PubMed] [Google Scholar]
- Monteiro A. S., Ferrier D. E. K.2006Hox genes are not always colinear. Int. J. Biol. Sci. 2, 95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H., Hibino T., Oji T., Hara Y., Amemiya S.2003Larval stages of a living sea lily (stalked crinoid echinoderm). Nature 421, 158–160 [DOI] [PubMed] [Google Scholar]
- Nakayama-Ishimura A., Chambon J. P., Hofie T., Satoh N., Sasakura Y.2009Delineating metamorphic pathways in the ascidian Ciona intestinalis. Dev. Biol. 326, 357–367 (doi:10.1016/j.ydbio.2008.11.026) [DOI] [PubMed] [Google Scholar]
- Nederbragt A., te Welscher P., van den Driesche S., van Loon A. E., Dictus W. J.2002Novel and conserved roles for orthodenticle/otx and orthopedia/otp orthologs in the gastropod mollusc Patella vulgata. Dev. Genes Evol. 212, 330–337 (doi:10.1007/s00427-002-0246-z) [DOI] [PubMed] [Google Scholar]
- Nielsen C.2005aLarval and adult brains. Evol. Dev. 7, 483–489 (doi:10.1111/j.1525-142X.2005.05051.x) [DOI] [PubMed] [Google Scholar]
- Nielsen C.2005bTrochophora larvae: cell-lineages, ciliary bands and body regions. 2. Other groups and general discussion. J. Exp. Zool. (Mol. Dev. Evol.) 304B, 401–447 [DOI] [PubMed] [Google Scholar]
- Nielsen C., Nørrevang A.1985The trochaea theory: an example of life cycle phylogeny. In The origins and relationships of lower invertebrates (eds Conway Morris S., George J. D., Gibson R., Platt H. M.), pp. 28–41 Oxford, UK: Clarendon Press [Google Scholar]
- Oliveri P., Walton K. D., Davidson E. H., McClay D. R.2006Repression of mesodermal fate by foxa, a key endoderm regulator of the sea urchin embryo. Development 133, 4173–4181 (doi:10.1242/dev.02577) [DOI] [PubMed] [Google Scholar]
- Olson E. N.2006Gene regulatory networks in the evolution and development of the heart. Science 313, 1922–1927 (doi:10.1126/science.1132292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechenik J. A.2004Biology of the invertebrates New York, NY: McGraw-Hill [Google Scholar]
- Pehrson J. R., Cohen L. H.1986The fate of the small micromeres in sea-urchin development. Dev. Biol. 113, 522–526 (doi:10.1016/0012-1606(86)90188-0) [DOI] [PubMed] [Google Scholar]
- Peterson K. J., Cameron R. A., Davidson E. H.1997Set-aside cells in maximal indirect development: evolutionary and developmental significance. BioEssays 19, 623–631 (doi:10.1002/bies.950190713) [DOI] [PubMed] [Google Scholar]
- Peterson K. J., Irvine S. Q., Cameron R. A., Davidson E. H.2000Quantitative assessment of Hox complex expression in the indirect development of the polychaete annelid Chaetopterus sp. Proc. Natl Acad. Sci. USA 97, 4487–4492 (doi:10.1073/pnas.97.9.4487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe H., Lartillot N., Brinkmann H.2005Multigene analyses of bilaterian animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa, and Protostomia. Mol. Biol. Evol. 22, 1246–1253 (doi:10.1093/molbev/msi111) [DOI] [PubMed] [Google Scholar]
- Pichaud F., Desplan C.2002Pax genes and eye organogenesis. Curr. Opin. Genet. Dev. 12, 430–434 (doi:10.1016/S0959-437X(02)00321-0) [DOI] [PubMed] [Google Scholar]
- Raff R. A.2008Origins of the other metazoan body plans: the evolution of larval forms. Phil. Trans. R. Soc. B 363, 1473–1479 (doi:10.1098/rstb.2007.2237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert H.2005A tripartite organization of the urbilaterian brain: developmental genetic evidence from Drosophila. Brain Res. Bull. 66, 491–494 (doi:10.1016/j.brainresbull.2004.11.028) [DOI] [PubMed] [Google Scholar]
- Reitzel A. M., Sullivan J. C., Finnerty J. R.2006Qualitative shift to indirect development in the parasitic sea anemone Edwardsiella lineata. Integr. Comp. Biol. 46, 827–837 (doi:10.1093/icb/icl032) [DOI] [PubMed] [Google Scholar]
- Rieger R. M.1994The biphasic life-cycle—a central theme of metazoan evolution. Am. Zool. 34, 484–491 [Google Scholar]
- Rosa R., Prud'homme B., Balavoine G.2005caudal and even-skipped in the annelid Platynereis dumerilii and the ancestry of posterior growth. Evol. Dev. 7, 574–587 (doi:10.1111/j.1525-142X.2005.05061.x) [DOI] [PubMed] [Google Scholar]
- Ryan K., Butler K., Bellefroid E., Gurdon J.1998Xenopus eomesodermin is expressed in neural differentiation. Mech. Dev. 75, 155–158 (doi:10.1016/S0925-4773(98)00084-7) [DOI] [PubMed] [Google Scholar]
- Ryan J. F., Mazza M. E., Pang K., Matus D. Q., Baxevanis A. D., Martindale M. Q., Finnerty J. R.2007Pre-bilaterian origins of the Hox cluster and the Hox code: evidence from the sea anemone, Nematostella vectensis. PLoS ONE 2, e153 (doi:10.1371/journal.pone.0000153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierwater B., Eitel M., Jakob W., Osigus H. J., Hadrys H., Dellaporta S. L., Kolokotronis S. O., DeSalle R.2009Concatenated analysis sheds light on early metazoan evolution and fuels a modern ‘Urmetazoon’ hypothesis. PLoS Biol. 7, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver E. C., Kaneshige L. M.2006Expression of 'segmentation' genes during larval and juvenile development in the polychaetes Capitella sp. I and H. elegans. Dev. Biol. 289, 179–194 (doi:10.1016/j.ydbio.2005.10.025) [DOI] [PubMed] [Google Scholar]
- Seipp S., Schmich J., Kehrwald T., Leitz T.2007Metamorphosis of Hydractinia echinata—natural versus artificial induction and developmental plasticity. Dev. Genes Evol. 217, 385–394 (doi:10.1007/s00427-007-0151-6) [DOI] [PubMed] [Google Scholar]
- Sempere L. F., Martinez P., Cole C., Baguñà J., Peterson K. J.2007Phylogenetic distribution of microRNAs supports the basal position of acoel flatworms and the polyphyly of Platyhelminthes. Evol. Dev. 9, 409–415 [DOI] [PubMed] [Google Scholar]
- Sly B. J., Snoke M. S., Raff R. A.2003Who came first—larvae or adults? Origins of bilaterian metazoan larvae. Int. J. Dev. Biol. 47, 623–632 [PubMed] [Google Scholar]
- Smith J., Kraemer E., Liu H. D., Theodoris C., Davidson E.2008A spatially dynamic cohort of regulatory genes in the endomesodermal gene network of the sea urchin embryo. Dev. Biol. 313, 863–875 (doi:10.1016/j.ydbio.2007.10.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshnikova N., Duboule D.2009Epigenetic temporal control of mouse Hox genes in vivo. Science 324, 1320–1323 (doi:10.1126/science.1171468) [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Truman J. W., Riddiford L. M.2008The role of broad in the development of Tribolium castaneum: implications for the evolution of the holometabolous insect pupa. Development 135, 569–577 (doi:10.1242/dev.015263) [DOI] [PubMed] [Google Scholar]
- Svácha P.1992What are and what are not imaginal discs: reevaluation of some basic concepts (Insecta, Holometabola). Dev. Biol. 154, 101–117 [DOI] [PubMed] [Google Scholar]
- Tagawa K., Humphreys T., Satoh N.1998Novel pattern of Brachyury gene expression in hemichordate embryos. Mech. Dev. 75, 139–143 (doi:10.1016/S0925-4773(98)00078-1) [DOI] [PubMed] [Google Scholar]
- Tagawa K., Humphreys T., Satoh N.2000T-brain expression in the apical organ of hemichordate tornaria larvae suggests its evolutionary link to the vertebrate forebrain. J. Exp. Zool. 288, 23–31 (doi:10.1002/(SICI)1097-010X(20000415)288:1<23::AID-JEZ3>3.0.CO;2-H) [DOI] [PubMed] [Google Scholar]
- Tanaka K., Truman J. W.2005Development of the adult leg epidermis in Manduca sexta: contribution of different larval cell populations. Dev. Genes Evol. 215, 78–89 (doi:10.1007/s00427-004-0458-5) [DOI] [PubMed] [Google Scholar]
- Technau U., Scholz C.2003Origin and evolution of endoderm and mesoderm. Int. J. Dev. Biol. 47, 531–539 [PubMed] [Google Scholar]
- Valentine J. W., Collins A. G.2000The significance of moulting in Ecdysozoan evolution. Evol. Dev. 2, 152–156 (doi:10.1046/j.1525-142x.2000.00043.x) [DOI] [PubMed] [Google Scholar]
- Wagner G. P.2007The developmental genetics of homology. Nat. Rev. Genet. 8, 473–479 (doi:10.1038/nrg2099) [DOI] [PubMed] [Google Scholar]
- Wikramanayake A. H., Hong M., Lee P. N., Pang K., Byrum C. A., Bince J. M., Xu R., Martindale M. Q.2003Axial polarity and germ layer segregation in the basal cnidarian Nematostella vectensis: an ancient role for β-catenin. Integr. Comp. Biol. 43, 1027. [DOI] [PubMed] [Google Scholar]
- Williams E. A., Degnan B. M., Gunter H., Jackson D. J., Woodcroft B. J., Degnan S. M.2009Widespread transcriptional changes pre-empt the critical pelagic–benthic transition in the vetigastropod Haliotis asinina. Mol. Ecol. 18, 1006–1025 (doi:10.1111/j.1365-294X.2008.04078.x) [DOI] [PubMed] [Google Scholar]
- Wilson K. A., Andrews M. E., Raff R. A.2005Dissociation of expression patterns of homeodomain transcription factors in the evolution of developmental mode in the sea urchins Heliocidaris tuberculata and H. erythrogramma. Evol. Dev. 7, 401–415 (doi:10.1111/j.1525-142X.2005.05045.x) [DOI] [PubMed] [Google Scholar]
- Wray G. A.1996Parallel evolution of nonfeeding larvae in echinoids. Syst. Biol. 45, 308–322 [Google Scholar]
- Yuan D., Nakanishi N., Jacobs D. K., Hartenstein V.2008Embryonic development and metamorphosis of the scyphozoan Aurelia. Dev. Genes Evol. 218, 525–539 (doi:10.1007/s00427-008-0254-8) [DOI] [PubMed] [Google Scholar]