Abstract
This theme issue pursues an exploration of the potential of taking into account the environmental sensitivity of development to explaining the evolution of metazoan life cycles, with special focus on complex life cycles and the role of developmental plasticity. The evolution of switches between alternative phenotypes as a response to different environmental cues and the evolution of the control of the temporal expression of alternative phenotypes within an organism's life cycle are here treated together as different dimensions of the complex relationships between genotype and phenotype, fostering the emergence of a more general and comprehensive picture of phenotypic evolution through a quite diverse sample of case studies. This introductory article reviews fundamental facts and concepts about phenotypic plasticity, adopting the most authoritative terminology in use in the current literature. The main topics are types and components of phenotypic variation, the evolution of organismal traits through plasticity, the origin and evolution of phenotypic plasticity and its adaptive value.
Keywords: life cycle, genetic accommodation, metazoans, polyphenism, reaction norm
In the context of the modern synthesis, the role of environment in organic evolution can be roughly summarized by the well-known phrase: ‘environment proposes, natural selection disposes’, which expresses the one-way relationship between environment and adaptation in orienting the direction of evolutionary change. The organism is thus seen as the ‘passive object of evolutionary forces’; one, the environment, generating ‘problems’ at random with respect to the organization and the performances of the organism, and the other, the organism's internal genetic machinery, generating ‘solutions’ at random with respect to the ‘problems’ posed by the environment (discussed in Lewontin 2000). The relationship between genotype and environment is thus restricted to selection by the latter on the phenotypes controlled by the former.
In recent years, a growing sense of dissatisfaction with this picture as the ultimate description of evolutionary change has been emerging. It appears that reducing the origin of variation to genetic mutation and recombination not only overlooks a growing mass of data on inheritable epigenetic variation (Jablonka & Lamb 2005; Gilbert & Epel 2009), but also fails to explain evolutionary routes of change that can be fully understood only by taking into account the environmental influences on the phenotype throughout the developmental processes (Minelli & Fusco 2008).
Phenotypic evolution depends on phenotypic variation, and in metazoans, as in other multicellular organisms, phenotypic variation (when not explicitly restricted to a given developmental stage) is variation in developmental trajectories throughout the ontogeny (Fusco 2001). An individual organism's trajectory is the result of a unique interaction between its genome(s), the temporal sequence of external environments to which it is exposed during its life and random events at the level of molecular interactions in its tissues (Lewontin 2000). Thus, ‘development is larger than just developmental genetics’, and there is a plethora of environmentally induced components of developmental variation that are relevant for both the ecology and the evolution of a species (Gilbert et al. 2010). As proximate causes of phenotypic variation, genes and environment are thus inextricably linked (Crispo 2007).
The contributions to this volume aim at exploring the potential of an integrated developmental and environmental approach to explaining the evolution of metazoan life cycles, with special focus on complex life cycles and the role of developmental plasticity. What is the value of taking the environmental sensitivity of developmental processes into the picture to account for the evolution of the metazoan life cycle? Is there a common genetic and/or epigenetic background shared by environmentally sensitive and insensitive developmental pathways? This theme issue carries out this exploration through a quite diverse sample of case studies that, while showing the multiplicity of ecological and evolutionary facets of the subject, will collectively provide large scope for basic conceptual revisitations.
This introductory article aims at presenting the main themes covered by the volume as well as at clarifying concepts and terms that are relevant for the analysis and discussion of the subject.
1. Evolution through plasticity
Phenotypic plasticity can be defined as ‘the ability of individual genotypes to produce different phenotypes when exposed to different environmental conditions’ (Pigliucci et al. 2006). This includes the possibility to modify developmental trajectories in response to specific environmental cues, and also the ability of an individual organism to change its phenotypic state or activity (e.g. its metabolism) in response to variations in environmental conditions (Garland & Kelly 2006).
Well-known textbook examples of plastic development are seasonal polyphenism in butterflies (e.g. Brakefield & Frankino 2007), caste polyphenism in social insects (e.g. Miura 2005), environmental sex determination in reptiles (e.g. Janzen & Phillips 2006) and predator-induced polyphenism in cladocerans (e.g. Laforsch & Tollrian 2004), but also phenotypic changes like acclimation, learning and the immune system adaptation are part of the repertoire of an organism's plastic responses (reviewed in Gilbert & Epel 2009).
As with any organismal trait, the way in which an individual responds to environmental influences is subject to evolutionary change. The ecological role and evolution of phenotypic plasticity is a highly debated issue in current evolutionary research. The literature is vast, but the interested reader can find valuable introductions to the subject in some keystone reviews (e.g. Schlichting & Pigliucci 1998; Greene 1999; Pigliucci 2001; West-Eberhard 2003, 2005; DeWitt & Scheiner 2004). However, the main focus here is not on the evolution of plasticity, but rather on the evolution of phenotypic traits and organismal diversity through plasticity, i.e. the role of plasticity in evolution. Although it is generally acknowledged that phenotypic plasticity is an important property of developmental systems, that allows the organism to cope with environmental unpredictability and/or heterogeneity, its role in adaptive evolution remains contentious (e.g. de Jong 2005). For instance, although it is generally acknowledged that phenotypic plasticity can increase organism survival under specific conditions, there is no general agreement on whether plasticity can drive the evolution of novel traits and promote taxonomic diversity, or on whether it has more often the effect of accelerating or retarding evolutionary change (Price et al. 2003; West-Eberhard 2003).
We will enter this discussion by widening the view on plasticity: along with its role in adaptive evolution in a variety of ecological contexts, we will also consider its value as a source of variation in the evolution of novel life cycles. There are many ways to investigate the role of the evolution of developmental genes and gene networks in the evolution of multicellular organisms. To date, the evolution of switches between alternative phenotypes as a response to different environmental cues has often been treated as a separate problem from the evolution of the control of the temporal expression of alternative phenotypes within an organism's life cycle. However, these different dimensions of the complex relationships between genotype and phenotype are possibly interrelated and may reveal general principles at the level of developmental processes and/or at the level of the gene networks controlling them. Rephrasing the question in a phylogenetic perspective, can the origin of the sometimes very different phenotypes that correspond to ontogenetic stages also be found in alternative phenotypes within an ancestral polyphenism? Or, vice versa, is it likely that from the sequence of phenotypes in an ancestral complex ontogeny, a developmental system with multiple alternative phenotypic trajectories eventually evolved?
A definitive answer to these questions is beyond the reach of this collection of papers. This theme issue's contributions aim instead at fostering the emergence of a more general and comprehensive picture of phenotypic evolution by integrating different aspects of plasticity that heretofore have often been treated separately. These include the possible contribution of phenotypic plasticity to organismal diversification (Pfennig & McGee 2010), the evolution of novel traits (Moczek 2010), the evolution of life cycles with indirect development (Degnan & Degnan 2010) and the very origins of the metazoan clade (Arenas-Mena 2010).
2. Variation: types and components
Since the first formulation of the Darwinian theory of evolution (Darwin 1859), variation has occupied a central role (Hallgrímsson & Hall 2005), as it provides the ‘rough material’ for evolutionary sorting processes, namely natural selection and random drift (Fusco 2001). In recent years, structure and origin of phenotypic variation have been considered the required ‘missing piece’ to complement the standard (neo-Darwinian) theory of evolution (e.g. Müller 2007), and their formal inclusion in the theory has been regarded as an obligate step towards an extended new synthesis that will expand the explanatory reach of the current evolutionary theory (Pigliucci & Müller 2010).
Thus, understanding both the genetic and developmental causes of phenotypic variation and their evolutionary consequences is a major goal in evolutionary biology (Greene 1999). However, investigating these sorts of questions requires a precise characterization of the nature and the structure of variation.
(a). Types of variation
Intraspecific variation (Darwin's ‘individual variability’; Darwin 1859) is ubiquitous in the living world. This variation is generally referred to as phenotypic variation, less frequently as phenotypic polymorphism, and can be variously qualified and/or partitioned. In the stereotyped view of the modern synthesis, that ‘evolution consists of genetic changes in populations over time’ (Futuyma 2005, p. 190), the most obvious divide is the one between genetic and environmental sources of variation. Mayr (1963) introduced the term polyphenism to distinguish environmentally induced phenotypic variation (‘the occurrence of several phenotypes in a population, the differences between which are not the result of genetic differences’; Mayr 1963, p. 670) from genetically controlled phenotypic variation, or genetic polymorphism. The term polymorphism, without further qualifications, is often used as a shorthand for genetic polymorphism (whether or not this has recognizable phenotypic effects), or for a phenotypic polymorphism with solid genetic basis. A genetic polymorphism does not necessarily translate into phenotypic variation: this is the case of many selectively neutral genetic markers, like microsatellite DNA. Standing genetic variation that does not contribute to phenotypic variation under standard conditions, while having the potential to modify the phenotype following a change in environmental setting or genetic context, is called cryptic genetic variation (see McGuigan & Sgrò (2009) for a recent review on its role in evolution).
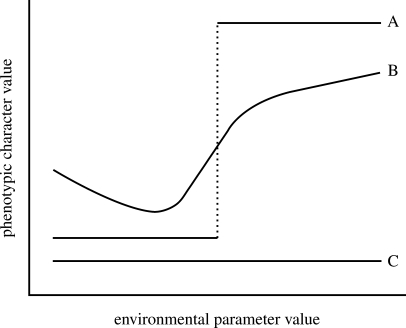
The term reaction norm refers to the set of phenotypes that can be produced by an individual genotype when exposed to different environmental conditions (Schlichting & Pigliucci 1998). This is often represented in the form of a mathematical function that associates the values of one or more environmental parameters (in a biologically relevant interval) to the values of one or more phenotypic characters (figure 1). A plastic character presents a reaction norm with a sizable codomain, whereas non-plastic characters present an environmentally invariant value (a flat reaction norm). A non-plastic character is said to be monophenic. The attribute ‘plastic’ is generally associated with a character, but it can also be referred to a natural population, a laboratory strain, a sex or a cohort within a species. Usually, the term polyphenism is restricted to the case in which two or more distinct phenotypes (without intermediates) are elicited by the environmental cue; thus polyphenism is a particular case of phenotypic plasticity (West-Eberhard 2003). The phenotypic discontinuity that characterizes a polyphenism can either be due to a real discontinuity in the reaction norm (as in reaction norm A of figure 1), reflecting an environmentally induced threshold-like switch from one developmental pathway to another, or be due to the effect of discontinuities in the values of relevant environmental parameters to which the organism is exposed, resulting in the expression of discrete phenotypes representing portions of an otherwise continuous reaction norm (Nijhout 2003).
Figure 1.
Schematic representation of the reaction norms for three characters (A–C): A and B are plastic (plastic reaction norm), whereas C is a non-plastic character (non-plastic reaction norm). A is a polyphenic character, while C is monophenic.
Environmental and genetic influences are inseparably entangled in both development (as phenotype determinants) and evolution (as it is through their interaction that selectable phenotypic variation emerges). Although they are often perceived as distinct phenomena, several lines of evidence suggest that they should be considered together as interdependent components of the processes that generate phenotypic variation and drive its evolution.
First, the relative contributions of the possible proximate causes of phenotypic variation form a continuum, where genetic and environmental components have opposite gradients of relative magnitude (Greene 1999). This relation is embodied in the simplest version of the classic quantitative genetics expression for variation components of a phenotypic trait, measured as statistical variance: VP = VG + VE + VG×E, where the population's phenotypic variance (VP) is partitioned into genetic (VG), environmental (VE) and genotype-by-environment (VG×E) variance. We speak of ‘genetic polymorphism’ when VG dominates over the other components and of ‘phenotypic plasticity’ when VE is the major term in the right-hand side of the equation. Non-parallel reaction norms for different genotypes of the same species indicate the presence of genotype-by-environment interactions (VG×E ≠ 0).
Second, beyond the relative weight of the different components in the observed phenotypic variation, genetic and environmental effects may exhibit very diverse forms of interaction, manifested in different compartments of the developmental system and at different levels of the regulation network. For instance, it would be misleading to classify a priori a polyphenism as a ‘non-genetic’ phenomenon, as individual variation in the reaction norm that underlies the response to the external environmental cues may have a firm genetic basis (Greene 1999).
Third, to a certain degree, the effects of genes and environments might be interchangeable. This is referred to as gene–environment equivalence or gene–environment interchangeability (West-Eberhard 2003). Some specific environmental conditions can induce phenotypes (phenocopies) that mimic otherwise genetically specified traits, and, vice versa, mutant genes can cause phenotypes (genocopies) to mimic environmentally elicited phenotypes. Depending on the context (e.g. species, population, genotype, environment), the activation of the same alternative physiological responses, or the same developmental choice among alternative developmental pathways, can either be controlled by a genetic polymorphism or elicited by an environmental cue (e.g. melanism in butterflies, Nijhout (1991); sex determination in reptiles, Janzen & Phillips (2006)). This can be explained at the biochemical level by the fact that the same regulative effect on the expression of a target gene can be produced either by an environmental stimulus that causes changes in the relative concentration of a relevant transcription factor, or by a genetic modification of the corresponding cis-regulative sequence (Zuckerkandl & Villet 1988).
Fourth, from the point of view of the process of adaptation of the developmental system, both environmental cues and genetic constitution can be viewed as operationally equivalent sources of information about which developmental path is likely to give rise to the best phenotype with which to confront the coming selective conditions (Leimar 2009; see also below).
A few broad-ranging conceptual instruments have been offered to bridge the gap between the different components of phenotypic variation and phenotypic evolution. The most widely recognized among them is genotype–phenotype mapping that since its original definition (Alberch 1991) has undergone considerable empirical improvement and theoretical expansion (Pigliucci 2010). Another concept, less used but with great potential, is phenotypic landscape (Rice 2004, 2008), a mathematical representation of the values of a phenotypic trait as a function of a series of underlying causal factors that can be either genetic constitution, physiological states, developmental modules or environmental parameters (Nijhout et al. 2010). These conceptual tools are of great value for the study of phenotype heritability, stability and evolvability.
(b). Types of plasticity
Plasticity phenomena can be classified in different ways, e.g. based on the nature of the interested trait (e.g. morphological, physiological and behavioural), the nature of the environmental cue (diet, population density, temperature and photoperiod) interpreted by the plastic developmental system or the relevant organism's performance (predator avoidance/defence, dispersal and resource exploitation) in the ecological context. Another distinction is whether phenotype determination is reversible (as the conspecific-dependent sex determination in the gobiid fish Trimma; Sunobe & Nakazono 1993) or irreversible (as in the seasonal polyphenism of the caterpillars of the geometrid moth Nemoria; Greene 1989). Still another interesting aspect of plasticity is whether the phase of sensitivity to the environmental cue is early in development (e.g. embryonic, as in temperature-dependent sex determination in turtles; Crews 2003) or late (e.g. post-embryonic, as in the wing polyphenism in the migratory grasshopper Locusta, regulated by population density during nymphal stages; Applebaum & Heifetz 1999).
From the point of view of the adaptive evolution of plasticity, a significant distinction can be drawn between direct and indirect effects of the environment on development. In the first case, plasticity is due to the effects of environmental variables that directly affect a developmental or a physiological process (e.g. temperature can directly influence developmental processes affecting chemical reaction kinematics and the physical properties of membranes). In such situations, plasticity is possibly non-adaptive. Conversely, we speak of indirect effects when the environmental cues elicit responses that are mediated by other physiological and developmental events. In this way, the environmental conditions that induce a given phenotype do not need to be the same conditions to which the phenotype is an adaptation (e.g. photoperiod, altering the pattern of hormone secretion in insects, can elicit a change in the developmental pathway that leads to the production of the phenotype best adapted to cope with the coming temperature and nutrition conditions). This provides scope for a time delay between the eliciting signal and the developmental response, so that the former can actually perform as a predictor of a forthcoming selective regime (Nijhout 2003; see also below).
Even under a rigorous definition of plasticity, thus disregarding more generic uses of the term, as for instance the predisposition of a living form to be variably moulded by natural selection (sometimes referred to as ‘evolutionary plasticity’, partially overlapping with the more recent concept of ‘evolvability’), the diversity of biological phenomena that this term encompasses is still notable (see Gilbert & Epel 2009 for a comprehensive treatment of plastic developmental processes). The following possibly not exhaustive list of phenomena highlights the diversity of biological processes in which plasticity is involved. The categories recognized here should not be regarded as strictly mutually exclusive.
Opportunistic-switch plasticity. When an environmental parameter relevant for the species has an unpredictable temporal dynamic on a time scale comparable to the organism's whole life cycle (or to a defined section of its ontogeny), developmental plasticity permits the production of alternative phenotypes with high fitness through a set of different situations. For instance, depending on the available kind of food, the tadpoles of the Mexican spadefoot toad Spea multiplicata can develop into either of two morphologically distinct environmentally induced morphs, differing in jaw and digestive apparatus and food preferences, each best adapted to exploit the available resources (Pfennig 1990, 1992). Such diet-induced plasticity is taxonomically widespread. Several forms of defensive polyphenism can be ascribed to this category as well.
Across-generations plasticity. Bivoltine or multivoltine species living in an environment that predictably changes during the year may present in the succeeding generations two or more different phenotypes that are repeated year after year. This is the case of the seasonal polyphenism in the bivoltine butterfly Bicyclus anynana, with two adult morphs adapted to the dry and wet season, respectively (Roskam & Brakefield 1998), and also the case of cyclomorphosis in many multivoltine species of rotifers, cladocerans and bryozoans (Greene 1999). Phenotypic variation in multivoltine species can involve morphology, life-history traits and modes of reproduction as well, resulting in a complex multi-generation life cycle (Minelli & Fusco 2010) as in the case of many aphids, with alternation of winged and wingless, amphigonic and parthenogenetic forms (Brisson 2010).
Coexisting morphs plasticity. The developmental switch between two or more possibly coexisting alternative phenotypic forms with the same genotype is induced by environmental parameters that affect the developmental trajectories. In many species of the scarab beetle genus Onthophagus, a sigmoid allometric relationship between body size at metamorphosis (this size depending in turn on larval nutrition) and the size of species-specific cephalic and thoracic exoskeletal projections (‘horns’) gives rise to the presence of horned and hornless males in the same population. Horns constitute male secondary sexual characters, and the two morphs adopt different reproductive strategies (Moczek 2010). The phenomenon of environmentally controlled sex determination belongs to this category, as well.
Caste polyphenism. Different phenotypes, with different social and reproductive role, determined by nutrition factors, are at the basis of the caste system in the societies of many social species (e.g. among hymenopterans and termites; Khila & Abouheif 2010). Distribution and regulation of the controlling environmental factors are at the basis of the ‘development’, ‘physiology’ and ‘reproduction’ of the society as a whole. For this reason, although caste polyphenism could perfectly fit into the category above, it deserves to be treated as a special class among the plasticity phenomena.
Lifelong plasticity. The capacity of an individual to respond to a variety of stimuli (changing its physiology, behaviour, synaptic connections, immune repertoire, etc.) in an adaptive direction is also called physiological adaptation, to be distinguished from evolutionary adaptation, the adaptive cross-generational change in the composition of a population (Garland & Kelly 2006). Examples of environmentally induced adaptive changes that occur within individual organisms during their lifetime are acclimatization, training, learning, seasonal change in fur thickness in mammals and feather moulting in birds.
Non-adaptive plasticity. Insofar as external physico-chemical parameters can exert influence on any material system, some degree of non-adaptive plasticity is an inevitable property of organisms (Newman et al. 2006). Plastic variation is expected to occur whenever an organism is exposed to environmental conditions not previously experienced in its evolutionary history (Garland & Kelly 2006), and against which the developmental system cannot be adaptively buffered. Even a perfectly horizontal (non-plastic) reaction norm is expected to bend at some point towards the extremities.
(c). Plasticity: unity in the diversity
All these apparently disparate natural history accounts are not grouped together under the umbrella term ‘plasticity’ solely on the basis of a vague definition of the latter. They really represent the many facets of the unique fact that the interactions between ‘genes’ and ‘environment’ are not only reflected in the selection process, but are also inextricably implicated in the production of the variants to be eventually winnowed by it. This is prominently made apparent in the arbitrariness of any classification of plasticity phenomena.
For instance, the apparently neat distinction between the possible modifications of a developmental trajectory elicited by specific environmental cues (‘plastic development’) and the ability of an individual organism to alter its performances in response to changes in environmental conditions (‘physiological adaptation’) relies on an equally clear demarcation between developmental and physiological phenomena. It implies the possibility to recognize an ‘endpoint’ in development, i.e. a precise time in ontogeny when developmental processes stop to give way to physiological and behavioural processes. No general biology-based criterion is available for such a demarcation, and conceivable operational divides between developmental and ‘post-developmental’ phenomena are in principle of no use in other instances, e.g. to investigate the evolution of plastic responses. As a point of fact, the evolution of alternative developmental pathways for distinct environmental settings may share significant similarities with the evolution of physiological adaptations (Arenas-Mena 2010). Attributing biological value to a conventional demarcation can result in a ‘conceptual trap’, i.e. a concept that can bias further investigations (Fusco 2008).
Another example of how deceiving a traditional categorization can be is provided by indirect modes of development, where different life stages of the same organism are very diverse and separated by metamorphosis. These can be considered forms of sequential ontogenetic polyphenism (Nijhout 1999), at variance with alternative polyphenism, where individuals proceed along one or another of a set of possible developmental trajectories. Metamorphosis and polyphenism, although phenomenologically different developmental events, are subject to extremely similar regulatory interactions in holometabolous insects. For instance, the developmental switches producing the alternative phenotypes are mediated by the same endocrine factors (juvenile hormone and ecdysteroids) that control metamorphosis (Nijhout 1999). Evolutionary relationships between alternative and sequential polyphenism are further discussed by Minelli & Fusco (2010).
To get an idea of how entangled different aspects of a plastic response can be, it is perhaps worth considering the case of the polyphenism in the migratory locust Schistocerca gregaria (review in Gilbert & Epel 2009). Schistocerca gregaria comes in two alternative adult forms: a solitary, sedentary form with shorter wings and a gregarious, migratory form with longer wings. The perception of the proper level of tactile stimuli (that is correlated to population density), principally on the surface of posterior leg femurs, can switch the last part of post-embryonic development towards one or the other of the two adult forms. But things are not that simple. The transition between the two morphs may take several stadia for some morphological characters, while being very rapid for behavioural changes (Rogers et al. 2003). Furthermore, the gregarious migratory morph can be retained for several generations after the crowding has stimulated the first gregarious morphs to appear. This ‘transgenerational effect’ is mediated by a chemical present in the foam that surrounds the eggs at deposition (Miller et al. 2008). Thus, polyphenism in Schistocerca is at the same time a case of opportunistic plasticity (in response to the mechanical stimulus owing to crowding) and a case of across-generations plasticity (where phenotype is epigenetically inherited after the primary environmental cue has disappeared), and two different stimuli, one tactile, the other chemical, one operating in late post-embryogenesis, the other operating during embryogenesis, can elicit the same phenotype.
3. The evolution of plasticity
The evolutionary origins and destiny of (genetic) polymorphism is a classic textbook subject in population genetics and evolutionary biology (e.g. Futuyma 2005). On the contrary, the evolutionary origins and pathways of change for polyphenism, or more generally for phenotypic plasticity, are mainly discussed in the specialist literature (see West-Eberhard 2003). Here, however, the choice and use of terminology is not completely settled and often is a subject of debate (see Crispo 2007).
Among the several aspects of the subject, three are particularly relevant here: (i) the evolutionary origin of adaptive plasticity, (ii) the two-way evolutionary transition between the predominance of environmental effects and genetic control on the phenotype (the polyphenism-to-polymorphism, or polymorphism-to-polyphenism transitions), and (iii) the organismal and environmental conditions that favour one form of phenotype determination over the other (the adaptive value of polyphenism versus polymorphism).
(a). Origins of adaptive plasticity and polyphenism
Because developmental and physiological processes are normally sensitive to environmental variables such as temperature, pH and the availability of nutrients, non-adaptive or just incidentally adaptive phenotypic plasticity ‘is possibly the primitive character state for most if not all traits’ (Nijhout 2003).
Depending on the effect that phenotypic plasticity has on fitness, evolutionary change can take different pathways: (i) stabilization of the phenotype through mechanisms that buffer it against environmental variation, actually eliminating the plasticity, (ii) genetic assimilation of phenotypes leading to a genetic polymorphism, and (iii) exaptation of plasticity, possibly through further elaboration on the relationship between environmental signal and developmental response.
In the evolution of a primitive plastic response, a key transition is from direct to indirect effects of environment on the phenotype, i.e. the ‘decoupling’ between the reception of the environmental stimulus and the organismal response. In insects, for instance, the production of alternative phenotypes is mediated by hormonal regulation. As developmental hormones are directly regulated by neurosecretory factors, when they are not neurosecretory hormones themselves, this implies that developmental responses are under the control of the central nervous system, which, by integrating information about the organism's internal and external environment, eventually regulates development through hormone secretion. Thus, ‘development can become responsive to a wide diversity of environmental signals, without the need to have developmental processes themselves be sensitive to the environment’ (Nijhout 2003).
(b). Directions of change
Since the first classic writings on the subject (e.g. Baldwin 1896; Waddington 1942), the scattered literature on the evolution of plasticity has been characterized by a certain degree of terminological confusion that only recently has started to be cleared out, thanks to some valuable reviews (e.g. Nijhout & Davidowitz 2003; West-Eberhard 2003; Braendle & Flatt 2006; Crispo 2007), in part under the need to theoretically accommodate the results of new experimental works (e.g. Suzuki & Nijhout 2006). Among the main sources of confusion there is the use of one and the same term (e.g. canalization, see below) to indicate both a process and its result, and an inadequate factorization of the causes of variation, unable to distinguish its different components (e.g. genetic versus environmental, or within one environment versus between different environments) (Nijhout & Davidowitz 2003).
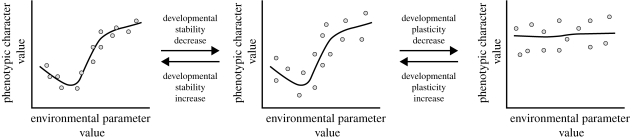
In analysing the possible developmental origins of phenotypic variation, Nijhout & Davidowitz (2003) introduced a useful schematization. This is based on the key concept of target phenotype, a property of an individual defined as ‘the phenotype that would be specified by a given genetic makeup and environmental conditions in complete absence of variation of the determinants, and in absence of noise of whatever kind’. With respect to the target phenotype, there are thus two main types of phenotypic variation: (i) consistent variation of the target phenotype as function of environmental (reaction norm) or genetic (allelic sensitivity) variation and (ii) individual variation around the target phenotype, as the effect of random perturbations of development (developmental instability). Distinguishing these different kinds of phenotypic variation is a requirement for discriminating different possible evolutionary routes of change for phenotypic variation (figure 2).
Figure 2.
Schematic representation of possible direction of evolutionary change in phenotypic variation with respect to environmental sensitivity. Lines represent the reaction norms of target phenotypes (see text) and circles show the variation around the target phenotype. With respect to the central panel, the left panel depicts a situation with a higher developmental homeostasis, while the right panel depicts a situation with a higher environmental canalization (modified from Nijhout & Davidowitz 2003).
A recently introduced general term to indicate the evolutionary processes by which the target phenotype varies its sensitivity to environmental or genetic variation is genetic accommodation (West-Eberhard 2003). Older and more familiar terms used to describe plasticity evolution can be considered as special cases of genetic accommodation. Canalization (Waddington 1942) is the term used for the process of change in the direction of a reduction of the sensitivity of an organism's phenotype either to allelic (genetic canalization) or environmental (environmental canalization) variation. The term canalization is used also to refer to the condition of a developmental system that is buffered against genetic and/or environmental variation. Genetic accommodation resulting in a decrease in environmental sensitivity (environmental canalization) is also called genetic assimilation (Waddington 1953), whereas genetic accommodation resulting in an increase in environmental sensitivity is partially coincident with the so-called Baldwin effect (Baldwin 1896; but see Crispo (2007) for a detailed analyses of the history and usage of these terms).
A general model for the process of genetic accommodation can be summarized as follows (Braendle & Flatt 2006): (i) a genetic or an environmental change triggers the expression of a novel phenotype uncovering previously cryptic, heritable genetic or epigenetic variation, thus exposing it to natural selection, (ii) the initially rare variant phenotype increases in frequency (e.g. because of the persistence of the eliciting environmental factors), so that a subpopulation of the original population expresses the novel phenotype, and (iii) selection on existing variation for trait expression causes it to become more strictly controlled genetically or to remain plastic.
Otherwise, evolutionary change can affect the scattering of phenotypes around the target phenotype within a particular environment. Homeorhesis (developmental homeostasis), the property of an individual organism to stabilize development within a particular environment, can either increase or decrease in evolution, with effects on the level of developmental (in)stability (Nijhout & Davidowitz 2003), but virtually independent from developmental plasticity.
(c). Conditions for the evolution of plasticity
The problem of identifying under which natural conditions either an increase or a decrease in plasticity would be favoured has been the subject of both empirical (e.g. Terblanche & Kelynhans 2009) and theoretical enquiry (e.g. Moran 1992).
Similarly to other problems in evolutionary biology, a possible approach is through the evaluation of the balance between the relative weights of costs and benefits of different modes of phenotype determination in different contexts (DeWitt et al. 1998; Crispo 2007). Plasticity can be adaptive in heterogeneous or unpredictably unstable environments, or in lineages with high gene flow among populations in divergent environments. On the other hand, it can be costly in terms of the energy necessary for the maintenance of sensory and regulatory mechanisms of developmental switch between different phenotypes, and can also have genetic costs associated with negative effects of pleiotropy or epistasis.
Another perspective on the adaptive value of a plastic response to a fluctuating environment comes from the evaluation of the length of the organism's life cycle (OC) with respect to the length of the cycle of environmental variation (EC) to which it is exposed (Greene 1999). For relatively long-lived organisms (OC > EC), environmental variability may be best dealt with through the ability to modulate physiological or behavioural responses to the changing conditions (physiological adaptation). On the contrary, species with lifespan close to the period of environmental variation (OC ≈ EC) may expect natural selection to adjust allele frequency in loci involved in morph determination, resulting in genetic polymorphism. For multivoltine species in seasonally varying environments (OC < EC), the selective environment and the population genetic structure responding to the selective events may be chronically out of phase. This condition can favour the evolution of plasticity (seasonal polyphenism) rather than polymorphism.
A third element for evaluating the conditions that determine the adaptive value of plasticity versus polymorphism in phenotype determination is the relative reliability of the relevant cue in predicting the forthcoming selective regime (Leimar 2009). Both genetic cues, in the form of allelic variation at polymorphic loci, and environmental cues can be viewed as ‘sources of information’ for the developing organism to adapt the phenotype to specific selective conditions. However, their reliability depends on the context. For instance, spatial variation in selective conditions associated with spatial variation in the frequencies of alleles that determine the state of adaptive phenotypic traits make these alleles highly informative cues. In conditions where the accuracy of genetic cue predictors is low (typically, in unpredictably unstable environments), if the organism has the potential to sense an environmental cue and to respond to it, we may expect environmental rather than genetic phenotype determination to evolve (e.g. Charnov & Bull 1977; Lively 1986; Pfennig 1990).
The nature of selective agents is a key factor for the evolution or physiological plasticity (Garland & Kelly 2006). In case of a relatively long-lasting selective event (i.e. non-instantaneous with respect to the time needed for possible plastic responses), individual organisms have the opportunity to trigger a plastic phenotypic change in the course of the selective event itself. If the direction of the plastic change results in an increased probability of survival and/or reproduction, the individuals exhibiting such a response will have a higher fitness. In this case, natural selection is expected to promote an evolutionary increase in adaptive physiological plasticity.
4. Concluding remarks
It has been argued that genetic accommodation in the strict sense refers to nothing but standard evolutionary change of genotype frequencies by selection, after mutational or environmental changes have uncovered previously cryptic genetic variation. Thus, the phenomena of phenotypic plasticity in evolution can be easily reduced to standard evolutionary genetic processes (see discussion in Braendle & Flatt 2006; Pigliucci et al. 2006).
We think that this argument ignores a growing mass of data on the pervasiveness of plasticity phenomena at all levels of biological organization and on inheritable (selectable) epigenetic variation (Gilbert & Epel 2009; Love 2010). Beyond that, the (non-warranted) possibility to reduce a set of well-distinct phenomena to the interplay of a smaller number of more basic processes does not imply that such a reduction is useful per se. In this case, disregarding plasticity phenomena, the explanatory power of a theory of the origin of phenotypic variation would be tangibly reduced, with obvious consequences on the structure of the evolutionary theory as a whole.
Even from this rapid excursion into development and evolution of phenotypic plasticity, it should be apparent that the scope for its inclusion in the mainstream evolutionary theory is enormous. However, it is still more evident that currently we have only a shallow knowledge of the complexity of the interrelations between different processes of phenotype determination and their evolutionary consequences. We have only just started.
Acknowledgements
We thank F. Nijhout and D. Pfennig for providing valuable comments on a previous version of this article. The volume has benefited from the precious help provided by numerous colleagues who reviewed the articles. For this, we thank W. Arthur, R. B. R. Azevedo, L. Bonato, C. Braendle, I. Brigandt, A. L. Cardini, C. DiTeresi, M. G. Hadfield, J. Heinze, B. S. Heming, R. A. Jenner, J. Lennox, G. B. Müller, C. Nielsen, R. Otte, J. Oettler, J. S. Robert, A. Schmidt-Rhaesa, K. Sterelny and M. A. Wund. We also thank a number of authors who, in addition to contributing an article, also served as reviewers and D. Maruzzo who helped us to edit the manuscripts.
Footnotes
One contribution of 12 to a Theme Issue ‘From polyphenism to complex metazoan life cycles’.
References
- Alberch P.1991From genes to phenotype: dynamical systems and evolvability. Genetica 84, 5–11 (doi:10.1007/BF00123979) [DOI] [PubMed] [Google Scholar]
- Applebaum S. W., Heifetz Y.1999Density-dependent physiological phase in insects. Ann. Rev. Ent. 44, 317–341 (doi:10.1146/annurev.ento.44.1.317) [DOI] [PubMed] [Google Scholar]
- Arenas-Mena C.2010Indirect development, transdifferentiation and the macroregulatory evolution of metazoans. Phil. Trans. R. Soc. B 365, 653–669 (doi:10.1098/rstb.2009.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J. M.1896A new factor in evolution. Am. Nat. 30, 441–451, 536–553 (doi:10.1086/276408) [Google Scholar]
- Braendle C., Flatt T.2006A role for genetic accommodation in evolution? Bioessays 28, 868–873 (doi:10.1002/bies.20456) [DOI] [PubMed] [Google Scholar]
- Brakefield P. M., Frankino W. A.2007Polyphenisms in Lepidoptera: multidisciplinary approaches to studies of evolution. In Phenotypic plasticity in insects. Mechanisms and consequences (eds Whitman D. W., Ananthakrishnan T. N.), pp. 121–151 Plymouth, UK: Science Publishers [Google Scholar]
- Brisson J. A.2010Aphid wing dimorphisms: linking environmental and genetic control of trait variation. Phil. Trans. R. Soc. B 365, 605–616 (doi:10.1098/rstb.2009.0255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E. L., Bull J. J.1977When is sex environmentally determined? Nature 266, 828–830 (doi:10.1038/266828a0) [DOI] [PubMed] [Google Scholar]
- Crews D.2003Sex determination: where environment and genetics meet. Evol. Dev. 5, 50–55 (doi:10.1046/j.1525-142X.2003.03008.x) [DOI] [PubMed] [Google Scholar]
- Crispo E.2007The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61, 2469–2479 (doi:10.1111/j.1558-5646.2007.00203.x) [DOI] [PubMed] [Google Scholar]
- Darwin C. R.1859On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- Degnan S. M., Degnan B. M.2010The initiation of metamorphosis as an ancient polyphenic trait and its role in metazoan life cycle evolution. Phil. Trans. R. Soc. B 365, 641–651 (doi:10.1098/rstb.2009.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong G.2005Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phytol. 166, 101–118 (doi:10.1111/j.1469-8137.2005.01322.x) [DOI] [PubMed] [Google Scholar]
- DeWitt T. J., Scheiner S. M. (eds) 2004Phenotypic plasticity. Functional and conceptual approaches. New York, NY: Oxford University Press [Google Scholar]
- DeWitt T. J., Sih A., Sloan Wilson D.1998Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 78–81 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- Fusco G.2001How many processes are responsible for phenotypic evolution? Evol. Dev. 3, 279–286 (doi:10.1046/j.1525-142x.2001.003004279.x) [DOI] [PubMed] [Google Scholar]
- Fusco G.2008Morphological nomenclature between patterns processes: segments segmentation as a paradigmatic case. Zootaxa 1950, 96–102 [Google Scholar]
- Futuyma D. J.2005Evolution. Sunderland, MA: Sinauer [Google Scholar]
- Garland T., Jr, Kelly S. A.2006Phenotypic plasticity and experimental evolution. J. Exp. Biol. 209, 2344–2361 (doi:10.1242/jeb.02244) [DOI] [PubMed] [Google Scholar]
- Gilbert S. F., Epel D.2009Ecological developmental biology: integrating epigenetics, medicine, and evolution. Sunderland, MA: Sinauer [Google Scholar]
- Gilbert S. F., McDonald E., Boyle N., Buttino N., Gyi L., Mai M., Prakash N., Robinson J.2010Symbiosis as a source of selectable epigenetic variation: taking the heat for the big guy. Phil. Trans. R. Soc. B 365, 671–678 (doi:10.1098/rstb.2009.0245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E.1989Diet-induced developmental polymorphism in a caterpillar. Science 243, 643–646 (doi:10.1126/science.243.4891.643) [DOI] [PubMed] [Google Scholar]
- Greene E.1999Phenotypic variation in larval development and evolution: polymorphism, polyphenism, and developmental reaction norms. In The origin and evolution of larval forms (eds Wake M., Hall B.), pp. 379–410 New York, NY: Academic Press [Google Scholar]
- Hallgrímsson B., Hall B. K. (eds) 2005Variation—a central concept in biology. New York, NY: Academic Press (Elsevier) [Google Scholar]
- Jablonka E., Lamb M.2005Evolution in four dimensions. Cambridge, MA: MIT Press [Google Scholar]
- Janzen F. J., Phillips P. C.2006Exploring the evolution of environmental sex determination, especially in reptiles. J. Evol. Biol. 19, 1775–1784 (doi:10.1111/j.1420-9101.2006.01138.x) [DOI] [PubMed] [Google Scholar]
- Khila A., Abouheif E.2010Evaluating the role of reproductive constraints in ant social evolution. Phil. Trans. R. Soc. B 365, 617–630 (doi:10.1098/rstb.2009.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforsch C., Tollrian R.2004Inducible defenses in multipredator environments: cyclomorphosis in Daphnia cucullata. Ecology 85, 2302–2311 (doi:10.1890/03-0286) [Google Scholar]
- Leimar O.2009Environmental and genetic cues in the evolution of phenotypic polymorphism. Evol. Ecol. 23, 125–135 (doi:10.1007/s10682-007-9194-4) [Google Scholar]
- Lewontin R.2000The triple helix: gene, organism and environment. Cambridge, MA: Harvard University Press [Google Scholar]
- Lively C. M.1986Canalization versus developmental conversion in a spatially variable environment. Am. Nat. 128, 561–572 (doi:10.1086/284588) [Google Scholar]
- Love A. C.2010Idealization in evolutionary developmental investigation: a tension between phenotypic plasticity and normal stages. Phil. Trans. R. Soc. B 365, 679–690 (doi:10.1098/rstb.2009.0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E.1963Animal species and evolution. Cambridge, MA: Harvard University Press [Google Scholar]
- McGuigan K., Sgrò C. M.2009Evolutionary consequences of cryptic genetic variation. Trends Ecol. Evol. 24, 305–311 (doi:10.1016/j.tree.2009.02.001) [DOI] [PubMed] [Google Scholar]
- Miller G. A., Islam M. S., Claridge T. D. W., Dodgson T., Simpson S. J.2008Swarm formation in the desert locust Schistocerca gregaria: isolation and NMR analysis of the primary maternal gregarizing agent. J. Exp. Biol. 211, 370–376 (doi:10.1242/jeb.013458) [DOI] [PubMed] [Google Scholar]
- Minelli A., Fusco G. (eds) 2008Evolving pathways. Key themes in evolutionary developmental biology. Cambridge, UK: Cambridge University Press [Google Scholar]
- Minelli A., Fusco G.2010Developmental plasticity and the evolution of animal complex life cycles. Phil. Trans. R. Soc. B 365, 631–640 (doi:10.1098/rstb.2009.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T.2005Developmental regulation of caste-specific characters in social-insect polyphenism. Evol. Dev. 7, 122–129 (doi:10.1111/j.1525-142X.2005.05014.x) [DOI] [PubMed] [Google Scholar]
- Moczek A. P.2010Phenotypic plasticity and diversity in insects. Phil. Trans. R. Soc. B 365, 593–603 (doi:10.1098/rstb.2009.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. A.1992The evolution and maintenance of alternative phenotypes. Am. Nat. 139, 971–989 (doi:10.1086/285369) [Google Scholar]
- Müller G. B.2007Evo-devo: extending the evolutionary synthesis. Nat. Rev. Genet. 8, 943–949 (doi:10.1038/nrg2219) [DOI] [PubMed] [Google Scholar]
- Newman S. A., Forgacs G., Müller G. B.2006Before programs: the physical origination of multicellular forms. Int. J. Dev. Biol. 50, 289–299 (doi:10.1387/ijdb.052049sn) [DOI] [PubMed] [Google Scholar]
- Nijhout H. F.1991The development and evolution of butterfly wing patterns. Washington, DC: Smithsonian Institution Press [Google Scholar]
- Nijhout H. F.1999Hormonal control in larval development and evolution. In The origin and evolution of larval forms (eds Wake M., Hall B.), pp. 217–254 New York, NY: Academic Press [Google Scholar]
- Nijhout H. F.2003Development and evolution of adaptive polyphenisms. Evol. Dev. 5, 9–18 (doi:10.1046/j.1525-142X.2003.03003.x) [DOI] [PubMed] [Google Scholar]
- Nijhout H. F., Davidowitz G.2003Developmental perspectives on phenotypic variation, canalization, and fluctuating asymmetry. In Developmental instability: causes and consequences (ed. Polak M.), pp. 3–13 New York, NY: Oxford University Press [Google Scholar]
- Nijhout H. F., Roff D. A., Davidowitz G.2010Conflicting processes in the evolution of body size and development time. Phil. Trans. R. Soc. B 365, 567–575 (doi:10.1098/rstb.2009.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig D. W.1990The adaptive significance of an environmentally-cued developmental switch in an anuran tadpole. Oecologia 85, 101–107 (doi:10.1007/BF00317349) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W.1992Polyphenism in spadefoot toad tadpoles as a locally-adjusted evolutionarily stable strategy. Evolution 46, 1408–1420 (doi:10.2307/2409946) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., McGee M.2010Resource polyphenism increases species richness: a test of the hypothesis. Phil. Trans. R. Soc. B 365, 577–591 (doi:10.1098/rstb.2009.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M.2001Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: The John Hopkins University Press [Google Scholar]
- Pigliucci M.2010Genotype ⇒ Phenotype mapping and the end of the ‘genes as blueprint’ metaphor. Phil. Trans. R. Soc. B 365, 557–566 (doi:10.1098/rstb.2009.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M., Müller G. B. (eds) 2010Evolution: the extended synthesis. Cambridge, MA: MIT Press [Google Scholar]
- Pigliucci M., Murren C. J., Schlichting C. D.2006Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 209, 2362–2367 (doi:10.1242/jeb.02070) [DOI] [PubMed] [Google Scholar]
- Price T. D., Qvarnström A., Irwin D. E.2003The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440 (doi:10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. H.2004Evolutionary theory: mathematical and conceptual foundations. Sunderland, MA: Sinauer Associates [Google Scholar]
- Rice S. H.2008Theoretical approaches to the evolution of development and genetic architecture. Ann. NY Acad. Sci. 1133, 67–86 (doi:10.1196/annals.1438.002) [DOI] [PubMed] [Google Scholar]
- Rogers S. M., Matheson T., Despland E., Dodgson T., Burrows M., Simpson S. J.2003Mechanosensory-induced behavioural gregarization in the desert locust Schistocerca gregaria. J. Exp. Biol. 206, 3991–4002 (doi:10.1242/jeb.00648) [DOI] [PubMed] [Google Scholar]
- Roskam J. C., Brakefield P. M.1998Seasonal polyphenism in Bicyclus (Lepidoptera: Satyridae) butterflies: different climates need different cues. Biol. J. Linn. Soc. 66, 345–356 [Google Scholar]
- Schlichting C. D., Pigliucci M.1998Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer [Google Scholar]
- Sunobe T., Nakazono A.1993Sex change in both directions by alternation of social dominance in Trimma okinawae. Ethology 94, 339–345 [Google Scholar]
- Suzuki Y., Nijhout H. F.2006Evolution of a polyphenism by genetic accommodation. Science 311, 650–652 (doi:10.1126/science.1118888) [DOI] [PubMed] [Google Scholar]
- Terblanche J. S., Kelynhans E.2009Phenotypic plasticity of desiccation resistance in Glossina puparia: are there ecotype constraints on acclimation responses? J. Evol. Biol. 22, 1636–1648 (doi:10.1111/j.1420-9101.2009.01784.x) [DOI] [PubMed] [Google Scholar]
- Waddington C. H.1942Canalization of development and the inheritance of acquired characters. Nature 150, 563–565 (doi:10.1038/150563a0) [DOI] [PubMed] [Google Scholar]
- Waddington C. H.1953Genetic assimilation of an acquired character. Evolution 7, 118–126 (doi:10.2307/2405747) [Google Scholar]
- West-Eberhard M. J.2003Developmental plasticity and evolution. New York, NY: Oxford University Press [Google Scholar]
- West-Eberhard M. J.2005Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102, 6543–6549 (doi:10.1073/pnas.0501844102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerkandl E., Villet R.1988Concentration–affinity equivalence in gene regulation: convergence of genetic and environmental effects. Proc. Natl Acad. Sci. USA 85, 4784–4788 (doi:10.1073/pnas.85.13.4784) [DOI] [PMC free article] [PubMed] [Google Scholar]