Abstract
While the incorporation of mathematical and engineering methods has greatly advanced in other areas of the life sciences, they have been under-utilized in the field of animal welfare. Exceptions are beginning to emerge and share a common motivation to quantify ‘hidden’ aspects in the structure of the behaviour of an individual, or group of animals. Such analyses have the potential to quantify behavioural markers of pain and stress and quantify abnormal behaviour objectively. This review seeks to explore the scope of such analytical methods as behavioural indicators of welfare. We outline four classes of analyses that can be used to quantify aspects of behavioural organization. The underlying principles, possible applications and limitations are described for: fractal analysis, temporal methods, social network analysis, and agent-based modelling and simulation. We hope to encourage further application of analyses of behavioural organization by highlighting potential applications in the assessment of animal welfare, and increasing awareness of the scope for the development of new mathematical methods in this area.
Keywords: fractal analysis, Theme, Markov chains, social networks, modelling, animal behaviour
1. Introduction
The analysis of behaviour is an area being greatly advanced through the application of mathematical and engineering methods, e.g. modelling (Sumpter 2006), artificial intelligence (Minsky 2006), remote behavioural detection methods (Fowler et al. 2001; Troscianko et al. 2004), operant equipment (Cook et al. 2004) and advanced statistics (Browne et al. 2007; Mundry & Sommer 2007). Such methodologies could be of great influence when behaviour is used to make inferences about an animal's welfare, but are currently vastly under-utilized for this purpose. Here, we review advances in the analysis of behavioural organization. We use the term behavioural organization to refer to the arrangement of behavioural states relative to each other in time and space, and in relation to other individuals' locations or behaviour.
Behaviour is used as a measure of animal welfare because an animal's state can be inferred from its actions. Classically, animal welfare has concerned the identification of negative welfare states; of markers of pain and stress, aggression, boredom and abnormal behaviour (Fraser 2008). Increasingly, there is also an interest in positive welfare states and potential markers of these, such as play and anticipation (Yeates & Main 2008). Many recent studies have focused on individual differences, through studies of personality and coping strategies, and interactions between maternal environment, early environment and later environments (e.g. Ruis et al. 2001; Coutellier et al. 2009). There is also interest in social interactions, whether positive, negative or neutral, and the effects of these on welfare (e.g. Ruis et al. 2001). Behaviour is recorded in situ to measure the welfare of animals in a particular environment, or in response to short-term changes in environment or a particular stressor or stimulus. The responses of animals and the choices they make in tests are used to infer the abilities of that animal or to try to understand what animals want or do not want (Dawkins 2003).
Several basic methods exist for scoring animal behaviour, where behaviour is considered to be a process in which events occur over a continuous time-stream. Behaviour can be recorded continuously or sampled at regular intervals, the behaviour of an individual or group can be recorded, it can be categorized by social interactions, vocalizations, locomotion, activity/inactivity, location or a complete comprehensive ethogram (see Martin & Bateson (1993) for a classic introductory text). In this review we present a simple example dataset of the behaviour of six chickens in a pen to demonstrate each of the mathematical techniques outlined (see boxes 1–4). For full details of the example dataset please see the electronic supplementary material.
A common approach to the study of behaviour in welfare science is to compile a comprehensive ethogram of each discrete type of behaviour. Correlations with other measures of welfare are used to identify behaviour associated with positive and negative welfare states. It is not unusual to find ethograms consisting of a large number of behaviour states, or events. In describing the play behaviour of equids, McDonnell & Poulin (2002) describe 38 states, which they divide into five distinct categories of play. As this example demonstrates, behaviour can be divided and further subdivided into progressively more specific types. Behaviour can also be subdivided into concurrently scored, overlapping behaviour patterns, to account for the possibility that an animal can be performing more than one behaviour at any one time, for example sitting and eating while interacting with a conspecific.
Ethogram approaches focus on discretization: approximating a continuous process by division into discrete parts. These discrete parts are commonly analysed individually, disregarding temporal and structural detail (organization). Often behavioural indicators of welfare show poor correlation with other measures of welfare, particularly physiological measures (Dawkins 2003). Clearly, current behavioural measures of welfare are not ideal and new methods of analysis are needed. There are reasons to believe that analysis of behaviour that incorporates measurement or understanding of behavioural organization might be of particular relevance when it is to be used as a measure of welfare.
The organization of behaviour encodes information on complexity, variability and flexibility, all of which are believed to be important in determining animal welfare. Animals with a large and flexible behaviour repertoire are expected to have better welfare. If an animal has more behavioural options available to them, we expect them to be better able to control their environment, for example they are able to re-prioritize motivations when necessary. A loss in controllability in the environment is linked with chronic stress and hence poor welfare (Wiepkema & Koolhaas 1993). It may be that welfare is compromised when animals are no longer in control and when their adaptive, re-prioritizing abilities have become compromised; when they are no longer able to cope (Bassett & Buchanan-Smith 2007).
Environmental complexity produces neural complexity, in turn producing behavioural complexity. Environmental complexity at the stage of brain development confers a host of benefits: decreasing stress reactivity; improving health and healing; delaying the onset of neurodegenerative diseases; and protecting against development of some forms of abnormal repetitive behaviour (for reviews see Lewis 2004; Fox et al. 2006). Many captive animals are reared in barren conditions and then expected to cope with unpredictable stressors. This creates a mismatch between the available behavioural complexity and the level of behavioural complexity that would allow effective coping.
A common outcome of stress is a reduction in the variability of physiological responses. For example, heart rate variability decreases in response to an acute stressor (Visser et al. 2002) and the diurnal cortisol profile becomes flattened and less variable in people who are chronically stressed (Miller et al. 2007). Since behaviour and physiology are intrinsically linked, it is possible that behaviour will follow a similar pattern. Alterations to the profile of response can be linked under the concept of allostasis, a model of physiological regulation. Allostasis considers that, when an animal is coping with its environment it has a wide regulatory range of mechanisms available, but once it gets into an allostatic state (system overload), the animal is forced into a narrower regulatory range and has an increased chance of veering off into either hypo- or hyper-reactive states (e.g. Wingfield 2005; Korte et al. 2007). Under this model, behaviour is expected to become either more or less variable than at baseline in response to stress overload.
One final reason to believe that behavioural complexity is important is that animals devote a large amount of resources through play to ensure they are behaviourally complex. While there are different theories to account for the evolution of play most point towards a more functional, behaviourally complex animal (either socially, physically or in terms of general flexibility; Spinka et al. 2001). Play is found universally among mammals and in birds, reptiles and even in an invertebrate (Burghardt 2005). In order to be this phylogenically widespread, play, and therefore also behavioural complexity, must confer significant benefits.
Here we discuss the possibilities that measures of behavioural organization present to welfare science, with respect to four analytical approaches: fractal analysis; temporal analysis (including Markov chains and time pattern approaches); social network analysis (SNA); and agent-based modelling and simulation (ABMS). The methods chosen have the ability either to measure behavioural organization or elucidate the rules that determine the organization, and some can be used to explore the consequences of the organization through modelling and simulation. With the exception of ABMS, this review will primarily concern measurement of behavioural organization. Each section will (i) outline the general principles and practices of each method; (ii) briefly review traditional uses of the methodology; (iii) present some examples of actual and possible applications to the study of animal behaviour and welfare; and (iv) highlight some of the advantages and disadvantages of the techniques.
2. Fractal analysis
2.1. Principles
Fractal analysis concerns the effects of scaling on the measurement of a continuous variable. Fractals are shapes with self-similar structure, i.e. that can be decomposed into smaller versions of themselves. Fractal analysis covers a range of methods that examine the degree to which objects or processes are fractal-like or self-similar, by comparing measurement of objects or processes at different resolutions. Fractal analysis can deal with objects and processes; the differentiation being that the former are measured on a physical scale and the latter a temporal scale. The classic example of fractal analysis is Mandelbrot's (1967, cited by Rutherford et al. 2004) examination of coastlines. Consider three different profiles of a coastline: one is smooth and could be represented by a continuous fluid line; one is made up of inlets that are formed inside larger inlets and so on; and a third coastline is rugged and craggy in nature. Both the smooth coastline and the inlets coastline have regular patterns, the rugged coastline does not. While the regular structure of the smooth coastline is easily quantified by traditional (Euclidean) geometry, the self-similar inlet coastline is not. Fractal geometry was conceived to be able to mathematically quantify the more complex type of regularity exhibited by the inlet coastline.
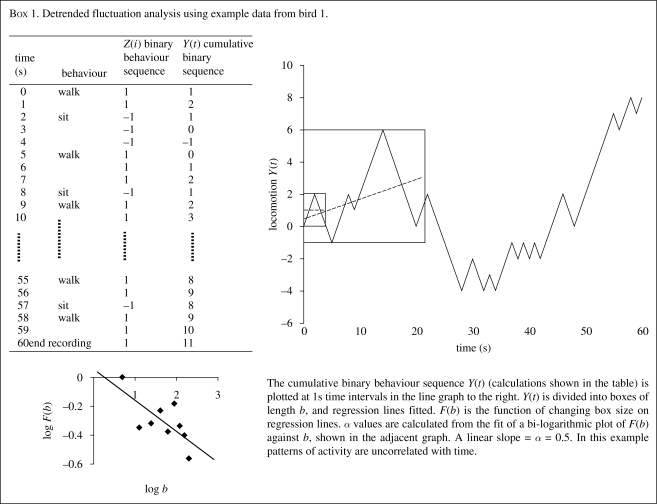
There are two types of behavioural data used in fractal analysis: (i) behavioural processes scored on a binary scale and (ii) pathway analysis (using x–y coordinates). Observations of the presence or absence of a state over time form the binary scale, e.g. whether an animal is active or inactive (Cole 1995), or fluctuation between two states (Alados & Huffman 2000). The method of fractal analysis most applied to such time series is detrended fluctuation analysis (DFA) (Peng et al. 1994, 1995a,b). In DFA, the correlation within binary sequences, Z(i), encoded 1 (e.g. for active), −1 (e.g. inactive or social), is graphically represented using a random walk, plotted as a function Y(t) over fixed time intervals, by summing the previous sequence (box 1). The function Y(t) is divided into series of progressively smaller sections, or boxes, b (i.e. measured at different time resolutions). Optimal box size can be established using the method outlined in Tavazoei & Haeri (2007). A regression line b(t) is fitted to the boxes and a mean taken of the residual variance of the whole sequence for each box size. Finally, the slope of the bi-log plot of the mean residual variance Y(t) and box size (b) is used to calculate the fractal exponent, α, as a function of bF(b) = bα. Regression lines should be fitted using the maximum likelihood method since least-square regressions in the log–log domain can lead to biased model estimates (Clauset et al. 2007). For a completely random sequence α = ½. As α increases above 0.5, the sequence becomes more self-similar or fractal-like. Lower α values indicate less regularity in the sequence and the absence of predictable patterns over time (or space in the case of pathway analysis).
For the study of animal pathways, Paulus & Geyer (1993) validated a type of fractal analysis for rats' movements, which compared microscopically recorded events with macroscopic behaviour patterns by partitioning local and mass-scaling exponents, using a filter value, q (analogous to the Lagrange multiplier) so that both high and low local spatial scaling exponents (the fractal dimension based on smaller and larger scales) contribute to the fractal score. Another way fractal analysis has been applied to pathway analysis is as a measure of path tortuosity: where tortuosity is the degree of turning in a given space and time, often used to distinguish between goal-oriented and random search movements. Incorporation of fractality into a model of Brownian motion could yield a model of a random search and while such models do exist (Dicke & Burrough 1988; Sugihara & May 1990), they are yet to be verified (Benhamou 2004).
A final example of the use of fractal analysis is in identifying hierarchical or functional levels of organization in behaviour. Using fractal analysis, Fritz et al. (2003) identified three functional levels under different spatial resolutions in wandering albatrosses' flight patterns (although see Edwards et al. (2007) for a critique). Under high resolutions, birds were moving in accordance with wind fluctuations; under medium resolutions birds were moving towards food sources, and on larger scales (lower resolutions) flight patterns corresponded with movement between patches. Hierarchical levels can be identified by the consistency in α (the scaling exponent) over a given range of k (resolutions). Outside this range, there will be a shift and a sudden change in the value of α.
2.2. General examples
Fractal analysis found early application in geology, but has many varied applications describing: regularity of heart beats (Yamamoto & Hughson 1991); structure of vocalizations (Pickover & Khorasani 1986); changes in cell mass and complexity (Smith et al. 1989); gait and movement analysis (Riley & Turvey 2002); disease spread (Cazelles et al. 2007); population dynamics (Hastings & Sugihara 1993); changes in economic markets (Gatti et al. 2008); and the structure of particles (Shibuichi et al. 1996).
2.3. Animal behaviour applications
Using DFA, more structured temporal sequences of behaviour (i.e. with higher α values) have been interpreted as a sign of stress, and have been observed with the stresses of pregnancy, disease (Alados & Huffman 2000) and exposure to toxins (Alados & Weber 1999). To date, five studies have examined the effects of stressors on temporal regularity of behaviour using DFA as a welfare indicator. One study found laying hens responded to proposed stressors of a novel arena and a restraint procedure with less regular vigilance patterns (lower α values), but did not find changes in response to blood sampling (Rutherford et al. 2003). In a separate study, there were no differences found between the structure of behaviour in differentially food-restricted broiler chickens (Hocking et al. 2007). María and co-workers (2004) found that locomotor patterns of chickens became less regular with age (lower α values) and were significantly altered by food limitation, where patterns became more regular (higher α values). In response to other stressors, a small sand-bath and introduction of strangers, chickens locomotor patterns were less regular (lower α value) and, in the sand-bath, perching and foraging patterns were also less regular (lower α value) and resting was more regular (higher α value). A fourth study examined pigs exposed to a chronic mild stress treatment. It found that pigs had more regular (higher α values) postural behaviour after being exposed to stressors (Rutherford et al. 2006). Less regular patterns (lower α values) were found in the movements of quails selected for low stress when compared with high stress in an open field test (Kembro et al. 2008). The studies outlined demonstrate that there are not consistent directional relations between stress and the fractal quality of behaviour as Alados supposes. Changes in regularity probably reflect adaptive shifts in the structure of behaviour in response to acute stress, so that the fractal exponent will depend on the type and duration of the stressor applied, the resolution of the behaviour examined, and which behaviour is being analysed.
The fractal dimension of pathways can also be altered by stressors, and this has been studied in fish exposed to toxins (toxins lowered the fractal dimension; Kane et al. 2004), and isolated and group-housed rats (isolated rats had straighter movement pathways and more predictable sequences than group-housed controls; Paulus et al. 1998). Fractal analysis of pathways could be used to identify animals with low variability in patterns of movement, such as those with locomotive stereotypies.
The potential use of the fractal dimension to identify levels of hierarchical organization is currently being examined in the grazing paths of cows. Early results indicate an effect of group size on the fractality at high resolution (<9 m) and an effect of paddock area at low resolution (>9 m; S. Tada 2008, personal communication). Circadian and ultradian rhythms in activity levels of free-living ruminants have been examined using a similar mathematical method (Scheibe et al. 1999). The fractal dimension exhibits consistency at the level of the individual (Rutherford et al. 2006), so has potential application in studies of animal personality or coping strategies.
The fractal quality of behaviour could be a valuable addition to behavioural indictors of welfare because it characterizes a type of organization that is frequently exhibited by natural systems: self-similarity. It can indicate how variable behaviour is and can be used to identify layers of behavioural structure. However, to date, links between the fractal quality and other indicators of animal welfare are mixed, and in order to find patterns in changes to regularity further studies are necessary. In order for fractal analysis to be valid much data are required and a lengthy period of time should ideally be measured. Hence, the analysis might be more suited to behaviour processes that can be measured simply in a binary manner but where interest lies in patterns over an extended time period (e.g. sleep; Lo et al. 2004). When the focus of data collection is placed on the quantity of data collected rather than observations rich in detail, such as can be the case for automated methods of behavioural data collection, fractal analysis can help condense large amounts of data.
2.4. Advantages and disadvantages
2.4.1. Advantages
Identifies self-similarity, a type of organization that is commonly exhibited by natural systems but not easily quantified using traditional mathematics.
Examines long-range patterns, so condenses information from data scored over extended time periods.
Can identify hierarchical or functional levels of behavioural organization.
2.4.2. Disadvantages
Measures fluctuation between two states, such that types of behaviour must be considered independently.
Much data are required for valid fractal analysis.
As with many welfare indicators, it can be difficult to interpret in relation to welfare. There is no simple relationship between the fractal quality of behaviour and stress.
3. Temporal analyses
3.1. Principles
Behaviour is a continuous process and yet often when it is analysed it is separated into discrete events where structural detail, such as temporal arrangement, is ignored. Temporal analyses retain information on structure, effectively reconstructing events into meaningful sequences. We outline two such analyses below: Markov chain-based methods and time-pattern methods.
3.1.1. Markov chain methods
A Markov chain is a series of events where the next event depends only upon the current state, or short chain of states. The Markovian property is used to determine sequential organization or determinism, by considering transitional probabilities between states (Gillespie 1992). In contrast to fractal analyses, which consider long-range correlations, Markov chain-based methods are concerned with the pattern of contiguous states: which specific state follows which. The number of discrete states (or greatest number of states) that best predicts the next state is the order of the chain. When only transitions from one event to the next are considered, this is known as first order. It may be the case that second- (three sequential events), third- (four sequential events), or even fourth- (five sequential events) order chains better fit behavioural data. In practice, real behavioural data rarely meet the requirements of tests of high-order dependencies because there are many combinations of transitions between behavioural elements which will not be observed (Haccou & Meelis 1992). States can be grouped together so that lower order chains can be constructed from higher order ones (for method see Cane 1959).
The most basic Markov model is a discrete-time, finite chain with constant transitional probabilities. Although there is not the scope to discuss these in detail, readers should be aware that other Markov-based models exist, which may be more suitable for behavioural data (for review of Markov models see Barbu & Limnios 2008). These include: continuous-time Markov models, although these are often based on a discrete-time approximation (Yin & Zhang 2005); hidden Markov models, where the state is unobservable but observable states can be used to infer the hidden state (e.g. foraging behaviour might be used to infer hunger); or semi-Markovian models, where the transitional probability depends in part on the current state, but also on another property such as the time spent in that state.
Markov chain methods are bottom-up, since more global features of the data are constructed or measured from the observed data, and as such can be computationally challenging. The computation is challenging because transition matrices between behavioural states can rapidly become large when all combinations of behavioural states are considered. In the earlier example of play behaviour in horses (McDonnell & Poulin 2002), there were 38 types of play behaviour. Possible combinations of transitions between these play types are: 1444 first-order; 54 872 second-order; and 2 085 136 third-order chains. This type of computation is possible but time-expensive.
3.1.2. Time-pattern approaches
Some pattern metrics use a more efficient top-down approach, applying global rules such as data-mining algorithms to observed time-series data. Many time-pattern algorithms have been formulated (e.g. SPADE, Patin et al. 2007; PrefixSpan, Pei et al. 2001) and while it is generally agreed that such algorithms have wide applications, to date their application to the study of behaviour has been limited. There has been some time pattern-based software development specifically focused on behaviour. Magnusson's Theme programme (Noldus Information Technology, Wageningen, The Netherlands) is by far the most widely applied to the study of animal behaviour and hence we focus on this methodology here. Other softwares designed for analysis of behaviour, such as GSEQ (Bakeman & Quera 1995), or the application of the time-pattern algorithms derived from data-mining techniques, could have similar utility in the study of behaviour sequences.
Theme decides on time patterns using pattern-detection algorithms based on: (i) the order of events; (ii) the time distances between them; (iii) events relative position on a time scale; and (iv) the probability of each event occurring (Magnusson 2000, 2005). Because the algorithms consider the duration and length of time between events, Theme can also detect patterns with an intervening event. If the between- or within-event duration is considerably longer or shorter than other observations of the pattern, it becomes unrecognizable by the algorithm and will not be counted as a time pattern, just as a sentence would become unintelligible if phonemes were slowed or quickened beyond a threshold level (Magnusson 2005).
To identify time patterns, the first stage is to establish the bout criterion interval, where a bout is defined as a temporal cluster of events, and all events within that bout make up a time pattern. Theme's pattern-detection algorithm searches for the longest time between the end of element X1, which lasts a duration d1, and the beginning of the next element X2, which lasts a duration d2, with the constraint that X2 appears after X1 within time frame [t + d1, t + d2] significantly more than expected given that X2 has a constant probability of occurring n times, randomly distributed throughout the observation period [t1, t2]. The processes are repeated with the newly identified time patterns treated as elements until no more time patterns are found (for more details, see Magnusson 2000, 2004, 2005). The sensitivity of the pattern-detection algorithm can be altered using various parameters entered by the experimenter (see Magnusson 2004; electronic supplementary material in Bonasera et al. 2008); however, there exists no objective procedure for determining these parameters.
3.2. General examples
Markov models have been used widely in genomics and phylogenetic analysis (Larget & Simon 1999; Krogh et al. 2001) and in artificial intelligence where they are used both for analysing original behaviour and then regenerating it artificially (Brand et al. 1997). The Markov property is a central mathematical model, and contributes to statistical (Hastings 1970) and computational advances (Keller-McNulty et al. 1991). Briefly, other examples of Markov-based analyses include: particle structure and movement (Hannenhalli & Russell 2000); analysis of disease spread (McBryde et al. 2007; Keeling & Ross 2008); meteorology and climate analysis (Valdez & Young 1985; Young 1994); distribution and movement of plants and animals (Miall 1973); analysis of speech and acoustics (Hawes & Foley 1973); and sequence analysis of sports footage (Leonardi et al. 2004).
Unlike other methods in this review, Theme's time patterns were specifically designed to analyse behaviour. Outside of animal behaviour, Theme has been used in the analysis of sports sequences (Borrie et al. 2002; Jonsson et al. 2006); in human psychology and psychiatry research (Grammer et al. 1998; Lyon & Kemp 2004); and to understand patterns in language (Anolli et al. 2005).
3.3. Animal behaviour applications
The application of Markovian methods is not new to the study of behaviour or welfare (e.g. Cane 1959; Wechsler & Bachmann 1998). Markov chains are mentioned in classic behavioural methods texts (Martin & Bateson 1993; Slater 1993). Markov models have previously been used in animal welfare science to understand eliminative behaviour (Wechsler & Bachmann 1998) and oral/nasal/facial activity in pigs (Dailey & McGlone 1997). Pigs were found to follow a pattern of ‘enter dunging area, sniff, posture, defecate, urinate and sniff’, when eliminating and had fewer transitions that involved the top of the snout when feeding outdoors than when feeding indoors. Such details could be useful in the design of housing systems or to understand development of abnormal feeding behaviour. A Markovian method, the χ2 test for sequential dependency (Haccou & Meelis 1992; also see box 2), has been applied to repetitive behaviour in captive starlings (Asher et al. 2009). Starlings were found to have less-repetitive behaviour patterns in large and medium-sized long-shaped cages. They have also been used to analyse perseveration in the Gambling task, an operant task examining flexibility in pattern learning, in animals and humans (Garner et al. 2003).
Theme has been used to study human–animal interactions (Kerepesi et al. 2005, 2006), behaviour in feed-restricted hens (Merlet et al. 2005; Hocking et al. 2007) and feeding behaviour in chicks (Martaresche et al. 2000). Most recently, a study of drug-induced stereotypic behaviour in mice used Theme as a method of objectively quantifying stereotypy (Bonasera et al. 2008).
For both methodologies permutation methods need to be applied to test the likelihood of obtaining the observed pattern under randomness given environmental and behavioural constraints (visual inspection is recommended for this comparison in Theme). To this end, we suggest the use of Monte Carlo methods to account for impossible and improbable transitions, an approach taken by Bonasera and co-workers (2008). While temporal analyses could be applied to almost any study of behaviour, the obvious usage is scoring repetitive behaviour, which is more deterministic because it is predictable in time and space.
3.4. Advantages and disadvantages
3.4.1. Advantages
Retains information concerning structure; order in the case of Markov chains, and order and timing in the case of time patterns.
Groups discrete behaviour events into patterns according to their co-occurrence.
3.4.2. Disadvantages
Requires investment to determine which model best suits behavioural data, e.g. in which order of analysis to use in Markovian methods and in Theme how to decide the criterion levels of all different variables.
Care needs to be taken to ensure that baseline randomness is controlled for, given real-word constraints.
4. Social network analysis
4.1. Principles
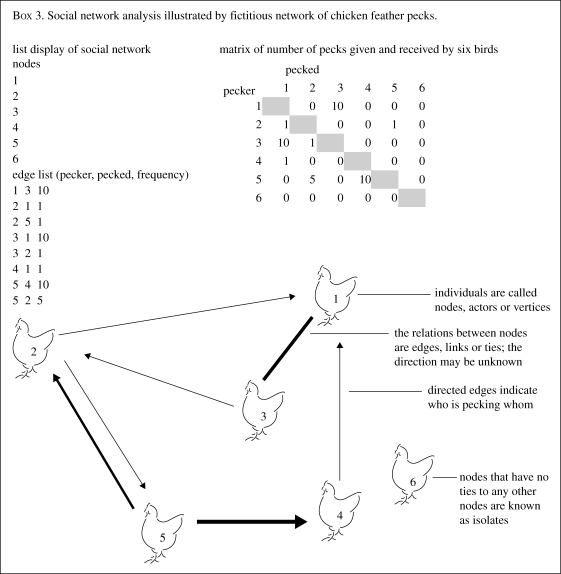
Formally, a social network consists of a finite set of nodes and the relations defining them: where a node (or actor) is a social entity; an edge (or tie) is a defined linkage between two nodes; and a relation is a collection of edges of specific kind among members of a group. SNA theorists have developed a set of concepts and parameters that enable the researcher to characterize and compare each member of the network systematically, detect relevant subgroups within a network as well as characterize the network as a whole (see box 3 or for review Hanneman 2005). Wasserman & Faust (1994) describe five key aspects of social networks: (i) models, theories or applications are expressed in terms of relational concepts; (ii) nodes and their relationships are viewed as interdependent rather than independent; (iii) edges between nodes allow the transfer or flow of resources (e.g. information or infection); (iv) the network structure provides opportunities or constraints in individual action; (v) structure is conceptualized as a lasting pattern of relations among nodes.
Depending on the choice of data collection, social networks can be complete, partial or ego-centric. A complete network contains all possible nodes and edges within a population. This can be time-consuming, lead to large, unwieldy datasets and there is potential for missing data. An ego-centric network focuses on a sample individual from a random population and examines all the edges that node has with other individuals in the population, although sampling errors and lack of representativeness can result in a reduction in validity. Partial networks are usually based on snowball-sampling, focusing on the edges between one node and others in the population and then gaining information on the interactions of those nodes and so on. Data collected using snowball methods can be more difficult to analyse (due to sampling biases).
After data collection, analyses centre on the transformation of raw data into network data. An analytical requirement of SNA is that the number of nodes and the period of time examined are finite, and therefore SNA often represents a snapshot of a network bound in space and time, in what is called the ‘boundary-specification problem’. Bounding the network to a limited number of nodes requires defining the relevant interactions in topological space. Dynamic network models are increasingly being developed (e.g. Vernon & Keeling 2009, where nodes were farms) to capture rare but important contacts between nodes.
The mathematical foundations of SNA are the graph, statistical and probability theories. Graph theory provides appropriate representation of network data in the form of matrices where all possible combinations of interactions between a set of actors can be represented (box 3). Statistical and probability models are used to test theoretical propositions and allow the model to show some error or lack of fit. Network data are considered to be interdependent and rarely normally distributed, hence parametric methods cannot be applied. Formal statistical tests based on permutation and bootstrapping procedures are now well established to allow testing of hypotheses and the measurement of type 1 errors. Multiple relationships within and between networks can also be tested using specific statistical models like random graph models (for a description of the most important quantitative models for analysing network data, see Carrington et al. 2005).
4.2. General examples
Social sciences developed the network framework as early as in the 1930s and became popular in the 1950s to study human social behaviour (Moreno 1934; Scott 2000). The uses of SNA are diverse in social sciences and some recent ones include: examining flow of information within an organization for management purposes (Nerkar & Paruchuri 2005); modelling fundamental biological theories, such as the evolution of language (Ke et al. 2008), or the spread of innovations (Goldstone et al. 2008); and investigating conflicts of interest among authors and potential reviewers (Aleman-Meza et al. 2006). Physicists and mathematicians have realized the potential of this methodology to explore the network structure of natural phenomena (Watts & Strogatz 1998), including biological systems, such as yeast genetic interactions and protein interactions (Tong et al. 2004; Zhang et al. 2008).
4.3. Animal behaviour applications
Animal interaction data that are typically collected include counts of particular type of interaction or proximity, both either with or without identification of individuals. There are many methods for analysing such data other than SNA that have typically centred on standard statistical tests (e.g. odds ratio of being involved in an interaction, tests of association, comparisons of group means) or multiple regression techniques (Minta 1992; Hardy & Field 1998). Such approaches can become laborious if many individuals or groups are involved, and individuals or groups are not statistically treated as interdependent. The type of information that can be extracted from interaction data without individual identification is limited. For instance, counts of aggression may not be representative of group aggression, but rather due to one or two aggressive individuals. Spatial distribution scores (such as nearest neighbour (Henderson et al. 2001) or cluster scores (Collins et al. in preparation)) are a useful measure of global positioning but do not provide details of the nature of any interactions. For example, are animals close because they are fighting or performing affiliative behaviours? When individuals are identified standard methods do not provide the flexibility that SNA does. For instance, dominance hierarchies typically rely upon a linear relation between all animals within a group which is consistent between contexts. Calculation of dominance hierarchies can be difficult and unreliable without many dyadic interactions (Poisbleau et al. 2006). SNA requires identification of individuals, which can be difficult in large groups; however, it does present a number of advantages over the methods outlined above: it has a network rather than a linear structure allowing for fluidity and dynamics in relations; it treats animals as interdependent accounting for the fact that the behaviour of one animal in a group affects the behaviour of another; it recognizes different layers of relational structure and can be used to define groups and subgroups (Wolf et al. 2007); it can examine the interactions within and between groups and elucidate the stability of social networks over time (e.g. Drewe et al. 2009). Social networks may be constructed from positional or more detailed analyses of behavioural interactions.
After its early application to the social behaviour of primates (Sade 1972), SNA has seldom been applied to animal networks until recent years (Lusseau 2003; Lusseau & Newman 2004; Croft et al. 2005; Sundaresan et al. 2007; Manno 2008). Recent reviews (Krause et al. 2007; Wey et al. 2008) and a newly published textbook (Croft et al. 2008), written specifically for behavioural biologists, synthesize the literature available on applications of this methodology. To date, and to the best of our knowledge, the only study of social networks in the field of animal welfare investigated captive primates (rhesus macaques, Macacca mulatta; McCowan et al. 2008). The conclusions of this study were that aggression could be minimized to prevent severe outbreaks of deleterious aggression in group-housed macaques through the understanding of social behaviour using SNA.
There are two primary reasons that suggest that SNA has utility in the study of animal welfare: (i) to understand transmission probabilities, and (ii) to understand the direct impact of social relations upon welfare. Much of the abnormal behaviour that concerns welfare scientists, such as tail-biting, feather pecking and stereotypic behaviour, spreads either actively due to social learning, or passively through animals encountering a wounded tail or damaged plumage. SNA could improve understanding of behavioural transmission. For example, in large commercial groupings SNA could be used to estimate the number of animals in a group that are likely to encounter each other within a given time-frame. It could also be used to study the effects of mixing animals or removing an individual or individuals from a stable social structure, such as occurs in many commercial pig and cattle farms, on a large scale during culling activities, and in wildlife relocation (e.g. Williams & Lusseau 2006). A key benefit of SNA is its ability to examine simultaneously more than one type of social interaction. Further, the influence of external environmental processes such as rainfall on the interaction patterns of individuals may be examined using SNA (Drewe et al. 2009). The network approach therefore complements other more commonly used techniques for the study of animal behaviour.
It has been established for many years that a network of social relationships is one of the most important determinants of quality of life in humans. For example, Berkman & Syme (1979) showed a strong relationship between social network and life expectancy. This has been supported by later studies (e.g. House et al. 1988) and meta-analyses (Uchino et al. 1996), which further suggest that the quality of relationships is important, as well as the quantity. Social housing has been found to affect rats' and pigs' ability to deal with stressors (Ruis et al. 1999, 2001). The importance of social housing and ‘stable social groups’ is recognized as important in determining animal welfare, for example recommendations for the care of animals in research frequently state that animals should be socially housed wherever possible. However, at present, we do not have a sufficient understanding of what constitutes a good social network as regards promoting good welfare, for any single captive species.
4.4. Advantages and disadvantages
4.4.1. Advantages
Organizes relational data assuming that individuals are interdependent and members of a super-structure called a social network.
Allows the analysis of relational data at different levels accounting for the interdependency of the data items.
Analysis may be performed at individual, group or population level.
4.4.2. Disadvantages
Alternative statistical techniques are required, i.e. parametric methods cannot be applied.
The elements of the network must be defined in time and space, hence identifying individuals is essential.
Impact of missing network data on analytical results is difficult to assess.
5. Agent-based modelling and simulation
5.1. Principles
ABMS or individual-based modelling and simulation is a type of computation using independent autonomous individuals or ‘agents’ to describe a system as a whole. The approach is bottom-up and generative. The rules that describe the entire system's behaviour are qualitatively different from those that govern its constituent units and yet the rules governing the units produce the rules of the system (Vicsek 2000). A set of rules or heuristics entered by the author of the model determine how the agents ‘behave’ and the emergent group behaviour of the agents is revealed. Models can be based on real data and the best validated models are those that are not only based on parameters from real datasets, but are also tested on independent datasets for robustness (Bryson et al. 2007). If the model accurately describes observed behaviour of real animals then the logic of the programme (the heuristics, parameters and their relative weighting) is supposed to reflect causal mechanisms. In reality a model can only reveal whether the heuristics and parameters entered are sufficient to generate observed behaviour. ABMS is used to describe how a system functions by subjecting the theoretical individual (agent) to a wide range of environmental conditions and observing the effects, at both the individual and population level. This is achieved by running simulations. The simulations reveal the consequences that arise from the combination of heuristics and parameters.
There is no standard ABMS methodology. The problem to be investigated, and, to a large extent, the author's style, will decide the form the model takes (see box 4 for worked example). This flexibility is a major advantage of ABMS, but also a significant drawback because there is no set precedent (although for suggestion of unifying framework see Grimm et al. 2005) and therefore is open to error. Authors may construct models with parameters which they believe to correspond to some real world parameter which actually corresponds to something else. Watts (1998) identifies three stages for models of movement behaviour: (i) initialization, to determine parameters and heuristics; (ii) refinement, to run simulations with different features, check the model mimics real behaviour and alter parameters/heuristics where necessary; (iii) validation, to compare the model with real systems and assess the predictive ability of the model. ABM heuristics are often borrowed from other areas of science; for instance, the Hills equation, which is more frequently used to describe protein molecule binding, has been used to model flock behaviour (Collins & Sumpter 2007). Some models incorporate artificial neural networks or genetic programming, or endow agents with motivations or emotions (Watts 1998; Abu Maria & Abu Zitar 2007; Broekens et al. 2007). They could also incorporate the other methods outlined in this review; for instance, we include basic information about social relations in the example of ABMS in box 4. ABMS can allow for heterogeneity in populations, therefore incorporating behavioural ecology and game theories concerning evolutionary stable strategies (Maynard-Smith 1972). It should, however, be noted that complicated models requiring parameter values for many different effects and relationships are more likely to contain errors and simulations will be subject to large variation. Conversely, complex models can be more intuitive to build, and once built can be reduced to a simplified form. Models that are too complex to analyse analytically can be evaluated using hypothesis testing (Bryson et al. 2001).
5.2. General examples
ABMS has been used to describe: financial markets (Jefferies et al. 2001); bacterial growth (Ben-Jacob & Levine 2006); evolutionary and social processes (Macy & Willer 2002; Bowles & Gintis 2004); and disease dynamics (Eubank et al. 2004; Yang et al. 2008). It is also a growing part of software design (Jennings 2000).
5.3. Animal behaviour applications
Agent-based models differ from more traditional analytical models that are top-down and at population level. Some such models have been applied to animal welfare; for example, Grafen's (2002) State-free optimization model is concerned with motivation in choice tests and Rands et al.'s (2003) work on foraging pairs has implications for obesity in domesticated species such as the horse. Animals have also been modelled as consumers, making choices between resources, using an approach loosely based on microeconomics (Kirkden & Pajor 2006).
The most extensive use of ABMS in animal behaviour is in the study of collective behaviour with examples in insect societies, fish schools and human crowds. The visualization of animal agents in simulated environments has led to their description as animats, the first example of which was Reynold's boids that could mimic behaviour of flocks, herds or schools using just three simple rules-of-thumb (Reynolds 1987). Examples of ABMS in animal welfare have almost exclusively considered the effects of physical environment on farm animal behaviour: the effect of lighting on broiler chickens (Kristensen et al. 2006) and the effect of space and resource availability in pigs and broilers (Stricklin et al. 1995, 1998; Gonyou et al. 1997; Collins & Sumpter 2007). As making real-life changes to the physical environment is costly, such models are appealing and the number and scope of environments tested can far exceed those that could be tested in the real world.
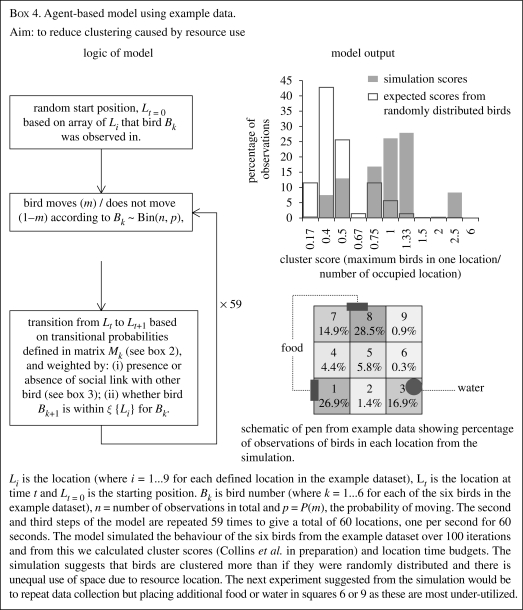
Much scope exists in application of ABMS from behavioural ecology (and other areas) to animal welfare and for conversion of analytical type models into agent-based ones. For instance, Inglis & Langton's (2006) analytical model accounts for the finding that behavioural complexity reduces in response to environmental complexity. An extension to this model could examine the effects of environmental complexity on group behaviour and test these predictions on group-housed animals. White & Smith (2007) demonstrated how ABMS can be used to evaluate the existing behaviour scoring methods. Since the methodology exists to incorporate genetic programming into ABMS, the phenotypic effects of selective breeding could be examined, thus moving towards the goal of breeding animals that cope better with captive environments. Used as Monte Carlo methods, simulations could be utilized to make statistical inference and distinguish between hypotheses. For instance, in recent work we compared clustering behaviour observed in real hens with simulated hens which spent the same proportion of time in each location as our real hens (Collins et al. in preparation) in a method similar to that used in box 4. We were able to ascertain that hens were being socially cohesive and were not just clustering due to shared resource use.
5.4. Advantages and disadvantages
5.4.1. Advantages
Can run experiments in number and varieties that would be impossible in the real world, thus reducing costs and the number of animals required.
Can be used to understand the complexity of systems and predict the effects of a large number of variables.
5.4.2. Disadvantages
There is an absence of a coherent framework.
Complicated models can be unreliable unless relationships between parameters are well understood.
Models are essentially best estimates if they are not testable, or based on real data.
6. Discussion
Collection of behavioural data is considered standard in the assessment of animal welfare and yet is not exploited to its full potential. Animal behaviour is complex, with simultaneously occurring, differentially contributing processes that do not follow simple linear patterns. Through this review it has become apparent that we currently know very little about the relation between deep structural characteristics of behaviour and animal welfare. We cannot be certain of the utility of behavioural organization methods when compared with typical ethogram approaches, but this can only be elucidated with a more consistent body of empirical evidence.
In addition to the potential advantages of analysis of behavioural organization in assessing welfare, such methods can also directly impact upon welfare. Group-housing of animals used in research could be encouraged by the application of SNA because it can control statistically for the fact one animal may have an impact upon another. Simulations, such as those used in ABMS, require fewer animals and allow experiments to be conducted in number and variety impossible in the real world.
Some of the methods mentioned are based on a bottom-up generative approach and others on top-down rules. Bottom-up approaches can be computationally expensive but may be expected to be particularly useful when there is much variability between individuals and the interactions between them. Top-down approaches, even stochastic ones, treat each individual unit in the same manner and therefore are likely to have most utility when animals share similar behavioural patterns and priorities.
Each of the techniques mentioned in this review captures a different aspect of complexity: fractal analysis captures long-range organization; temporal methods capture short-term behaviour patterns and the determinism of these patterns; SNA captures social complexity; and ABMS can decompose complex behaviour into heuristic rules. For certain data types it is possible that more than one of the methods outlined could be correctly applied. Hocking et al. (2007) used the Theme program and DFA to analyse the behaviour of feed-restricted broiler chickens. Theme was found to complement traditional ethogram analysis but DFA was less useful, possibly due to differences in the range of these analyses.
For ease of comparison we have briefly summarized the type of data that could be inputted into the four methods of analysis and the possible outputs (table 1). Comparisons between the methods are probably best explained using examples. While the four methods in this review have not been applied to the same behavioural data they have all been applied to the study of football, for example: the fractal dimension of player two-dimensional coordinates on a pitch has been found to be higher (i.e. behaviour is more regular) when teams were defending (Kim 2006); more consistency has been found in the t-patterns of international football over club matches (Borrie et al. 2002); Brazilian football players were found to be a small-world network (Onody & de Castro 2004); and an ABMS performed well in simulated football matches and could be extended to predict effective strategies against specific teams (Jolly et al. 2007).
Table 1.
Comparison of inputs and outputs of methods of analysing behavioural organization.
| analysis | input | output |
|---|---|---|
| fractal analysis | most usually binary (1,0) data or x–y coordinates from scan sampling | measures self-similarity patterns in data along a random to organized continuum |
| temporal analysis | discrete behavioural events measured along a continuous time scale | can measure the predictability of chains of events and identify and measure chains of events |
| social network analysis | presence/absence or frequencies of interactions or associations between individuals in a group | information concerning social structure and individuals' position within this structure |
| agent-based modelling | model of a system of autonomous parts driven by parameters from real data | deconstruction of systems into heuristic parts and simulations to predict effects of changes to the system |
We used the same example dataset throughout this review to illustrate the use of different methods. It is hoped that these examples provide an intuitive understanding of the methods outlined rather than a true reflection of how these techniques might work in practice. In box 1 we calculated the fractal dimension of activity in a single bird and found a low fractal exponent. This indicates that the birds' activity was randomly distributed over time. In order to be able to offer a true interpretation we would need to find the time distribution of activity in birds in stressful and non-stressful situations. However, the bird fractal dimension demonstrated that the bird had variability in behaviour which shows it is not limited to the simple motor patterns associated with abnormal repetitive behaviour. In box 2 we demonstrated a lack of Markovian determinism in the sequences of direction moved by one hen. Again this suggests the bird is not limited to repetitive movement. From all birds in the dataset, we visualized the structure of relations between birds using a sociogram in box 3. All but one of the six hens pecked or were pecked by another bird. If this were a real dataset with multiple groups examined it would be interesting to examine the progression of pecking over time or look at the effects of the overall social network structure or individual position within the network on pecking behaviour. Finally, in the ABMS we simulated the movements of birds based on heuristics from the example dataset. The simulated birds were clustering more than expected at random and under-utilized certain areas of the pen that did not contain resources.
Animal behaviour, like many natural and man-made systems, is complex and multi-dimensional. The principles for understanding the complexity of systems transcend traditional scientific disciplines and therefore research on complex systems is likely to be most effective by taking a multi-disciplinary approach. The four types of analysis outlined in this review were either developed or have since been utilized for purposes other than behavioural analysis. Developments in behavioural analysis will continue to become increasingly important alongside advancements in physics and engineering (e.g. Spink et al. 2001; Kramer & Kinter 2003; Pack et al. 2007; Donohue et al. 2008) that are continuing to improve the quantity, and sometimes quality, of behavioural data collected. Through this review we hope to have encouraged a multi-disciplinary approach to behavioural analysis and illustrated some of the realized and potential advantages such an approach may bring.
Acknowledgements
We thank Paul Peterson, Bill Browne and Emily O'Connor and four anonymous referees for their helpful comments on the manuscript. This work was financially supported by the BBSRC.
Glossary
- Bootstrapping:
A method for making statistical inference based on repeated randomly sampling from an actual sample.
- Bottom-up:
A strategy for information processing derived from piecing together component parts.
- Euclidean:
An area of mathematics relating to standard geometry based on the mathematician Euclid's axioms.
- Monte Carlo methods:
A suite of heuristic mathematical techniques where possible random variation in all parameters is examined and can be used to assess the cause of observed effects.
- Random permutations:
The random ordering of discrete items.
- Random walk:
A sequence of random movements of fixed step length where each movement depends only on the direction of the previous movement.
- Top-down:
Information processing based on decomposition of a system into component units. The opposite of bottom-up.
References
- Abu Maria K., Abu Zitar R. 2007. Emotional agents: a modeling and an application. Inf. Softw. Technol. 49, 695–716. ( 10.1016/j.infsof.2006.08.002) [DOI] [Google Scholar]
- Alados C. L., Huffman M. A. 2000. Fractal long-range correlations in behavioural sequences of wild chimpanzees: a non-invasive analytical tool for the evaluation of health. Ethology 106, 105–116. ( 10.1046/j.1439-0310.2000.00497.x) [DOI] [Google Scholar]
- Alados C. L., Weber D. N. 1999. Lead effects on the predictability of reproductive behavior in fathead minnows (Pimephales promelas): a mathematical model. Environ. Toxicol. Chem. 18, 2392–2399. ( 10.1897/1551-5028(1999)018%3C2392:LEOTPO%3E2.3.CO;2) [DOI] [PubMed] [Google Scholar]
- Aleman-Meza B., Nagarajan M., Ramakrishnan C., Ding L., Kolari P., Sheth A., Arpinar I. B., Joshi A., Finn T. 2006. Semantic analysis on social networks: experiences in addressing the problem of conflcit of interest detection. In Proc. Int. Conf. on the World Wide Web, Edinburgh, UK New York, NY: ACM Press. [Google Scholar]
- Anolli L., Duncan S., Jr, Magnusson M. S., Riva G. 2005. Conversation patterns in Icelandic and Italian people: similarities and differences in rhythm and accommodation. In The hidden structure of interaction: from neurons to culture patterns. Amsterdam, The Netherlands: IOS Press. [Google Scholar]
- Asher L., Davies G. T. O., Bertenshaw C. E., Cox M. A. A., Bateson M. 2009. The effects of cage volume and cage shape on the condition and behaviour of captive European starlings (Sturnus vulgaris). Appl. Anim. Behav. Sci. 116, 286–294. ( 10.1016/j.applanim.2008.10.008) [DOI] [Google Scholar]
- Bakeman R., Quera V. 1995. Analyzing interaction: sequential analysis with SDIS and GSEQ. New York, NY: Cambridge University Press. [Google Scholar]
- Barbu V. S., Limnios N. 2008. Semi-Markov chains and hidden semi-Markov models toward applications: their use in reliability and DNA analysis. New York, NY: Birkhäuser. [Google Scholar]
- Bassett L., Buchanan-Smith H. M. 2007. Effects of predictability on the welfare of captive animals. Appl. Anim. Behav. Sci. 102, 223–245. ( 10.1016/j.applanim.2006.05.029) [DOI] [Google Scholar]
- Benhamou S. 2004. How to reliably estimate the tortuosity of an animal's path: straightness, sinuosity, or fractal dimension? J. Theor. Biol. 229, 209–220. ( 10.1016/j.jtbi.2004.03.016) [DOI] [PubMed] [Google Scholar]
- Ben-Jacob E., Levine H. 2006. Self-engineering capabilities of bacteria. J. R. Soc. Interface 3, 197–214. ( 10.1098/rsif.2005.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman L. F., Syme S. L. 1979. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda county residents, vol. 109, pp. 186–204. Oxford, UK: Oxford University Press. [DOI] [PubMed] [Google Scholar]
- Bonasera S., Schenk A., Luxenberg E., Tecott L. 2008. A novel method for automatic quantification of psychostimulant-evoked route-tracing stereotypy: application to Mus musculus. Psychopharmacology 196, 591–602. ( 10.1007/s00213-007-0994-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrie A., Jonsson G. K., Magnusson M. S. 2002. Temporal pattern analysis and its applicability in sport: an explanation and exemplar data. J. Sports Sci. 20, 845–852. ( 10.1080/026404102320675675) [DOI] [PubMed] [Google Scholar]
- Bowles S., Gintis H. 2004. The evolution of strong reciprocity: cooperation in heterogeneous populations. Theor. Popul. Biol. 65, 17–28. ( 10.1016/j.tpb.2003.07.001) [DOI] [PubMed] [Google Scholar]
- Brand M., Oliver N., Pentland A. 1997. Coupled hidden Markov models for complex action recognition. In Proc. IEEE Computer Society Conference on Computer Vision and Pattern Recognition, San Juan, Puerto Rico, pp. 994–999. New York, NY: IEEE. [Google Scholar]
- Broekens J., Kosters W. A., Verbeek F. J. 2007. Affect, anticipation, and adaptation: affect-controlled selection of anticipatory simulation in artificial adaptive agents. Adapt. Behav. 15, 397–422. ( 10.1177/1059712307084686) [DOI] [Google Scholar]
- Browne W. J., McCleery R. H., Sheldon B. C., Pettifor R. A. 2007. Using cross-classified multivariate mixed response models with application to life history traits in great tits (Parus major). Stat. Modell. 7, 217–238. ( 10.1177/1471082x0700700301) [DOI] [Google Scholar]
- Bryson J., Lowe W., Stein L. A. 2001. Hypothesis testing for complex agents. In Workshop on performance metrics for intelligent systems (eds Meystel A. M., Messina E. R.). Washington, DC: NIST Special Publication. [Google Scholar]
- Bryson J. J., Ando Y., Lehman H. 2007. Agent-based modelling as scientific method: a case study analysing primate social behaviour. Phil. Trans. R. Soc. B 362, 1685–1698. ( 10.1098/rstb.2007.2061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt G. M. 2005. The genesis of animal play: testing the limits. Cambridge, MA: MIT Press. [Google Scholar]
- Cane V. 1959. Behaviour sequences as semi-Markov chains. J. R. Stat. Soc. Ser. B 21, 36–58. [Google Scholar]
- Carrington P. J., Scott J., Wasserman S. 2005. Models and methods in social network analysis. New York, NY: Cambridge University Press. [Google Scholar]
- Cazelles B., Chavez M., de Magny G. C., Guegan J. F., Hales S. 2007. Time-dependent spectral analysis of epidemiological time-series with wavelets. J. R. Soc. Interface 4, 625–636. ( 10.1098/rsif.2007.0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauset A., Shalizi C. R., Newman M. E. J. 2007. Power-law distributions in empirical data. (http://arxiv.org/abs/0706.1062v1) [Google Scholar]
- Cole B. J. 1995. Fractal time in animal behavior: the movement activity of Drosophila. Anim. Behav. 50, 1317–1324. ( 10.1016/0003-3472(95)80047-6) [DOI] [Google Scholar]
- Collins L. M., Sumpter D. J. T. 2007. The feeding dynamics of broiler chickens. J. R. Soc. Interface 4, 65–72. ( 10.1098/rsif.2006.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. M., Asher L., Pfeiffer D. U., Nicol C. J. In preparation Clustering, synchrony and activity levels in laying hens: the effect of environmental resources on social dynamics. [Google Scholar]
- Cook R. G., Geller A. I., Zhang G. R., Gowda R. 2004. Touch screen-enhanced visual learning in rats. Behav. Res. Meth. Instrum. 36, 101–106. [DOI] [PubMed] [Google Scholar]
- Coutellier L., Friedrich A. C., Failing K., Marashi V., Wurbel H. 2009. Effects of foraging demand on maternal behaviour and adult offspring anxiety and stress response in C57BL/6 mice. Behav. Brain Res. 196, 192–199. ( 10.1016/j.bbr.2008.08.042) [DOI] [PubMed] [Google Scholar]
- Croft D. P., James R., Ward A. J. W., Botham M. S., Mawdsley D., Krause J. 2005. Assortative interactions and social networks in fish. Oecologia 143, 211–219. ( 10.1007/s00442-004-1796-8) [DOI] [PubMed] [Google Scholar]
- Croft D. P., James R., Krause J. 2008. Exploring animal social networks. Princeton, NJ: Princeton University Press. [Google Scholar]
- Dailey J. W., McGlone J. J. 1997. Oral/nasal/facial and other behaviors of sows kept individually outdoors on pasture, soil or indoors in gestation crates. Appl. Anim. Behav. Sci. 52, 25–43. ( 10.1016/S0168-1591(96)01099-4) [DOI] [Google Scholar]
- Dawkins M. S. 2003. Behaviour as a tool in the assessment of animal welfare. Zoology 106, 383–387. ( 10.1078/0944-2006-00122) [DOI] [PubMed] [Google Scholar]
- Dicke M., Burrough P. A. 1988. Using fractal dimensions for characterizing tortuosity of animal trails. Physiol. Entomol. 13, 393–398. ( 10.1111/j.1365-3032.1988.tb01122.x) [DOI] [Google Scholar]
- Donohue K. D., Medonza D. C., Crane E. R., O'Hara B. F. 2008. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed. Eng. Online 7, 14 ( 10.1186/1475-925x-7-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe J. A., Madden J. R., Pearce G. P. 2009. The social network structure of a wild meerkat population. 1. Inter-group interactions. Behav. Ecol. Sociobiol. 63, 1295–1306. ( 10.1007/s00265-009-0782-x) [DOI] [Google Scholar]
- Edwards A. M., et al. 2007. Revisiting Levy flight search patterns of wandering albatrosses, bumblebees and deer. Nature 449, 1044–1048. ( 10.1038/nature06199) [DOI] [PubMed] [Google Scholar]
- Eubank S., Guclu H., Kumar V. S. A., Marathe M. V., Srinivasan A., Toroczkai Z., Wang N. 2004. Modelling disease outbreaks in realistic urban social networks. Nature 429, 180–184. ( 10.1038/nature02541) [DOI] [PubMed] [Google Scholar]
- Fowler S. C., Birkestrand B. R., Chen R., Moss S. J., Vorontsova E., Wang G., Zarcone T. J. 2001. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J. Neurosci. Methods 107, 107–124. ( 10.1016/S0165-0270(01)00359-4) [DOI] [PubMed] [Google Scholar]
- Fox C., Merali Z., Harrison C. 2006. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav. Brain Res. 175, 1–8. ( 10.1016/j.bbr.2006.08.016) [DOI] [PubMed] [Google Scholar]
- Fraser D. 2008. Understanding animal welfare: the science and its cultural context. UFAW Animal Welfare Series Oxford, UK: Wiley-Blackwell. [Google Scholar]
- Fritz H., Said S., Weimerskirch H. 2003. Scale-dependent hierarchical adjustments of movement patterns in a long-range foraging seabird. Proc. R. Soc. Lond. B 270, 1143–1148. ( 10.1098/rspb.2003.2350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner J. P., Mason G. J., Smith R. 2003. Stereotypic route-tracing in experimentally caged songbirds correlates with general behavioural disinhibition. Anim. Behav. 66, 711–727. ( 10.1006/anbe.2002.2254) [DOI] [Google Scholar]
- Gatti D. D., Di Guilmi C., Gallegati M., Gaffeo E., Giulioni G., Palestrini A. 2008. Scaling laws in the macroeconomy. Adv. Complex Syst. 11, 131–138. ( 10.1142/S0219525908001532) [DOI] [Google Scholar]
- Gillespie D. T. 1992. Markov processes: an introduction for physical scientists. San Diego, USA: Academic Press. [Google Scholar]
- Goldstone R. L., Roberts M. E., Gureckis T. M. 2008. Emergent processes in group behavior. Curr. Direct. Psychol. Sci. 17, 10–15. ( 10.1111/j.1467-8721.2008.00539.x) [DOI] [Google Scholar]
- Gonyou H. W., Zhou J. Z., Stricklin W. R. 1997. Applying animal technology to the design and evaluation of pens for growing-finishing pigs. In Livestock Environment V, Proc. 5th Int. Livest. Environ. Symp., Minneapolis, vol. 2, pp. 836–842. [Google Scholar]
- Grafen A. 2002. A state-free optimization model for sequences of behaviour. Anim. Behav. 63, 183–191. ( 10.1006/anbe.2001.1871) [DOI] [Google Scholar]
- Grammer K., Kruck K. B., Magnusson M. S. 1998. The courtship dance: patterns of nonverbal synchronization in opposite-sex encounters. J. Nonverbal Behav. 22, 3–29. ( 10.1023/A:1022986608835) [DOI] [Google Scholar]
- Grimm V., et al. 2005. Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science 310, 987–991. ( 10.1126/science.1116681) [DOI] [PubMed] [Google Scholar]
- Haccou P., Meelis E. 1992. Statistical analysis of behavioural data: an approach based on time-structured models. Oxford, UK: Oxford University Press. [Google Scholar]
- Hanneman R. A. 2005. Spatial dynamics of human populations: some basic models. See http://faculty.ucr.edu/~hanneman/spatial/index.html. [Google Scholar]
- Hannenhalli S. S., Russell R. B. 2000. Analysis and prediction of functional sub-types from protein sequence alignments. J. Mol. Biol. 303, 61–76. ( 10.1006/jmbi.2000.4036) [DOI] [PubMed] [Google Scholar]
- Hardy I. C. W., Field S. A. 1998. Logistic analysis of animal contests. Anim. Behav. 56, 787–792. ( 10.1006/anbe.1998.0833) [DOI] [PubMed] [Google Scholar]
- Hastings W. K. 1970. Monte-Carlo sampling methods using Markov chains and their applications. Biometrika 57, 97–109. ( 10.1093/biomet/57.1.97) [DOI] [Google Scholar]
- Hastings H. M., Sugihara G. 1993. Fractals: a user's guide for the natural sciences. Oxford, UK: Oxford Science Publications, Oxford University Press. [Google Scholar]
- Hawes L. C., Foley J. M. 1973. Markov analysis of interview communication. Speech Monogr. 40, 208–219. [Google Scholar]
- Henderson J. V., Lines J. A., Wathes C. M., White R. P., Nicol C. J. 2001. Behaviour of domestic ducks exposed to mobile predator stimuli. 2. The association of individual duckling attributes with relative position in a flock. Br. Poult. Sci. 42, 439–448. ( 10.1080/00071660120070659) [DOI] [PubMed] [Google Scholar]
- Hocking P. M., Rutherford K. M. D., Picard M. 2007. Comparison of time-based frequencies, fractal analysis and T-patterns for assessing behavioural changes in broiler breeders fed on two diets at two levels of feed restriction: a case study. Appl. Anim. Behav. Sci. 104, 37–48. ( 10.1016/j.applanim.2006.04.023) [DOI] [Google Scholar]
- House J. S., Landis K. R., Umberson D. 1988. Social relationships and health. Science 241, 540 ( 10.1126/science.3399889) [DOI] [PubMed] [Google Scholar]
- Inglis I. R., Langton S. 2006. How an animal's behavioural repertoire changes on response to a changing environment: a stochastic model. Behaviour 143, 1563–1596. ( 10.1163/156853906779367044) [DOI] [Google Scholar]
- Jefferies P., Hart M. L., Hui P. M., Johnson N. F. 2001. From market games to real-world markets. Eur. Phys. J. B 20, 493–501. ( 10.1007/s100510170228) [DOI] [Google Scholar]
- Jennings N. R. 2000. On agent-based software engineering. Artif. Intell. 117, 277–296. ( 10.1016/S0004-3702(99)00107-1) [DOI] [Google Scholar]
- Jolly K. G., Ravindran K. P., Vijayakumar R., Kumar R. S. 2007. Intelligent decision making in multi-agent robot soccer system through compounded artificial neural networks. Robot. Auton. Syst. 55, 589–596. ( 10.1016/j.robot.2006.12.011) [DOI] [Google Scholar]
- Jonsson G. K., Anguera M. T., Blanco-Villasenor A., Losada J. L., Hernandez-Mendo A., Arda T., Camerino O., Castellano J. 2006. Hidden patterns of play interaction in soccer using SOF-CODER. Behav. Res. Methods 38, 372–381. [DOI] [PubMed] [Google Scholar]
- Kane A. S., Salierno J. D., Gipson G. T., Molteno T. C. A., Hunter C. 2004. A video-based movement analysis system to quantify behavioral stress responses of fish. Water Res. 38, 3993–4001. ( 10.1016/j.watres.2004.06.028) [DOI] [PubMed] [Google Scholar]
- Ke J., Gong T., Wang W. S. Y. 2008. Language change and social networks. Commun. Comput. Phys. 3, 935–949. [Google Scholar]
- Keeling M. J., Ross J. V. 2008. On methods for studying stochastic disease dynamics. J. R. Soc. Interface 5, 171–181. ( 10.1098/rsif.2007.1106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-McNulty S., McNulty M. S., Gustafson D. A. 1991. Stochastic-models for software science. J. Syst. Softw. 16, 59–68. ( 10.1016/0164-1212(91)90032-2) [DOI] [Google Scholar]
- Kembro J. M., Satterlee D. G., Schmidt J. B., Perillo M. A., Marin R. H. 2008. Open-field temporal pattern in ambulation in Japanese quail selected for contrasting adrenocortical responsiveness to brief manual restraint. Poult. Sci. 87, 2186–2195. ( 10.3382/ps.2008-00108) [DOI] [PubMed] [Google Scholar]
- Kerepesi A., Jonsson G. K., Miklosi A., Topal J., Csanyi V., Magnusson M. S. 2005. Detection of temporal patterns in dog-human interaction. Behav. Processes 70, 69–79. ( 10.1016/j.beproc.2005.04.006) [DOI] [PubMed] [Google Scholar]
- Kerepesi A., Kubinyi E., Jonsson G. K., Magnusson M. S., Miklosi A. 2006. Behavioural comparison of human-animal (dog) and human-robot (AIBO) interactions. Behav. Processes 73, 92–99. ( 10.1016/j.beproc.2006.04.001) [DOI] [PubMed] [Google Scholar]
- Kim S. 2006. Player's positional dependence of fractal behaviors in a soccer game. Fractals-Complex Geom. Patterns Scaling Nat. Soc. 14, 71–76. ( 10.1142/S0218348X06003003) [DOI] [Google Scholar]
- Kirkden R. D., Pajor E. A. 2006. Using preference, motivation and aversion tests to ask scientific questions about animals' feelings. Appl. Anim. Behav. Sci. 100, 29–47. ( 10.1016/j.applanim.2006.04.009) [DOI] [Google Scholar]
- Korte S. M., Olivier B., Koolhaas J. M. 2007. A new animal welfare concept based on allostasis. Physiol. Behav. 92, 422–428. ( 10.1016/j.physbeh.2006.10.018) [DOI] [PubMed] [Google Scholar]
- Kramer K., Kinter L. B. 2003. Evaluation and applications of radiotelemetry in small laboratory animals. Physiol. Genom. 13, 197–205. ( 10.1152/physiolgenomics.00164.2002) [DOI] [PubMed] [Google Scholar]
- Krause J., Croft D. P., James R. 2007. Social network theory in the behavioural sciences: potential applications. Behav. Ecol. Sociobiol. 62, 15–27. ( 10.1007/s00265-007-0445-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen H. H., Aerts J. M., Leroy T., Wathes C. M., Berckmans D. 2006. Modelling the dynamic activity of broiler chickens in response to step-wise changes in light intensity. Appl. Anim. Behav. Sci. 101, 125–143. ( 10.1016/j.applanim.2006.01.007) [DOI] [Google Scholar]
- Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. L. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580. ( 10.1006/jmbi.2000.4315) [DOI] [PubMed] [Google Scholar]
- Larget B., Simon D. L. 1999. Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol. Biol. Evol. 16, 750–759. [Google Scholar]
- Leonardi R., Migliorati P., Prandini M. 2004. Semantic indexing of soccer audio-visual sequences: a multimodal approach based on controlled Markov chains. IEEE Trans. Circuit. Syst. Video Technol. 14, 634–643. ( 10.1109/TCSVT.2004.826751) [DOI] [Google Scholar]
- Lewis M. H. 2004. Environmental complexity and central nervous system development and function. Mental Retard. Dev. Disabil. Res. Rev. 10, 91–95. ( 10.1002/mrdd.20017) [DOI] [PubMed] [Google Scholar]
- Lo C. C., Chou T., Penzel T., Scammell T. E., Strecker R. E., Stanley H. E., Ivanov P. C. 2004. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc. Natl Acad. Sci. USA 101, 17545–17548. ( 10.1073/pnas.0408242101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusseau D. 2003. The emergent properties of a dolphin social network. Proc. R. Soc. Lond. B 270, S186–S188. ( 10.1098/rsbl.2003.0057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusseau D., Newman M. E. J. 2004. Identifying the role that animals play in their social networks. Proc. R. Soc. Lond. B 271, 477–481. ( 10.1098/rsbl.2004.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M., Kemp A. S. 2004. Increased temporal patterns in choice responding and altered cognitive processes in schizophrenia and mania. Psychopharmacology 172, 211–219. [DOI] [PubMed] [Google Scholar]
- Macy M. W., Willer R. 2002. From factors to actors: computational sociology and agent-based modeling. Ann. Rev. Sociol. 28, 143–166. ( 10.1146/annurev.soc.28.110601.141117) [DOI] [Google Scholar]
- Magnusson M. S. 2000. Discovering hidden time patterns in behavior: T-patterns and their detection. Behav. Res. Methods Instrum. 32, 93–110. [DOI] [PubMed] [Google Scholar]
- Magnusson M. S. 2004. Theme: powerful tool for the detection and analysis of hidden patterns in behavior. Reference Manual Version 5.0. Wageningen, The Netherlands: Noldus Information Technology. [Google Scholar]
- Magnusson M. S. 2005. Understanding social interaction: discovering hidden structure with model and algorithms. The hidden structure of interaction: from neurons to culture patterns (eds Anolli L., Duncen S. J., Magnusson M. S., Riva G.). Amsterdam, The Netherlands: IOS Press. [Google Scholar]
- Manno T. G. 2008. Social networking in the Columbian ground squirrel, Spermophilus columbianus. Anim. Behav. 75, 1221–1228. ( 10.1016/j.anbehav.2007.09.025) [DOI] [Google Scholar]
- María G. A., Escós J., Alados C. L. 2004. Complexity of behavioural sequences and their relation to stress conditions in chickens (Gallus gallus domesticus): a non-invasive technique to evaluate animal welfare. Appl. Anim. Behav. Sci. 86, 93–104. ( 10.1016/j.applanim.2003.11.012) [DOI] [Google Scholar]
- Martaresche M., Le Fur C., Magnusson M. S., Faure J. M., Picard M. 2000. Time structure of behaviour patterns realted to feed pecking in chicks. Physiol. Behav. 70, 443–451. ( 10.1016/S0031-9384(00)00283-3) [DOI] [PubMed] [Google Scholar]
- Martin P., Bateson P. 1993. Measuring behaviour: an introductory guide. New York, NY: Cambridge University Press. [Google Scholar]
- Maynard-Smith J. 1972. On evolution. Edinburgh, UK: Edinburgh University Press. [Google Scholar]
- McBryde E. S., Pettitt A. N., Cooper B. S., McElwain D. L. S. 2007. Characterizing an outbreak of vancomycin-resistant Enterococci using hidden Markov models. J. R. Soc. Interface 4, 745–754. ( 10.1098/rsif.2007.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan B., Anderson K., Heagarty A., Cameron A. 2008. Utility of social network analysis for primate behavioral management and well-being. Appl. Anim. Behav. Sci. 109, 396–405. ( 10.1016/j.applanim.2007.02.009) [DOI] [Google Scholar]
- McDonnell S. M., Poulin A. 2002. Equid play ethogram. Appl. Anim. Behav. Sci. 78, 263–290. ( 10.1016/S0168-1591(02)00112-0) [DOI] [Google Scholar]
- Merlet F., Puterflam J., Faure J. M., Hocking P. M., Magnusson M. S., Picard M. 2005. Detection and comparison of time patterns of behaviours of two broiler breeder genotypes fed ad libitum and two levels of feed restriction. Appl. Anim. Behav. Sci. 94, 255–271. ( 10.1016/j.applanim.2005.02.014) [DOI] [Google Scholar]
- Miall A. D. 1973. Markov chain analysis applied to an ancient alluvial plain succession. Sedimentology 20, 347–364. ( 10.1111/j.1365-3091.1973.tb01615.x) [DOI] [Google Scholar]
- Miller G. E., Chen E., Zhou E. S. 2007. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol. Bull. 133, 25–45. ( 10.1037/0033-2909.133.1.25) [DOI] [PubMed] [Google Scholar]
- Minsky M. L. 2006. The emotion machine: commonsense thinking, artificial intelligence, and the future of the human mind. New York, NY: Simon & Schuster. [Google Scholar]
- Minta S. C. 1992. Tests of spatial and temporal interaction among animals. Ecol. Appl. 2, 178–188. ( 10.2307/1941774) [DOI] [PubMed] [Google Scholar]
- Moreno J. L. 1934. Who shall survive? Foundations of sociometry, group psychotherapy and sociodrama. Washington, DC: Nervous and Mental Disease Publishing Co. [Google Scholar]
- Mundry R., Sommer C. 2007. Discriminant function analysis with non-independent data: consequences and an alternative. Anim. Behav. 74, 965–976. ( 10.1016/j.anbehav.2006.12.028) [DOI] [Google Scholar]
- Nerkar A., Paruchuri S. 2005. Evolution of R&D capabilities: the role of knowledge networks within a firm. Manage. Sci. 51, 771–785. ( 10.1287/mnsc.1040.0354) [DOI] [Google Scholar]
- Onody R. N., de Castro P. A. 2004. Complex network study of Brazilian soccer players. Phys. Rev. E 70, ( 10.1103/PhysRevE.70.037103) [DOI] [PubMed] [Google Scholar]
- Pack A. I., et al. 2007. Novel method for high-throughput phenotyping of sleep in mice. Physiol. Genom. 28, 232–238. ( 10.1152/physiogenomics.00139.2006) [DOI] [PubMed] [Google Scholar]
- Patin G., Sighireanu M., Touili T. 2007. Spade: verification of multithreaded dynamic and recursive programs. Lect. Notes Comput. Sci. 4590, 254 ( 10.1007/978-3-540-73368-3_28) [DOI] [Google Scholar]
- Paulus M. P., Geyer M. A. 1993. Quantitative assessment of microstructure of rat behavior. 1. F(D), the extension of the scaling hypothesis. Psychopharmacology 113, 177–186. ( 10.1007/BF02245695) [DOI] [PubMed] [Google Scholar]
- Paulus M. P., Bakshi V. P., Geyer M. A. 1998. Isolation rearing affects sequential organization of motor behavior in post-pubertal but not pre-pubertal Lister and Sprague-Dawley rats. Behav. Brain Res. 94, 271–280. ( 10.1016/S0166-4328(97)00158-7) [DOI] [PubMed] [Google Scholar]
- Pei J., Han J., Mortazavi-Asl B., Pinto H., Chen Q., Dayal U., Hsu M. C. 2001. PrefixSpan: mining sequential patterns efficiently by prefix-projected pattern. Data Eng. 17, 215–224. [Google Scholar]
- Peng C. K., Buldyrev S. V., Havlin S., Simons M., Stanley H. E., Goldberger A. L. 1994. Mosaic organization of DNA nucleotides. Phys. Rev. E 49, 1685–1689. ( 10.1103/PhysRevE.49.1685) [DOI] [PubMed] [Google Scholar]
- Peng C. K., Havlin S., Hausdorff J. M., Mietus J. E., Stanley H. E., Goldberger A. L. 1995a. Fractal mechanisms and heart rate dynamics: long-range correlations and their breakdown with disease. J. Electrocardiol. 28, 59–65. ( 10.1016/S0022-0736(95)80017-4) [DOI] [PubMed] [Google Scholar]
- Peng C. K., Havlin S., Stanley H. E., Goldberger A. L. 1995b. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time-series. Chaos 5, 82–87. ( 10.1063/1.166141) [DOI] [PubMed] [Google Scholar]
- Pickover C. A., Khorasani A. 1986. Fractal characterization of speech wave-form graphs. Comput. Graph. 10, 51–61. ( 10.1016/0097-8493(86)90068-3) [DOI] [Google Scholar]
- Poisbleau M., Jenouvrier S., Fritz H. 2006. Assessing the reliability of dominance scores for assigning individual ranks in a hierarchy. Anim. Behav. 72, 835–842. ( 10.1016/j.anbehav.2006.01.024) [DOI] [Google Scholar]
- Rands S. A., Cowlishaw G., Pettifor R. A., Rowcliffe J. M., Johnstone R. A. 2003. Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434. ( 10.1038/nature01630) [DOI] [PubMed] [Google Scholar]
- Reynolds C. W. 1987. Flocks, herds and schools: a distributed behavioural model. Comput. Graph. 21, 25–34. ( 10.1145/37402.37406) [DOI] [Google Scholar]
- Riley M. A., Turvey M. T. 2002. Variability and determinism in motor behavior. J. Motor Behav. 34, 99–125. [DOI] [PubMed] [Google Scholar]
- Ruis M. A. W., te Brake J. H. A., Buwalda B., De Boer S. F., Meerlo P., Korte S. M., Blokhuis H. J., Koolhaas J. M. 1999. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology 3, 285–300. [DOI] [PubMed] [Google Scholar]
- Ruis M. A. W., te Brake J. H. A., Engel B., Buist W. G., Blokhuis H. J., Koolhaas J. M. 2001. Adaptation to social isolation: acute and long-term stress responses of growing gilts with different coping characteristics. Physiol. Behav. 73, 541–551. ( 10.1016/S0031-9384(01)00548-0) [DOI] [PubMed] [Google Scholar]
- Rutherford K. M. D., Haskell M. J., Glasbey C., Jones R. B., Lawrence A. B. 2003. Detrended fluctuation analysis of behavioural responses to mild acute stressors in domestic hens. Appl. Anim. Behav. Sci. 83, 125–139. (doi:0.1016/s0168-1591(03)00115-1) [Google Scholar]
- Rutherford K. M. D., Haskell M. J., Glasbey C., Jones R. B., Lawrence A. B. 2004. Fractal analysis of animal behaviour as an indicator of animal welfare. Anim. Welf. 13, S99–S103. [Google Scholar]
- Rutherford K. M. D., Haskell M. J., Glasbey C., Lawrence A. B. 2006. The responses of growing pigs to a chronic-intermittent stress treatment. Physiol. Behav. 89, 670–680. ( 10.1016/j.physbeh.2006.08.006) [DOI] [PubMed] [Google Scholar]
- Sade D. S. 1972. Sociometrics of Macaca-mulatta: linkages and cliques in grooming matrices. Folia Primatol. 18, 196–223. ( 10.1159/000155480) [DOI] [PubMed] [Google Scholar]
- Scheibe K. M., Berger A., Langbein J., Streich W. J., Eichhorn K. 1999. Comparative analysis of ultradian and circadian behavioural rhythms for diagnosis of biorhythmic state of animals. Biol. Rhythm Res. 30, 216–233. ( 10.1076/brhm.30.2.216.1420) [DOI] [Google Scholar]
- Scott J. 2000. Social network analysis: a handbook. London, UK: Sage. [Google Scholar]
- Shibuichi S., Onda T., Satoh N., Tsujii K. 1996. Super water-repellent surfaces resulting from fractal structure. J. Phys. Chem. 100, 19512–19517. ( 10.1021/jp9616728) [DOI] [PubMed] [Google Scholar]
- Slater P. J. B. 1993. Describing sequences of behavior. In Perspectives in ethology: behavior and evolution, vol. 10 (eds Bateson P. P. G., Klopfer P. H., Thompson N. S.), pp. 131–153. New York, NY: Springer. [Google Scholar]
- Smith T. G., Marks W. B., Lange G. D., Sheriff W. H., Neale E. A. 1989. A fractal analysis of cell images. J. Neurosci. Methods 27, 173–180. [DOI] [PubMed] [Google Scholar]
- Spink A. J., Tegelenbosch R. A. J., Buma M. O. S., Noldus L. 2001. The ethovision video tracking system: a tool for behavioral phenotyping of transgenic mice. Physiol. Behav. 73, 731–744. ( 10.1016/S0031-9384(01)00530-3) [DOI] [PubMed] [Google Scholar]
- Spinka M., Newberry R. C., Bekoff M. 2001. Mammalian play: training for the unexpected. Q. Rev. Biol. 76, 141–168. ( 10.1086/393866) [DOI] [PubMed] [Google Scholar]
- Stricklin W. R., Zhou J. Z., Gonyou H. W. 1995. Selfish animats and robot ethology: using artificial animals to investigate social and spatial-behavior. Appl. Anim. Behav. Sci. 44, 187–203. ( 10.1016/0168-1591(95)00613-W) [DOI] [Google Scholar]
- Stricklin W. R., de Bourcier P., Zhou J. Z., Gonyou H. W. 1998. Artificial pigs in space: using artificial intelligence and artificial life techniques to design animal housing. J. Anim. Sci. 76, 2609–2613. [DOI] [PubMed] [Google Scholar]
- Sugihara G., May R. M. 1990. Applications of fractals in ecology. Trends Ecol. Evol. 5, 79–86. ( 10.1016/0169-5347(90)90235-6) [DOI] [PubMed] [Google Scholar]
- Sumpter D. J. T. 2006. The principles of collective animal behaviour. Phil. Trans. R. Soc. B 361, 5–22. ( 10.1098/rstb.2005.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan S. R., Fischhoff I. R., Dushoff J., Rubenstein D. I. 2007. Network metrics reveal differences in social organization between two fission–fusion species, Grevy's zebra and onager. Oecologia 151, 140–149. ( 10.1007/s00442-006-0553-6) [DOI] [PubMed] [Google Scholar]
- Tavazoei M. S., Haeri M. 2007. An optimization algorithm based on chaotic behavior and fractal nature. J. Comput. Appl. Math. 206, 1070–1081. ( 10.1016/j.cam.2006.09.008) [DOI] [Google Scholar]
- Tong A. H. Y., et al. 2004. Global mapping of the yeast genetic interaction network. Science 303, 808–813. ( 10.1126/science.1091317) [DOI] [PubMed] [Google Scholar]
- Troscianko T., Holmes A., Stillman J., Mirmehdi M., Wright D., Wilson A. 2004. What happens next? The predictability of natural behaviour viewed through CCTV cameras. Perception 33, 87–101. ( 10.1068/p3402) [DOI] [PubMed] [Google Scholar]
- Uchino B. N., Cacioppo J. T., Kiecolt-Glaser J. K. 1996. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol. Bull. 119, 488–531. ( 10.1037/0033-2909.119.3.488) [DOI] [PubMed] [Google Scholar]
- Valdez M. P., Young K. C. 1985. Number fluxes in equilibrium raindrop populations: a Markov-chain analysis. J. Atmos. Sci. 42, 1024–1036. ( 10.1175/1520-0469(1985)042%3C1024:NFIERP%3E2.0.CO;2) [DOI] [Google Scholar]
- Vernon M. C., Keeling M. J. 2009. Representing the UK's cattle herd as static and dynamic networks. Proc. R. Soc. B 276, 469–476. ( 10.1098/rspb.2008.1009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicsek T. 2000. Complexity: the bigger picture. Nature 418, 131 ( 10.1038/418131a) [DOI] [PubMed] [Google Scholar]
- Visser E. K., van Reenen C. G., van der Werf J. T., Schilder M. B., Knaap J. H., Barneveld A., Blokhuis H. J. 2002. Heart rate and heart rate variability during a novel object test and a handling test in young horses. Physiol. Behav. 76, 289–296. ( 10.1016/S0031-9384(02)00698-4) [DOI] [PubMed] [Google Scholar]
- Wasserman S., Faust K. 1994. Structural analysis in the social sciences. In Social network analysis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Watts D. J., Strogatz S. H. 1998. Collective dynamics of ‘small-world’ networks. Nature 393, 440–442. ( 10.1038/30918) [DOI] [PubMed] [Google Scholar]
- Watts J. M. 1998. Animats: computer-simulated animals in behavioral research. J. Anim. Sci. 76, 2596–2604. [DOI] [PubMed] [Google Scholar]
- Wechsler B., Bachmann I. 1998. A sequential analysis of eliminative behaviour in domestic pigs. Appl. Anim. Behav. Sci. 56, 29–36. ( 10.1016/S0168-1591(97)00075-0) [DOI] [Google Scholar]
- Wey T., Blumstein D. T., Shen W., Jordan F. 2008. Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim. Behav. 75, 333–344. ( 10.1016/j.anbehav.2007.06.020) [DOI] [Google Scholar]
- White D. J., Smith V. A. 2007. Testing measures of animal social association by computer simulation. Behaviour 144, 1447–1468. ( 10.1163/156853907782418259) [DOI] [Google Scholar]
- Wiepkema P. R., Koolhaas J. M. 1993. Stress animal welfare. Anim. Welf. 2, 195–218. [Google Scholar]
- Williams R., Lusseau D. 2006. A killer whale social network is vulnerable to targeted removals. Biol. Lett. 2, 497–500. ( 10.1098/rsbl.2006.0510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J. C. 2005. The concept of allostasis: coping with a capricious environment. J. Mammal. 86, 248–254. ( 10.1644/BHE-004.1) [DOI] [Google Scholar]
- Wolf J. B. W., Mawdsley D., Trillmich F., James R. 2007. Social structure in a colonial mammal: unravelling hidden structural layers and their foundations by network analysis. Anim. Behav. 74, 1293–1302. ( 10.1016/j.anbehav.2007.02.024) [DOI] [Google Scholar]
- Yamamoto Y., Hughson R. L. 1991. Coarse-graining spectral-analysis: new method for studying heart-rate-variability. J. Appl. Physiol. 71, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Yang Y., Atkinson P., Ettema D. 2008. Individual space-time activity-based modelling of infectious disease transmission within a city. J. R. Soc. Interface 5, 759–772. ( 10.1098/rsif.2007.1218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates J. W., Main D. C. J. 2008. Assessment of positive welfare: a review. Vet. J. 175, 293–300. ( 10.1016/j.tvjl.2007.05.009) [DOI] [PubMed] [Google Scholar]
- Yin G., Zhang Q. 2005. Discrete-time Markov chains: two-time-scale methods and applications. New York, NY: Springer. [Google Scholar]
- Young K. C. 1994. A multivariate chain model for simulating climatic parameters from daily data. J. Appl. Meteorol. 33, 661–671. ( 10.1175/1520-0450(1994)033%3C0661:AMCMFS%3E2.0.CO;2) [DOI] [Google Scholar]
- Zhang B., Park B. H., Karpinets T., Samatova N. F. 2008. From pull-down data to protein interaction networks and complexes with biological relevance. Bioinformatics 24, 979–986. ( 10.1093/bioinformatics/btn036) [DOI] [PubMed] [Google Scholar]