Abstract
Alien predators can have catastrophic effects on ecosystems and are thought to be much more harmful to biodiversity than their native counterparts. However, trophic cascade theory and the mesopredator release hypothesis predict that the removal of top predators will result in the reorganization of trophic webs and loss of biodiversity. Using field data collected throughout arid Australia, we provide evidence that removal of an alien top-predator, the dingo, has cascading effects through lower trophic levels. Dingo removal was linked to increased activity of herbivores and an invasive mesopredator, the red fox (Vulpes vulpes), and to the loss of grass cover and native species of small mammals. Using species distribution data, we predict that reintroducing or maintaining dingo populations would produce a net benefit for the conservation of threatened native mammals across greater than 2.42 × 106 km2 of Australia. Our study provides evidence that an alien top predator can assume a keystone role and be beneficial for biodiversity conservation, and also that mammalian carnivores more generally can generate strong trophic cascades in terrestrial ecosystems.
Keywords: biodiversity conservation, dingo, keystone species, mesopredator, top predator, trophic cascade
1. Introduction
Restoring and maintaining the ecological function of top-order predators is a topical and contentious issue worldwide (Soulé et al. 2005). Typically, top predators are strongly interactive species that exert top-down control on ecosystems through their direct predatory and competitive interactions with herbivores and smaller predators (Soulé et al. 2003). Losses of large predators from ecosystems have been linked to eruptions of herbivores and smaller predators and to cascades of ‘indirect’ effects on lower trophic levels (Crooks & Soulé 1999; Terborgh et al. 2001). Abundant herbivores can deplete plant biomass and affect animals that depend on plants for food or shelter (Berger et al. 2001; Hebblewhite et al. 2005), while smaller predators (mesopredators) can suppress populations of small prey species (Crooks & Soulé 1999). Increasingly, ecologists are realizing that top predators have pervasive or keystone effects on ecosystems that, if harnessed, could be used to manipulate ecological processes and species abundances to achieve biodiversity conservation goals (Beyer et al. 2007; Ripple & Beschta 2007).
Trophic cascade theory predicts that top predators have alternating positive and negative effects on lower trophic levels and may indirectly enhance plant biomass (Hairston et al. 1960). A related concept, the mesopredator release hypothesis (MRH), predicts that reduced abundance of top-order predators results in increased abundance or activity of smaller predators (mesopredators) and consequently has detrimental impacts on the prey of the smaller predators (Crooks & Soulé 1999). But what if the top predator is an alien species? Current evidence suggests that alien predators are more harmful to biodiversity than native predators (Salo et al. 2007); yet in Australia, the continent's largest terrestrial predator, the dingo (Canis lupus dingo: 12–20 kg), has coexisted with native prey species since its arrival some 5000 years ago (Savolainen et al. 2004) and is widely regarded as an invasive pest (Fleming et al. 2001).
Dingoes became mainland Australia's largest predator soon after their arrival when the thylacine (Thylacinus cynocephalus: 35 kg), a marsupial predator, became extinct (Johnson & Wroe 2003). Since British colonization in 1788 dingoes have been exterminated from much of the continent because they attack livestock (Fleming et al. 2001), and other placental mammals including rabbits (Oryctolagus cuniculus), foxes (Vulpes vulpes) and house mice (Mus musculus) have been introduced (Rolls 1969). These invasive species have wrought havoc on Australia's native biota and contributed to the mass extinction and endangerment of desert-dwelling mammals weighing 50–5500 g (McKenzie et al. 2006). The desert mammal fauna is now dominated by invasive species and relict assemblages of large (more than 15 kg) and small (less than 100 g) native species (Letnic & Dickman 2006). Macroecological studies of the distributions of extant indigenous rodents and marsupials suggest that their persistence is associated with the presence of dingoes (Smith & Quin 1996; Johnson et al. 2007).
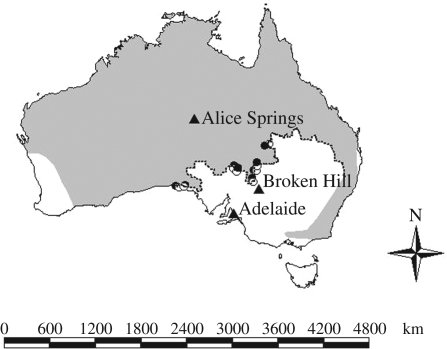
Although it is recognized that large carnivores can have strong effects on ecosystems, their role in regulating terrestrial trophic cascades remains contentious (Borer et al. 2005). Debate prevails in large part because of the paucity of empirical data; it is logistically very difficult to conduct experiments at spatial scales that are appropriate to observe the effects of large carnivores. As a consequence, most investigations of trophic cascades in terrestrial systems have studied arthropod predator–prey systems (Borer et al. 2005). However, it is likely that the trophic effects of arthropod predators differ from those of mammalian predators that have much greater metabolic demands (Borer et al. 2005). In arid Australia, the ‘dingo fence’ provides an unprecedented opportunity to conduct a large scale experiment (McKnight 1969). Extending over 5000 km (figure 1), this fence maintains dingoes on one side only and allowed us to examine whether dingoes act as trophic regulators.
Figure 1.
A map of Australia showing where dingoes are common (shaded), the dingo fence (dashed) and the location of the study sites in areas where dingoes were common (black circles) and areas where dingoes were rare (white circles). The triangles indicate the locations of selected towns.
Applying trophic cascade theory, we predicted that the effect of dingoes on other mammals should alternate with trophic group and scale with body size. Specifically, we expected kangaroos (Macropus spp.: 15–90 kg) and smaller invasive mesopredators, the red fox (3–7 kg) and feral cat (Felis catus: 2–5 kg), to increase in the absence of dingoes as they would experience less predation or harassment. Conversely, rabbits (1.5 kg), small mammals (Rodentia, Dasyuridae: less than 0.1 kg) and grasses (Poacae) were expected to benefit from the presence of dingo owing to reduced predation and competition from the mesopredators and kangaroos. We tested our predictions by comparing the activity or abundance of all groups at eight locations on either side of the dingo fence (figure 1) and pooled the results using meta-analysis to determine the effects of dingo absence on the response variables. To investigate predator and herbivore impacts, we also analysed predator diets and counted herbivore scats. We then evaluated the benefits for threatened mammal species that could accrue from maintaining or reintroducing dingo populations by using threatened species classifications published by the Australian Government and predictions made according to the MRH.
2. Material and methods
(a). Study sites
The dingo fence was constructed from 1900 to the 1960s to exclude dingoes from sheep rangelands (McKnight 1969). Dingoes are common on one side of the fence and rare on the other owing to intensive population control (Fleming et al. 2001). Although the specifications of the fence differ throughout its length, it is generally impenetrable to dingoes, foxes and kangaroos. Hybrids between dingoes and domestic dogs are rare in arid Australia (Elledge et al. 2006).
Each of our eight sites consisted of a pair of subsites situated on either side of the dingo fence in areas that on average receive less than 350 mm of annual rainfall (figure S1 and table S1 in the electronic supplementary material). Subsites were selected on the basis that vegetation types, landforms and average annual rainfall were similar (rainfall differences were less than 20 mm) and that primary land use was the same and subject to minimal predator control using the poison 1080. Study sites were located in sandy desert, stony desert and mallee (Eucalyptus spp.) woodland biomes. Each site was sampled once over a two-week period, with differences between subsites at each site assumed to be constant, regardless of season. Similarity of land-use intensity and rainfall history between paired subsites was tested by comparing differences in livestock activity, recent and long-term rainfall (table S1 in the electronic supplementary material). No systematic differences were found in livestock grazing activity (§3a and figure 2 in the electronic supplementary material), rainfall in the previous six months (paired t-test, t = 1.02, d.f. = 7, p = 0.340) or long-term annual rainfall (paired t-test, t = 0.44, d.f. = 7, p = 0.440). Sheep grazing was recorded on only one side of the dingo fence (dingoes removed) at three sites. Commercial kangaroo harvesting occurred at six of the sites but was only undertaken in the absence of dingoes.
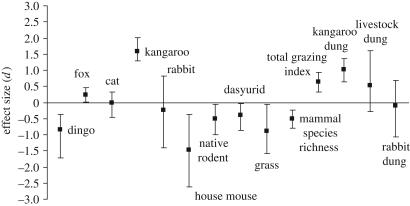
Figure 2.
The grand effect size (Hedge's d) of dingo removal ± 95 bias-corrected bootstrapped confidence intervals on each of the study variables. Negative values indicate variables that decreased in the absence of dingoes; positive values indicate variables that increased in the absence of dingoes. The mean effect size was considered statistically significant if the 95 per cent confidence intervals excluded zero.
At each site, we could not intersperse the dingo treatments (dingoes common, dingoes rare) on either side of the dingo fence (figure 1). The reason for this was that dingoes were rare ‘inside’ the dingo fence. Thus, our treatments were spatially segregated such that, in a strictly statistical sense, our experimental design does not allow inferences concerning the effect of dingoes (Hurlbert 1984). Because of this issue, we replicated the study at eight widely separated sites (figure 1) and pooled the results from these independent comparisons to determine whether the overall trends in the response variables on either side of the dingo fence were consistent (Oksanen 2001). We suggest that it is most unlikely that a result corroborating our a priori predictions regarding the effect of dingoes on the abundances of taxa at multiple trophic levels and in relation to body size could emerge as a consequence of any other source of variation.
(b). Abundance and species richness assessments
Assessments of dingo, fox and cat activity at each subsite were made using 25 track detection stations situated 1 km apart along vehicular tracks and counting footprints for three consecutive nights. The track stations were swept each morning. An index of abundance for each species at each subsite was expressed as the percentage of plots on which predator tracks were detected during the three-night track detection session.
Kangaroo and rabbit populations were assessed using three to four nocturnal spotlight transects (7–20 km long) at each subsite. Mammals were counted by an observer using a 50 W spotlight while sitting on the roof of a four-wheel-drive vehicle moving at 15 km h−1. Indices of kangaroo and rabbit abundance at each subsite were expressed as mean numbers of animals sighted per kilometre of spotlight survey.
At each subsite, small mammal abundance was assessed on seven to eight trapping grids comprising six pitfall traps (diameter = 150 mm, depth = 600 mm) equipped with a 10 m long drift fence (height = 30 mm) and 25 box traps baited with peanut butter, oats and treacle. To reduce disturbance from livestock, most grids were established at least 2.5 km from livestock watering points. Grids were at least 1 km apart and traps were checked for four consecutive mornings. Indices of rodent, dasyurid and house mouse abundance at each subsite were calculated as the mean number of animals captured per grid. Species richness at each subsite was calculated as the mean number of native small mammal species captured per grid.
The intensity of herbivory by kangaroos, rabbits, cattle, sheep and horses on each trapping grid was estimated by scoring the presence of fresh herbivore dung (dung with a black patina) on three 2 m × 100 m belt transects. A herbivory index was constructed for each taxon, with values ranging from 0 (no dung on any transects) to 3 (dung on all transects). An index of the total intensity of herbivory (TGP) on each grid was calculated as the sum of the herbivory indices for all species. A livestock grazing activity index (Livestock) was calculated as the sum of all cattle, sheep and horse data, excluding rabbits and kangaroos.
Surveys of grass cover were conducted using a step-point method (Landsberg et al. 2003). On each grid, three 80 m transects were sampled at 1 m intervals, giving 240 points per grid. At each point, ground cover was classified as: bare, live grass, dead grass, live forb, dead forb, live shrub or dead shrub. Grass cover was calculated as the percentage of points where living grasses were recorded.
Cumulative rainfall (mm) received at each subsite in the six months preceding trapping (Rain 6) was derived from a digital model of monthly rainfall for Australia (http://www.bom.gov.au/). The resolution of the rainfall grid was 25 km.
(c). Predator diet analyses
At each site, searches for predator scats were undertaken along roads and at watering points. On collection, scats were placed into paper bags and air-dried until they were sorted in the laboratory. In the laboratory, scats were oven dried overnight at 100oC, then placed individually in nylon bags and washed in a washing machine. Following washing, mammal remains in the scats were identified to the lowest possible taxonomic level using microscopic analysis of diagnostic residues (i.e. hair, teeth, claws) and compared against known reference specimens. The frequency of occurrence of mammals of three body size categories (small: less than 1000 g; medium: 1000–10 000 g; large: more than 10 000 g) in the diet of each predator species was calculated as the number of scats in which the dietary item was identified divided by the total number of scats sorted.
(d). Predicting the effect of dingoes on threatened mammal populations
We collated data on 19 nationally listed threatened mammal species that occur in areas receiving less than 350 mm rain per annum and excluded species that are present only on islands or in fox-free enclosures (table S2 in the electronic supplementary material). Data on threatening processes and the current distribution of each species were obtained from the Australian Government website (Department of the Environment, Water, Heritage and the Arts 2009). An expert panel of scientists, the Threatened Species Scientific Committee (TSSC), assesses the evidence for species being listed as critically endangered, endangered, vulnerable or conservation-dependent, makes recommendations for their population recovery and identifies the threatening processes thought to adversely affect each threatened species.
We used the threatening processes identified by the TSSC for each of the threatened mammal species to predict whether the presence of dingoes would be beneficial or detrimental for their populations (table S2 in the electronic supplementary material). Applying the MRH (Crooks & Soulé 1999), we predicted that if dingoes or wild dogs were identified as a threatening process the species would be affected detrimentally by dingoes. Conversely, if a species was identified as being threatened by foxes but not by dingoes, we predicted that the species may benefit from dingoes being present because dingoes are likely to suppress foxes and their impacts.
We restricted our predictions to areas receiving less than 350 mm annual rainfall because these drier areas are where dingoes are currently excluded and subdivided this area into 100 × 100 km grid cells. We mapped the current distributions of each of the threatened species by overlaying the grid on distribution maps published by the Australian government (Department of the Environment, Water, Heritage and the Arts 2009) and scoring the presence or absence of each taxon in each grid cell. Where available, we supplemented the distribution maps with our own field data on species distributions. We calculated the net benefit or detriment of dingoes being present in each grid cell by subtracting the number of species expected to be affected detrimentally by dingoes from the number of species expected to be affected positively. Negative scores indicate a detrimental effect of dingo presence and positive scores a beneficial effect. A score of zero indicates neutral benefit.
(e). Statistical analyses
Because sites differed with regard to biome and each was sampled at a different time, each site was treated as an independent comparison of the effect of dingo removal. Random effects meta-analysis with a hierarchical schema was used to test our a priori hypotheses that the effects of dingo removal on the abundance and species richness of taxa were consistent among sites and that the mean effect of dingo removal differed significantly from zero (Gurevitch & Hedges 1999). A random effects model was used because we expected the effect of dingoes to vary between sites owing to a range of possible reasons, including for example, differences in dingo abundance between sites and differences in rainfall and land-management both between and within sites. Hedge's d was used as the metric of effect size. Tests for homogeneity of effect sizes were conducted using the Q-statistic. The mean effect size was considered statistically significant if the bias-corrected bootstrapped 95 per cent confidence intervals calculated from 5000 simulations excluded zero (Gurevitch & Hedges 1999). Analyses were undertaken using MetaWin v. 2 (Rosenberg et al. 2000).
The strength of direct relationships between small mammal species richness and fox activity, cat activity, TGP, dingo activity, kangaroo grazing and rainfall in the previous six months on the study grids was investigated using linear mixed effects modelling. To account for biogeographical variation between sites, biome was included in all models as a random factor. Rainfall in the previous six months was included in all models because small mammal populations in arid Australia fluctuate markedly in response to prior rainfall (Letnic & Dickman 2006). We tested each predictor variable sequentially and used Akaike's Information Criterion to rank the models and compared the rankings against a null model comprising rainfall + biome. Prior to analyses, predictor variables were standardized and a 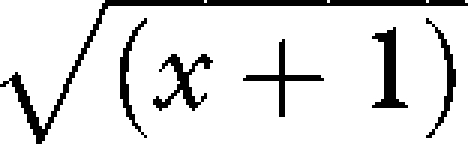 transformation was applied to correct skewed distributions. We used contingency tables to investigate differences in the frequency of mammal prey in different body size classes between predator species.
transformation was applied to correct skewed distributions. We used contingency tables to investigate differences in the frequency of mammal prey in different body size classes between predator species.
3. Results
(a). The effects of dingo exclusion
Dingoes were ubiquitous outside the dingo fence and were the most frequently recorded predator outside the fence at six of the eight subsites (table S1 in the electronic supplementary material). By contrast, the tracks of foxes or cats were recorded most frequently inside the fence, with dingo tracks seen at one site only. Fox scats were found at all sites on both sides of the dingo fence. Confirming the effectiveness of the fence, dingo activity was strongly depressed on the removal side (figure 2; Q = 12.38, d.f. = 7, p = 0.089). As predicted, dingo absence positively affected fox abundance (figure 2; Q = 5.01, d.f. = 5, p = 0.415) but had surprisingly little effect on cats (figure 2; Q = 5.11, d.f. = 5, p = 0.401).
As expected, dingo absence positively affected kangaroos (figure 2; Q = 3.17, d.f. = 7, p = 0.869), but unexpectedly had no effect on rabbits (figure 2; Q = 7.29, d.f. = 7, p = 0.399). Dingo absence had predictably negative effects on populations of small mammals, including native rodents (figure 2; Q = 7.55, d.f. = 7, p = 0.374), dasyurid marsupials (figure 2; Q = 7.24, d.f. = 7, p = 0.404) and the invasive house mouse (figure 2; Q = 6.80, d.f. = 6, p = 0.340). Dingo absence also had consistently negative effects on the species richness of native small mammals (figure 2; Q = 4.95, d.f. = 7, p = 0.666) and grass cover (figure 2; Q = 7.83, d.f. = 7, p = 0.347).
Total grazing activity (figure 2; Q = 5.515, d.f. = 7, p = 0.597) and grazing by kangaroos (figure 2; Q = 6.93, d.f. = 7, p = 0.436) were higher where dingoes were absent, corresponding with the trend in grass cover. Dingo removal had no effect on grazing activity by livestock (figure 2; Q = 7.950, d.f. = 6, p = 0.242) or rabbits (figure 2; Q = 5.236, d.f. = 5, p = 0.388).
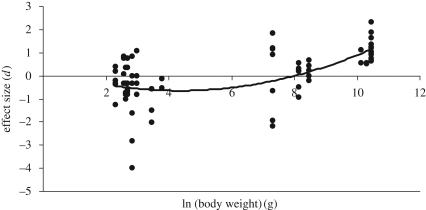
As predicted, the effect of dingo removal on mammal species scaled with body size (r2 = 0.338, F = 18.361, p < 0.001, d.f. = 2, 72; figure 3). Small species (less than 15 g) showed little response, those weighing 15–50 g tended to be less abundant and mammals more than 4000 g were much more abundant in the absence of dingoes.
Figure 3.
The effect (Hedge's d) of dingo removal on the abundances of 18 wild mammal species at eight locations plotted against the natural logarithm of their body sizes (g). Negative values indicate body sizes that decreased in the absence of dingoes; positive values indicate those that increased in the absence of dingoes.
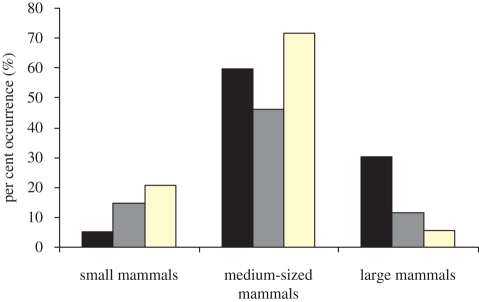
The pronounced differences in mammal assemblages on either side of the dingo fence corresponded with the diets of the predators (figure 4). Dingoes were more likely to consume large prey (more than 10 kg body weight) than foxes (dingo versus fox χ2 = 35.11, d.f. = 1, p < 0.001) or cats (dingo versus cat χ2 = 14.44, d.f. = 1, p < 0.001), while foxes (dingo versus fox χ2 = 19.12, d.f. = 1, p < 0.001) and cats (dingo versus cat χ2 = 17.48, d.f. = 1, p < 0.001) ate smaller mammals (less than 100 g) than dingoes. Dingoes were also more likely to consume more medium-sized (1–10 kg) prey than foxes (dingo versus fox χ2 = 13.29, d.f. = 1, p < 0.001), but not cats (dingo versus cat χ2 = 2.79, d.f. = 1, p = 0.095). Rabbits occurred in 45–71 per cent of the scats of all predators. Cats occurred in 0.7 per cent and 0.3 per cent of dingo and fox scats, respectively.
Figure 4.
The percentage occurrence of terrestrial mammals in the scats of dingoes (n = 451, black bar), foxes (n = 292, grey bar) and cats (n = 53, light yellow bar) in all the study areas combined.
Given these findings, we hypothesized that, without dingoes, predation by red foxes and grazing by herbivores would suppress small mammal species richness, and tested these expectations and alternative hypotheses using linear mixed effects models (table S3 in the electronic supplementary material). Three models were better predictors of small mammal species richness than the null model. The best predictive model for small mammal species richness showed a positive relationship with rainfall and a negative relationship with fox activity. The second ranked model showed positive associations between small mammal species richness, rainfall and dingo activity, while the third showed a positive association between small mammal species richness and rainfall and a negative relationship with livestock activity.
(b). Evaluating the biodiversity benefits of maintaining or reintroducing dingo populations
Taking the 19 arid-dwelling (less than 350 mm annual rainfall) native mammal species listed as threatened by the Australian Government (table S2 in the electronic supplementary material), we predicted how dingoes would affect their populations. We used the government's own assignation of threats from dingoes or wild dogs and foxes and the MRH as the rationale for our predictions (table S2 in the electronic supplementary material). If a species was identified as threatened by foxes but not by dingoes, we predicted that it would benefit from the fox-suppressive effects of dingoes. Foxes are ubiquitous in arid areas.
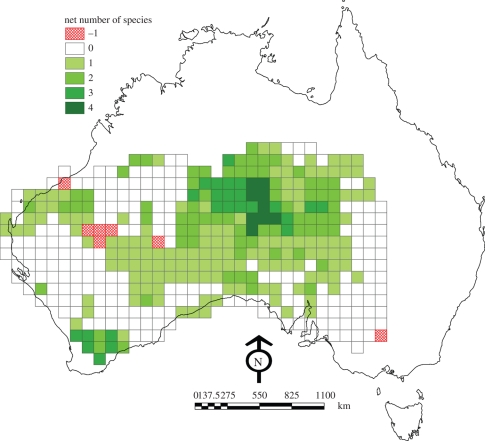
Our classification identified 16 threatened native mammal species that would probably benefit from dingoes being present and three that may be affected detrimentally. To explore the geographical extent of these effects, we then assessed how threatened species would be affected in the 293 map-grid cells (100 km × 100 km) where they now occur (figure 5). Results showed that net benefit should accrue to threatened mammals from the presence of dingoes in 82.6 per cent of cells, with net detriment occurring in 2.4 per cent of cells and no effect in 15 per cent (benefit versus neutral versus detriment, χ2 = 326.95, d.f. = 2, p < 0.001).
Figure 5.
The net number of threatened native mammal species predicted to be affected positively or negatively by the presence of dingoes in 100 × 100 km cells encompassing the area of Australia that receives less than 350 mm of annual rainfall.
4. Discussion
(a). The effects of dingo exclusion
Our field data demonstrated marked differences in the structure of ecosystems with and without dingoes that accord generally with trophic cascade theory and our a priori predictions (figure 2). Our results also provide evidence for links between the activity of dingoes, red foxes and the abundances and species richness of small mammals that accord with the MRH. These results are consistent with previous studies that have found inverse relationships between the presence of dingoes and the abundances of kangaroos (Caughley et al. 1980; Pople et al. 2000) and red foxes (Newsome et al. 2001; Letnic 2007; Johnson & Van Der Wal 2009) and positive relationships between dingoes and the persistence of small and medium-sized indigenous mammals (Smith & Quin 1996; Johnson et al. 2007; Letnic et al. in press). Viewed collectively, these studies constitute strong evidence that this recently introduced top predator regulates a trophic cascade in arid Australia and has positive benefits for the conservation of small native mammals. Our results also add to a growing body of evidence suggesting that mammalian carnivores can generate strong trophic cascades in terrestrial ecosystems (Hebblewhite et al. 2005; Beyer et al. 2007; Aunapuu et al. 2008; Fey et al. in press).
Like previous field studies on the effects of dingoes, our study relied on a pre-existing land-use framework, the dingo fence, for the ‘experimental’ treatment. Thus, potentially confounding factors, such as differences in grazing pressure, land use, geomorphology and climate on either side of the dingo fence may have contributed to the differences we observed (Fleming et al. 2001; Newsome et al. 2001). Could some source of variation on either side of the dingo fence, other than the presence/absence of dingoes, have caused the consistent effects that we observed with respect to both body size and trophic group? Although there were no systematic differences in recent or annual rainfall and livestock grazing activity between our paired sites, one potential weakness of this study involved differences in land use on either side of the dingo fence. The dingo fence was constructed with the aim of reducing dingo predation on sheep (McKnight 1969), and sheep grazing is limited largely to areas inside the dingo fence (Fleming et al. 2001). Similarly, commercial kangaroo harvesting is limited to areas inside the fence because kangaroos are rare outside, and the only inside sites where kangaroo harvesting did not occur were two sites in conservation reserves.
During the selection of our study sites, we attempted to avoid sampling in areas where sheep were grazed, but because sheep grazing is so widespread, we could not locate a sufficient number of sites where the vegetation type was similar on both sides of the dingo fence and where cattle grazing or no grazing was conducted on both sides. Thus, sheep were present inside the dingo fence at three of our eight subsites and cattle were grazed outside the fence at these site pairs. Sheep grazing is generally considered to be a more intensive land use than cattle grazing (Ludwig et al. 1997). Despite these differences, we consider it unlikely that sheep grazing or kangaroo harvesting could have produced the patterns in abundances that we observed on either side of the dingo fence. This is because the patterns in the abundances of kangaroos, red foxes, native rodents, M. musculus, dasyurids and grasses, and the species richness of native small species were consistent between sites despite there being differences in land uses. These land uses included cattle grazing, sheep grazing and conservation reserve. If anything, the strong association between sheep grazing, kangaroo harvesting and dingo exclusion suggests that the structuring effects of dingo predation extended to the human economy. The underlying reason for this is that dingo predation can make sheep grazing and kangaroo harvesting uneconomic (Fleming et al. 2001), and therefore stifles these activities. Consequently, we contend that the existence of sheep grazing and kangaroo harvesting are not confounding variables, but rather provide further evidence that dingoes do indeed regulate trophic cascades.
Because we conducted a snapshot study and did not experimentally manipulate dingo abundance, we can only speculate about the mechanisms that produced the patterns in abundance and species richness we observed. Considering trophic cascade theory, the differences in herbivore grazing activity and predator assemblages on either side of the dingo fence, and the diets of the three predator species, we hypothesize that differences in predation and grazing regimes on either side of the dingo fence were important factors driving the patterns in abundances we observed. Specifically, we hypothesize that dingoes exert top-down control on red fox and kangaroo populations, and that the following interactions ensue: (i) predation by dingoes reduces kangaroo abundance and prevents sheep grazing outside the dingo fence. In turn, grazing by abundant herbivores reduces grass cover and abundance in the absence of dingoes; (ii) dingoes reduce fox abundance through predation and competition. In turn, predation by foxes suppresses small mammal abundances and species richness in the absence of dingoes. The results of our linear mixed effects models provide support for the latter prediction, although it remains conceivable that both of these interaction pathways contributed to the decrease in small mammal abundances and species richness in the absence of dingoes. Controlled experiments are required to confirm or refute these patterns and to identify the ecological mechanisms that produce them, as it is possible that other interactions could produce similar effects.
Although the trends in the abundances of small mammals, kangaroos, foxes and grasses on either side of the dingo fence conformed with our a priori predictions, the abundances of cats and rabbits did not. A previous study (Newsome et al. 2001) and analyses of a subset of the data used in this paper (Letnic & Koch in press) found that rabbits in the sand dune habitats of the Strzelecki Desert were more abundant in the presence than in the absence of dingoes. However, when data from the sandy desert sites were pooled with the results from the other five sites examined in this study, rabbits did not, on average, respond to dingo removal. These observations suggest that the response of rabbits to dingo removal may be context dependent. Similarly, feral cats did not, on average, respond to dingo removal. Given that both dingoes and foxes are predators of rabbits and cats, it is plausible that interactions with both dingoes and foxes could have influenced their abundances (Newsome et al. 1989; Risbey et al. 2000). Another alternative is that rabbit and cat populations may be determined by other factors such as the availability of food resources and recent rainfall events more than by the presence of dingoes (Letnic et al. in press). Further investigation of the factors driving rabbit and cat populations in the presence and absence of dingoes is required.
(b). Dingoes and the conservation of mammals in arid Australia
Our simulation exercise predicted that maintaining or re-introducing dingoes should yield net conservation benefits for threatened mammals across 2.42 × 106 km2 of continental Australia, detrimental effects across 70 320 km2 and neutral effects across 2.08 × 106 km2 (figure 5). The major assumptions of our simulation exercise were that: (i) the threat classifications of the TSSC are accurate and (ii) dingoes mitigate the impacts of fox predation on threatened mammal species. Although these assumptions are not predicated on experimental data, we contend that, given the generally poor knowledge of the ecology of threatened mammal species in arid Australia, the predictions of the TSSC provide the best knowledge that is currently available. In addition, a growing body of field studies indicates that the phenomenon of mesopredator release is widespread among mammalian carnivore assemblages (e.g. Palomares et al. 1995; Crooks & Soulé 1999; Trewby et al. 2008) and appears to be particularly applicable to coexisting canids of differing body sizes such as dingoes and red foxes (Kamler et al. 2003; Berger & Gese 2007). Given increasing support for the MRH and data on dingo–fox interactions (Newsome et al. 2001; Letnic 2007; Johnson & Van Der Wal 2009; this study), we have no reason to believe that the outcomes of interactions between dingoes and foxes, and the prey of foxes, should not accord with the MRH.
Although dingoes probably contributed to the extinction of the thylacine and Tasmanian devil (Sarcophilus harrisii) soon after reaching mainland Australia (Johnson & Wroe 2003) and did not prevent the post-1788 mass extinction of mammals in Australia, the results of our field study, simulation exercise and previous desktop studies showing positive relationships between dingoes and the persistence of native mammals weighing less than 10 kg (Smith & Quin 1996; Johnson et al. 2007) suggest that dingoes are beneficial for the conservation of mammals in arid Australia. The most likely mechanism by which these benefits accrue is that dingoes suppress fox populations and thus indirectly reduce the impacts that foxes have on their prey (Johnson et al. 2007). A major implication of dingoes’ apparent fox-suppressive effect is that management programmes which reduce dingo populations may be detrimental to native mammal conservation by releasing fox populations from predation and competition with dingoes (Glen & Dickman 2005). Indeed, the positive link between the presence of dingoes and the persistence of native mammals (less than 10 kg) suggests that the ecological function of dingoes could be formally incorporated into biodiversity conservation programmes by maintaining or reintroducing populations of this canid predator throughout the low rainfall regions of the Australian continent.
(c). Conclusion
Our results provide, to our knowledge, the first field-based evidence that dingo removal has cascading effects through lower trophic levels and, in particular, that this leads to increased fox activity and widespread losses of native small mammals. They also provide evidence that an alien predator can assume a keystone role and, by its suppressive effects on the red fox, can facilitate the conservation of native mammal species at a subcontinental scale. Reintroducing dingoes into the current exclusion zones would no doubt cause decreases in the abundance of kangaroos and other large herbivores and would need the support of agricultural interests, but we believe the benefits for native species that are vulnerable to fox predation will be dramatic. Our study supports the notion that the dingo's functional role as a top predator is ecologically more significant than the classification of this species as an undesirable alien pest.
Acknowledgements
All animal census procedures were performed in accordance with Australian laws under University of Sydney Ethics Approval no. L04/8-2006/3/4447.
This research was funded by Australian Research Council grant DP0666574. Georgeanna Story analysed the predator scats. Peter Bird provided invaluable insights. Peter Banks and Tim Dempster provided comments on a draft manuscript.
References
- Aunapuu M., et al. 2008Spatial patterns and dynamic responses of Arctic food webs corroborate the exploitation ecosystems hypothesis (EEH). Am. Nat. 171, 249–262 (doi:10.1086/524951) [DOI] [PubMed] [Google Scholar]
- Berger K. M., Gese E. M.2007Does interference competition with wolves limit the distribution and abundance of coyotes? J. Anim. Ecol. 76, 1075–1085 (doi:10.1111/j.1365-2656.2007.01287.x) [DOI] [PubMed] [Google Scholar]
- Berger J., Stacey P. B., Bellis L., Johnson M. P.2001A mammalian predator–prey imbalance: grizzly bear and wolf extinction affect avian neotropical migrants. Ecol. Appl. 11, 947–960 [Google Scholar]
- Beyer H. L., Merrill E. H., Varley N., Boyce M. S.2007Willow on Yellowstone's northern range: evidence for a trophic cascade? Ecol. Appl. 17, 1563–1571 (doi:10.1890/06-1254.1) [DOI] [PubMed] [Google Scholar]
- Borer E. T., Seabloom E. W., Shurin J. B., Anderson K. E., Blanchette C. A., Broitman B., Cooper S. D., Halpern B. S.2005What determines the strength of a trophic cascade? Ecology 86, 528–537 (doi:10.1890/03-0816) [Google Scholar]
- Caughley G., Grigg G. C., Caughley J., Hill G. J. E.1980Does dingo predation control the densities of kangaroos and emus? Aust. Wildl. Res. 7, 1–12 (doi:10.1071/WR9800001) [Google Scholar]
- Crooks K. R., Soulé M. E.1999Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566 (doi:10.1038/23028) [Google Scholar]
- Department of the Environment, Water, Heritage and the Arts. EPBC Act list of threatened fauna. 2009 (http://www.environment.gov.au/biodiversity/threatened/species.html. ) [Google Scholar]
- Elledge A. E., Leung L. K. P., Allen L. R., Firestone K., Wilton A. N.2006Assessing the taxonomic status of dingoes Canis familiaris dingo for conservation. Mamm. Rev. 36, 142–156 (doi:10.1111/j.1365-2907.2006.00086.x) [Google Scholar]
- Fey K., Banks P. B., Oksanen L., Korpimäki E.In press Does removal of an alien predator from small islands in the Baltic Sea induce a trophic cascade?. Ecography (doi:10.1111/j.1600-0587.2008.05637.x) [Google Scholar]
- Fleming P., Corbett L., Harden R., Thompson P.2001Managing the impacts of dingoes and other wild dogs Canberra, Australia: Bureau of Resource Sciences [Google Scholar]
- Glen A. S., Dickman C. R.2005Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol. Rev. 80, 387–401 (doi:10.1017/S1464793105006718) [DOI] [PubMed] [Google Scholar]
- Gurevitch J., Hedges L. V.1999Statistical issues in ecological meta-analyses. Ecology 80, 1142–1149 [Google Scholar]
- Hairston N. G., Smith F. E., Slobodkin L. B.1960Community structure, population control, and competition. Am. Nat. 94, 421–425 (doi:10.1086/282146) [Google Scholar]
- Hebblewhite M., White C. A., Nietvelt C. G., McKenzie J. A., Hurd T. E., Fryxwell J. M., Bayley S. E., Paquet P. C.2005Human activity mediates a trophic cascade caused by wolves. Ecology 86, 2135–2144 (doi:10.1890/04-1269) [Google Scholar]
- Hurlbert S.1984Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 54, 187–211 (doi:10.2307/1942661) [Google Scholar]
- Johnson C. N., Wroe S.2003Causes of extinction of vertebrates during the Holocene of mainland Australia: arrival of the dingo, or human impact? Holocene 13, 941–948 (doi:10.1191/0959683603hl682fa) [Google Scholar]
- Johnson C. N., Van Der Wal J.2009Evidence that dingoes limit abundance of a mesopredator in eastern Australian forests. J. Appl. Ecol. 46, 641–646 (doi:10.1111/j.1365-2664.2009.01650.x) [Google Scholar]
- Johnson C. N., Isaac J. L., Fisher D. O.2007Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc. R. Soc. B 274, 341–346 (doi:10.1098/rspb.2006.3711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamler J. F., Ballard W. B., Gilliland R. L., Lemons P. R., II, Mote K.2003Impacts of coyotes on swift foxes in north-east Texas. J. Wildl. Manag. 67, 317–323 (doi:10.2307/3802773) [Google Scholar]
- Landsberg J., James C. D., Morton S. R., Muller W. J., Stol J.2003Abundance and composition of plant species along grazing gradients in Australian rangelands. J. Appl. Ecol. 40, 1008–1024 (doi:10.1111/j.1365-2664.2003.00862.x) [Google Scholar]
- Letnic M.2007The impacts of pastoralism on the fauna of arid Australia. In Animals of arid Australia: out on their own? (eds Dickman C. R., Lunney D., Burgin S.), pp. 65–75 Sydney, Australia: Royal Zoological Society of New South Wales [Google Scholar]
- Letnic M., Dickman C. R.2006Boom means bust: interactions between the El Nino/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. Biodivers. Conserv. 15, 3847–3880 (doi:10.1007/s10531-005-0601-2) [Google Scholar]
- Letnic M., Koch F.In press Are dingoes a trophic regulator in arid Australia? A comparison of mammal communities on either side of the dingo fence. Aust. Ecol. [Google Scholar]
- Letnic M., Crowther M. S., Koch F. Does a top predator provide an endangered rodent with refuge from predation by an invasive mesopredator? Anim. Conserv. In press. ( doi:10.1111/j.1469-1795.2009.00250.x) [Google Scholar]
- Ludwig J., Tongway D., Freudenberger D., Noble J., Hodgkinson K.1997Landscape ecology function and management: principles from Australia's rangelands Canberra, Australia: CSIRO [Google Scholar]
- McKenzie N. L., et al. 2006Analysis of factors implicated in the recent decline of Australia's mammal fauna. J. Biogeogr. 34, 597–611 (doi:10.1111/j.1365-2699.2006.01639.x) [Google Scholar]
- McKnight T. L.1969Barrier fencing for vermin control in Australia. Geogr. Rev. 59, 330–347 (doi:10.2307/213480) [Google Scholar]
- Newsome A. E., Parer I., Catling P. C.1989Prolonged prey suppression by carnivores: predator-removal experiments. Oecologia 78, 458–467 (doi:10.1007/BF00378734) [DOI] [PubMed] [Google Scholar]
- Newsome A. E., Catling P. C., Cooke B. D., Smyth R.2001Two ecological universes separated by the dingo fence in semi-arid Australia: interactions between landscapes, herbivory and carnivory, with and without dingoes. Rangeland J. 23, 71–98 (doi:10.1071/RJ01015) [Google Scholar]
- Oksanen L.2001Logic of experiments in ecology: is pseudoreplication a pseudoissue? Oikos 94, 27–38 (doi:10.1034/j.1600-0706.2001.11311.x) [Google Scholar]
- Palomeres F., Gaona P., Ferreras P., Delibes M.1995Positive effects on game species of top predators by controlling smaller predator populations: an example with lynx, mongooses, and rabbits. Conserv. Biol. 9, 295–305 (doi:10.1046/j.1523-1739.1995.9020295.x) [Google Scholar]
- Pople A. R., Grigg G. C., Cairns S. C., Beard L. A., Alexander P.2000Trends in numbers of kangaroos and emus on either side of the South Australian dingo fence: evidence for predator regulation. Wildl. Res. 27, 69–276 (doi:10.1071/WR99030) [Google Scholar]
- Ripple W. J., Beschta R. L.2007Restoring Yellowstone's aspen with wolves. Biol. Conserv. 138, 514–519 (doi:10.1016/j.biocon.2007.05.006) [Google Scholar]
- Risbey D. A., Calver M. C., Short J., Bradley J. S., Wright I. W.2000The impact of cats and foxes on the small vertebrate fauna of Heirisson Prong, Western Australia. II. A field experiment. Wildl. Res. 27, 223–235 (doi:10.1071/WR98092) [Google Scholar]
- Rolls E. C.1969They all ran wild: the story of pests on the land in Australia Sydney, Australia: Angus and Robertson [Google Scholar]
- Rosenberg M. S., Adams D. C., Gurevitch J.2000MetaWin: statistical software for meta-analysis, version 2 Sunderland, MA: Sinauer Associates [Google Scholar]
- Salo P., Korpimäki E., Banks P. B., Nordström M., Dickman C. R.2007Alien predators are more dangerous than native predators to prey populations. Proc. R. Soc. B 274, 1237–1243 (doi:10.1098/rspb.2006.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen P., Leitner T., Wilton A. N., Matisoo-Smith E., Lundeberg J.2004A detailed picture of the origin of the Australian dingo, obtained from the study of mitochondrial DNA. Proc. Natl Acad. Sci. USA 101, 12 387–12 390 (doi:10.1073/pnas.0401814101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. P., Quin D. G.1996Patterns and causes of extinction and decline in Australian conilurine rodents. Biol. Conserv. 77, 243–267 (doi:10.1016/0006-3207(96)00002-X) [Google Scholar]
- Soulé M. E., Estes J. A., Berger J., Martinez Del Rio C.2003Ecological effectiveness: conservation goals for interactive species. Conserv. Biol. 17, 1238–1250 (doi:10.1046/j.1523-1739.2003.01599.x) [Google Scholar]
- Soulé M. E., Estes J. A., Miller B., Honnold L.2005Strongly interactive species: conservation, policy, management and ethics. BioScience 55, 168–176 (doi:10.1641/0006-3568(2005)055[0168:SISCPM]2.0.CO;2) [Google Scholar]
- Terborgh J., et al. 2001Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 (doi:10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- Trewby I. D., et al. 2008Experimental evidence of competitive release in sympatric carnivores. Biol. Lett. 4, 170–172 (doi:10.1098/rsbl.2007.0516) [DOI] [PMC free article] [PubMed] [Google Scholar]