Abstract
Despite the increasing evidence of drastic and profound changes in many ecosystems, often referred to as regime shifts, we have little ability to understand the processes that provide insurance against such change (resilience). Modelling studies have suggested that increased variance may foreshadow a regime shift, but this requires long-term data and knowledge of the functional links between key processes. Field-based research and ground-truthing is an essential part of the heuristic that marries theoretical and empirical research, but experimental studies of resilience are lagging behind theory, management and policy requirements. Empirically, ecological resilience must be understood in terms of community dynamics and the potential for small shifts in environmental forcing to break the feedbacks that support resilience. Here, we integrate recent theory and empirical data to identify ways we might define and understand potential thresholds in the resilience of nature, and thus the potential for regime shifts, by focusing on the roles of strong and weak interactions, linkages in meta-communities, and positive feedbacks between these and environmental drivers. The challenge to theoretical and field ecologists is to make the shift from hindsight to a more predictive science that is able to assist in the implementation of ecosystem-based management.
Keywords: regime shift, resilience, intrinsic dynamics, coastal ecosystems
1. Introduction
Earth's ecosystems are being impacted by human activity on a global scale. These impacts are intense in the coastal zone, which represent some of our most valued ecosystems (Costanza et al. 1997). The current decline in these ecosystems occurs across a broad range of spatial and temporal scales, but rapid and broad-scale changes (regime shifts) are increasingly being described. Such changes can probably be predicted with some level of accuracy, when single environmental or biological drivers are sufficiently strong to force an ecological system into an alternative state. However, there is growing evidence that interactions between intrinsic ecological dynamics and chronic, cumulative or multiple stressor effects can also lead to the loss of resilience and an increased risk of regime shift. These ecosystem shifts are currently impossible to predict (de Young et al. 2008), but the implications are clear: homogenization of communities and ecosystems owing to reductions in foodweb complexity, diversity within functional groups, and biogenic habitat structure, as well as decreases in the size of organisms.
To date, investigations of regime shifts and implied loss of resilience have largely focused on interpreting historical trends (Mantua 2004; Hughes et al. 2005; Andersen et al. 2008; de Young et al. 2008). The development of a forecasting ability to identify increased risk of a regime shift is not only a scientific challenge but would also be a valuable tool for resource managers. Without this ability the best we can hope for is the management of ecosystem components to ensure their adaptive capacity. The costs of not anticipating or preventing unwanted regime shifts are high because of the ecological importance, multiple uses and economic values of intact ecosystems (Hughes et al. 2005). Coastal zones have been central to the development of human societies, and we have undoubtedly changed the nature of these ecosystems (Lotze et al. 2006; Airoldi & Beck 2007). Nevertheless rapid changes in these ecosystems are still occurring usually associated with human use, exploitation and the indirect impacts of climate change.
At present, a major barrier to forecasting shifts in resilience of these ecosystems is the disparity between theory and what is practically testable or measurable in the real world. Here we review recent studies to gain insight into the ecological mechanisms that underpin resilience, and suggest designs for field experiments that can help us understand and identify the feedback processes that hold the system in a particular state and thus characterize resilience.
2. Defining resilience and regime shifts
Regime shifts are drastic broad-scale changes in species composition and function (de Young et al. 2008). Regime shifts are often described by thresholds, step-trends, criticality, rapid transitions or tipping points. From an oceanographic perspective, a regime shift is the result of environmental forcing, but regime shifts can also occur because of changes in ecological relationships. Regime shifts reflect major changes in the functionality of ecological systems implying that detection of change is not just a matter of statistical significance. We are most likely to detect a regime shift because of the loss of specific species or groups that are functionally important and our ability to detect shifts will depend on sampling opportunities over different scales for different ecosystem components (de Young et al. 2008).
Resilience was first used in an ecological context by Holling (1973) and its definition has been extensively discussed (Gunderson 2000). Although it is often implicit that we are referring to broad-scale changes, resilience can apply at the local patch scale as well as the regional scale (Dayton et al. 1984), with patchiness and changes in spatial structure able to be generated by self-organization (Pascual & Guichard 2005). Essentially there are two fundamentally different definitions of resilience: (i) the potential for recovery from disturbance (Pimm 1991), sometimes called engineering resilience, and (ii) a variable that represents the movement of an ecosystem within and between stability domains (corresponding to the size of the basin of attraction in ecosystems with alternative stable states), also called ecological resilience (Ludwig et al. 1997; Gunderson 2000). Engineering resilience may lead to an empirical measurement of resilience, but is not directly related to the theory of regime shifts, whereas ecological resilience can be measured directly only when long time series of the variables describing and governing the system are available. Additionally, a strict mathematical evaluation of the size of the basins of attraction might be difficult for natural systems where observational and process noise are present (Sugihara 1994). Certainly, assessing resilience and stability of systems that can shift in state, function and values will require more than a single metric (Peterson et al. 1998).
Differences in definitions can create confusion over the implications of maintaining resilience. Degraded systems often show hysteretic responses in terms of recovery to more valued states (Scheffer et al. 2001). For example, macrobenthic communities in degraded ecosystems typically have low functionality and are dominated by small rapidly growing and highly mobile species, which recover quickly following local disturbance. Such degraded benthic communities thus have high engineering resilience (Thrush & Whitlatch 2001; Troell et al. 2005), though they lack the capacity of a system to adapt or transform, which is perhaps the most tractable definition of resilience from a resource management perspective (Gunderson 2000).
We can also consider ecological resilience as the ability of a system to maintain its identity in the face of both internal and external drivers (Cumming et al. 2005). This represents an insurance against potentially adverse changes in the delivery of ecosystem goods and services. Thus resilience is not only a property of an ecological system but is an important ecological service, offering insurance against loss of valued functions. Broadening the concept of resilience into social-ecological systems emphasizes the importance of defining the ecological and social context (Carpenter et al. 2001; Folke et al. 2004; Carpenter et al. 2005). Unfortunately, perspectives on values, states and trends are easily biased by shifting baselines that plague ecological comparisons when information on ecosystem history is limited (Dayton 1989; Dayton et al. 1998; Duarte et al. 2009).
The ecological background and the number of definitions highlight the importance of understanding ecological and environmental context and defining scales of space, time and biological organization when assessing resilience.
3. Indicators of regime shifts and changes to resilience in ecological time series
Increased temporal variability and various aspects of variability in the ecosystem have been investigated as potential early-warning signals of an approaching regime shift. Carpenter & Brock (2006) explored changes in the standard deviation of key biological- and linked-physical variables derived from a 300-year model simulation of a lake ecosystem subjected to variations in nutrient loading and fishing pressure. This analysis indicated that increasing standard deviation could be detected about a decade ahead of the regime shift. Rising variability has also been demonstrated in a time series derived from a social-ecological system model of temporal changes in pollutants and ecosystem services prior to the regime shift (Brock & Carpenter 2006). Another model-based analysis has indicated that changes in skewness in the distribution of time-series data is a model-independent, early-warning signal of either reduced resilience or increased external fluctuations that can tip ecosystems to alternative states (Guttal & Jayaprakash 2008). However, it is unlikely that such long and perfectly sampled time series can be generated in nature. Even when long-term observational data is available, the timing of changes in different-ecosystem response variables may be lagged, confusing early-warning signals. Long-term studies of the benthic communities off the NE coast of England have shown increased interannual variability and decreased multi-variate stability (Warwick et al. 2002), but only after abrupt changes in the water column associated with a regime shift (Reid et al. 2001).
The availability of data and analytical and interpretational problems often limit the identification of regime shifts (Collie et al. 2004), and thus the development of methods assessing resilience. However, a wide array of statistical techniques has been applied to identify thresholds and step changes in ecological time series (Rodionov 2005; Weijerman et al. 2005; Andersen et al. 2008; Zaldivar et al. 2008). Empirical testing, with real and thus short and noisy ecological time series, is needed to ground-truth forecasts of increased risk of a regime shift. Zaldivar et al. (2008) demonstrated the usefulness of recurrence-quantification analysis and recurrence plots for exploring whether regime shifts occur with shorter time series that include noise, but they did not propose a technique for analysing the likelihood of a regime-shift occurring. Trends of decreasing abundance in a key species or rate of a key function to levels below that found during natural cycles in abundance (as determined by CUSUM, control charts, and critical F-tests, (e.g. Anderson & Thompson 2004; Andersen et al. 2008) may indicate risk of a regime shift. A logical extension of these methods is that increasing deviance from natural levels indicates risk of regime shift, with the risk increasing with increased deviance from previously defined natural variability. Determining the ‘natural’ variability of a system, relative to variability in environmental forcing, is a major challenge, and the speed with which we can make these determinations is an essential factor influencing the ability of managers to act (Biggs et al. 2009; Contamin & Ellison 2009).
Most studies proposing indicators of regime shifts also emphasize the need for knowledge of ecological mechanisms and feedbacks (Brock & Carpenter 2006; Carpenter & Brock 2006). Thus regime shifts have been identified as a result of changes in productivity (Ware & Thomson 2005), shifts in the timing of events leading to decoupling of processes (Edwards & Richardson 2004), changes in recruitment and juvenile mortality (Casini et al. 2009) as well as prior shifts in key environmental factors (Weijerman et al. 2005). However, such profound knowledge of the important ecological mechanisms that underpin these patterns is often lacking, emphasizing that assessing risk of a regime shift will depend on the relative importance of the variables for which data are available.
4. Identifying thresholds and designing studies to improve our understanding of resilience
Measuring resilience requires determining whether there are thresholds that separate different stability domains, and at present the only sure way to detect a threshold in a natural system is to cross it (Carpenter 2003). However, we should be able to identify signs of shifts in ecosystems that forecast the risk of abrupt future change. This requires deriving tests from theoretical studies and identifying empirical information on the key processes and rates that control how communities and ecosystems respond to changes in environmental forcing. In particular, we need to determine how spatial structure in communities and ecosystems interacts with disturbance regimes and recovery dynamics (Pascual & Guichard 2005). We also need to be able to describe when positive feedbacks between fast and slow processes are most likely to decouple, because these links are key in defining dynamics (van Nes et al. 2007; Rietkerk & Van de Koppel 2008). Such profound knowledge of the important ecological mechanisms that underpin these patterns is often lacking, because they emerge from the interaction of processes operating on different space and time scales. The key issues that still need to be addressed are defining exactly how close a system is to a threshold and what can we actually measure in natural ecosystems to better understand resilience and forewarn us of drastic change.
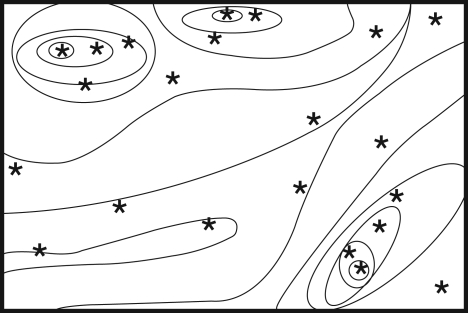
Empirically testable hypotheses that could contribute to our understanding of the mechanisms that underpin resilience must focus on the interactions of species and processes that bind and define the system, together with how these factors respond to stress within their environmental and ecological context (table 1). Given that resilience can be affected by chronic and cumulative effects, experiments within the landscape should be designed to capture variations in responses along stress gradients or encompass variations in connectivity (meta-community dynamics). A powerful way to understand such context dependency in experimental results is to array sites along environmental gradients (figure 1) (Hewitt et al. 2007). While resilience and regime shifts are temporal concepts, thresholds can occur spatially (e.g. patch boundaries) and understanding these spatial thresholds could provide useful insight into processes and interactions around thresholds.
Table 1.
Community dynamics, feedbacks and thresholds in resilience of coastal marine ecosystems.
| key processes | mechanisms | how increased |
|---|---|---|
| potentially containing thresholds | maintaining resilience | stress or disturbance can influence transitions |
| functional loss of key species | key species form habitats, and drive fluxes of energy and matter, patterns of species interaction | density, size or spatial arrangement of key species drop below threshold for functional performance. |
| loss of diversity within functional groups | diversity within functional groups maintains stable function in the face of change | stress or disturbance affects all species within functional group; other aspects of the natural history of individual species limit the potential for replacement |
| recovery to ambient conditions slow and variation in recovery of disturbed areas increases | intrinsic interactions between species and local habitat during recovery processes facilitate recovery dynamics. Neighbouring habitats supply colonists with diverse functional traits | variability in community structure increases moving away from a basin of attraction |
| decrease in β-diversity and meta-community connectivity | low β-diversity and high connectivity in a landscape ensure continuous supply of species to recover disturbed patches | late successional stage species are limited in distribution across the landscape |
Figure 1.
Experimental design with sites randomly allocated to strata across a ‘landscape’. This landscape could represent a stress or disturbance gradient or spatial structure in the density or size of key species or diversity within a functional group. This design and, more generally, the construction of gradients facilitate the use of co-variables to tease apart the effects of different factors on experimental processes and multi-scale analysis (Thrush et al. 1997; Thrush et al. 2000; Hewitt et al. 2007).
Cumming et al. (2005) developed a framework for the empirical measurement of resilience. Their approach involved the identification of a key set of variables that characterize changes in the system in terms of structure, functional networks and variation in space and time. This framework emphasizes the need for good information on natural history and ecological and environmental context within which experiments assessing resilience can be nested.
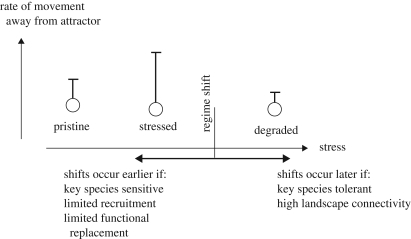
The ecological context in which the experiment will be conducted is particularly important (figure 2). For example, when simple systems are being studied we need to consider whether they reflect systems where physiological stressors restrict the range of available species (e.g. saltmarsh, high shore or desert habitats) or represent a disturbance end-member community dominated by fast-growing opportunistic species. This is consistent with resilience theory, in that the aspects of a system that confer resilience depend on context, including spatial configuration and change over time (Holling 2001). From a dynamics perspective we may have little idea a priori where the system subjected to experimental study sits relative to a threshold or within a basin of attraction, although ecological understanding should provide some insight.
Figure 2.
Movement of pristine communities into a stressed state and the resilience of degraded communities. The effect of key species or members of a functional group on the positioning of a regime shift along a stress or disturbance gradient is dependent on whether the species are sensitive or resistant. Furthermore, whether a regime shift or a gradual change occurs depends on how positive feedbacks are affected by stress or disturbance and the connectivity among patches.
Resilience of degraded communities often makes it difficult for the system to return to its previous, non-degraded state limiting the potential for restoration (Scheffer et al. 2001). Four explicit tests for the presence of a hysteresis effect have been proposed by Schroder et al. (2005): (i) discontinuity in the response to an environmental driver; (ii) lack of recovery potential after a perturbation; (iii) divergence owing to different initial conditions; and (iv) random divergence. They analysed 35 experimental studies using the minimum turnover of individuals in terms of lifespan to assess the stability of different states and found that although there was evidence of hysteresis effects in some systems, there was a range of potential system dynamics that resulted in context-dependent results.
(a). Ecological mechanisms that may underpin resilience
In most natural ecosystems a variety of processes interact and diverse communities consisting of multiple species and guilds with a wide range of biological traits are represented. This diversity is, at present, poorly accommodated in theoretical models, but integration requires the relative importance of processes, and feedbacks between them, to be empirically tested. Here we review recent applications to gain insight into the ecological mechanisms that may underpin resilience and are testable with well-designed field experiments. While it may be possible to directly measure engineering resilience (as the inverse of recovery rate), direct measures of ecological resilience may not be possible. Nevertheless, experiments can provide valuable insight into the processes, feedbacks and thresholds that underpin system dynamics and thus ecological resilience.
(b). Functional loss of key species
Key species that dominate local function are relatively easily identified by manipulative experiments (Paine 1980). Although the role of species may change with scale, the rate of processes in which a species plays a functional role are usually defined by a combination of size, density and spatial arrangement (Thrush & Dayton 2002). Thus key species do not have to go locally extinct to affect ecosystem performance.
Intertidal rocky-shore experiments provide many examples of successional processes ranging from inhibition to facilitation involving limpets, turf algae, barnacles and mussels, with many nuances influenced by environmental variables such as relative desiccation, shade, and sediment trapping. Dominant species are not necessarily resistant to stress, particularly stressors outside their evolutionary history. For example, an investigation of engineering resilience in fucoid-dominated rocky-shore communities highlighted that while the key structurally dominant species increased diversity, these high diversity treatments responded poorly to heat stress (Allison 2004). These effects highlight the importance of individual species in driving ecosystem responses and the fact that it is easier to detect diversity shifts in more diverse assemblages as they have more species to lose. Other experiments on the removal of dominant fucoids have failed to identify functional replacement, indicating little buffering should these species be lost or severely reduced in abundance (Schiel 2006).
In soft-sediment habitats, major shifts in ecosystem performance are often associated with changes in species that influence sediment stability or nutrient processing (Lohrer et al. 2004; Norkko et al. 2006a; van Nes et al. 2007). Coastal and estuarine habitats have long been subjected to the direct exploitation of resources and impacts associated with the development of adjacent catchments (Airoldi & Beck 2007). Historical reconstructions have highlighted that the loss of suspension-feeding bivalves from these systems have profoundly influenced trophic relationships and ecosystem function (Nichols et al. 1986; Lotze et al. 2006; Airoldi et al. 2008). While most studies of regime-shift focus on the loss of species and ecosystem services, shifts can also increase ecological values. Petersen et al. (2008) argue that hydrodynamic changes as a result of coastal engineering activity slightly increased the salinity of a Danish fjord, enabling colonization by suspension-feeding bivalves with concomitant changes in trophic relations and water clarity. More generally, however, eutrophication-induced hypoxia and anoxia reduce the role of deep-burrowing, and bioturbating taxa that are functionally important in organic matter recycling. Conely et al. (2007) have argued that loss of benthic fauna and altered energy pathways is the reason why major reductions of nutrients to coastal waters have not resulted in improvements in eutrophication status.
These examples illustrate that species that play major roles in influencing habitat, recovery rates or energy transfer can affect resilience (see also Ludwig et al. 1997). They also highlight the potential for a mismatch in temporal scales between field experiments and theoretical predictions, owing to contingent effects associated with the characteristics of the local species pool or the life-history characteristics of dominant species. Once the relationships between specific key species and ecosystem functions are defined, then experiments on the response of the key species to stressors should provide meaningful insight into the risk of a regime shift (figure 1). Albeit, identifying and predicting the responses of species to stressors may be complicated by variations in the sensitivity of a species to a stressor across landscapes (Fleeger et al. 2003; Johnston & Keough 2005; Thrush et al. 2008c).
(c). Diversity within functional groups and weak interactions
If a particular ecosystem function or community is not strongly influenced by a key species but rather by a diverse range of species within a functional group, then other approaches will be needed to define thresholds. Weak interactions by definition will be hard to determine from small, short experimental studies without high levels of statistical power. The key here will be in detecting shifts in the diversity within functional groups requiring long duration observations or experiments (Thrush et al. 2008a).
Whether systems dominated by strong or weak interactions are less ecologically resilient is still contentious. Weak interactions could mean that there is less likely to be a regime shift because the positive feedbacks with key dominants are not important and species substitutions can maintain specific functions. Here, hypotheses should focus on the influence of diversity within functional groups within the context of key functions for specific habitats. Weak interactions and rare species can influence resilience as individual species respond differently to changes in species composition or environmental factors (Walker et al. 1999; Jonsson & Malmqvist 2000). This emphasizes that resilience in some communities will be maintained by diversity within functional groups to ensure that the group encompasses a range of environmental response capabilities. Thus experiments conducted across locations with different within-functional group diversities should reveal where faster responses to changes in resource or environmental changes are occurring. The significance of particular groups of traits will depend on the exact nature of the stress to which the system is subjected and how individual species, often at low density, respond to that stress.
5. Adapting disturbance-recovery experiments to measure resilience
Recovery is the culmination of interactions between species (dominance, inhibition or facilitation) and extrinsic factors (e.g. colonist supply and environmental setting) making it a direct measure of engineering resilience and providing useful information to define ecological resilience (Dayton et al. 1992). In some circumstances, species colonizing after disturbance can resist the establishment of other species and limit recovery for decades (Dayton et al. 1984). Recovery from disturbance is also often scale-dependent, with the area disturbed not only influencing the rate and mode of colonist supply, but also the role of bio-physical and species interactions in the recovery of the disturbed patch (Thrush et al. 1996; Norkko et al. 2006b). Moreover, despite recovery being a key ecological process defining how ecological systems are likely to respond to changes in the disturbance regime, context-dependent results are common. Thus, if we are to use such experiments, we must develop a framework that focuses on explaining how processes operating on different scales influence recovery dynamics rather than merely documenting differences in recovery. To achieve this, context dependency must be woven into the experimental design.
Experiments arrayed across stress gradients are useful. This approach has recently been employed in assaying the resilience of a Spartina alterniflora salt marsh. Slocum & Mendelssohn (2008) postulated that more stressed locations would recover more slowly and the incidence of failure to recover should increase. The authors concluded that their small-scale disturbance experiments accurately assessed variations in stress responses along the stress gradient and highlighted some interesting indirect effects in stressed areas owing to variations in the Spartina patch morphology that limited vegetative expansion into denuded areas, possibly because the system had not yet crossed over into the more resilient but degraded state.
For a species-rich and functionally diverse system subject to a perturbation or stress we would predict that the recovery response from small-scale disturbance should lead to divergence in community composition (multi-variate variability, community dissimilarity). This divergence should increase as the pull of the key processes that define the attractor weaken. These effects should also strengthen as the spatial grain of experimental disturbance increases. There is some support for this hypothesis from studies of variability in benthic communities along stress gradients (Warwick & Clarke 1993). Unfortunately, detecting the edge of the basin of attraction for the noisy time series that characterize natural ecosystems can only be done by tracking the increased variability in community composition, emphasizing the usefulness of studying spatial gradients or employing small-scale disturbance experiments as assays. Interpretation of spatial or temporal trends must be done cognizant that changes to variance in the abundance of a species or a community parameter in response to stress are likely to be dependant on the spatial or temporal structure, e.g. changes in aggregation of populations in relation to mean abundance (Taylor 1961; Rosewell et al. 1990). Depending on the scale of experiments, interactions between biogenic heterogeneity and disturbance are likely to be highly nonlinear. Disturbance may well increase community heterogeneity up to a point, with heterogeneity later crashing when the habitat-forming or functionally important species are lost from the system.
van Nes & Scheffer (2007) provided a theoretical context to empirical testing by demonstrating with complex system models that the rate of recovery from small perturbations (engineering resilience) is a remarkably good indicator of ecological resilience. Their models predict that recovery rates should slow close to a threshold; resulting in increased spatial variance across the landscape (they do note, however, that this behaviour is not restricted to a regime shift). The potential utility of this approach has been assessed in tracking large-scale regime shifts. Litzow et al. (2008) were able to identify increased spatial variance one year ahead of a climate associated regime shift in the Gulf of Alaska, and three years ahead of the overfishing related regime shift on the Scotian shelf. Less specific evidence of alternate states and non-equilibrium communities can also be found in the divergence of communities in multi-variate space (Vandermeer et al. 2004). However, recent experimental studies indicate that the nature and duration of disturbance events relative to time scales of species response can influence community trajectories and the potential for multiple community states emerge (Houseman et al. 2008).
6. The role of connectivity among communities
Metacommunity structure and connectivity are likely to influence resilience across landscapes (Holyoak et al. 2005). Localized disturbance within the landscape limits the availability of potential colonists to other disturbed patches, this can slow recovery rates and in particular reduce the recovery of slow-growing and poorly dispersing species (Pascual & Guichard 2005; Crooks & Sanjayan 2006). This contributes to the loss of biogenic habitats and fragmentation across landscapes with the concomitant loss of diversity (Thrush et al. 2006). Thrush et al. (2008b) conducted a disturbance-recovery experiment across spatial gradients of community type and environmental conditions and demonstrated that community recovery rates were controlled by a combination of physical features and the geographical extent of specific ecological communities and their connectivity.
Beta-diversity can be viewed as either a measure of turnover in species richness (in space or time) or a measure of the isolation of an individual location's species richness from the regional species pool. In the latter metacommunity context, β-diversity can be considered an inverse measure of ecological connectivity. From this perspective, as a region approaches a threshold shifting the system into a species-poor state, we would predict a slight loss of species from the regional species pool (γ-diversity) and increasing variability in α-diversity across sites within the region, owing to cumulative impacts and increased local loss of habitat forming species as the patches of communities dominated by slow growing and poor dispersing species fragment across the landscape. Despite the slight decrease in γ-diversity, the changes in α-diversity should increase the average β-diversity and reduce the ecological connectivity between locations.
7. The take-home message
Predicting the limit to resilience and the concomitant loss of ecosystem goods and services is a profound challenge to both empirical and theoretical ecology. Empirical ecologists must meet this challenge with well-designed experiments and monitoring studies that encompass the interaction of processes operating over different scales of space and time. Empiricists and theoreticians together need to develop the ability to predict when cumulative effects pass ecological thresholds beyond which recovery is limited and ecosystem services are degraded. While no simple methods for achieving this are currently available, past empirical research points to some probable directions: experimental tests of hypotheses about potential factors generating thresholds in community dynamics (table 1); adaptation of disturbance-recovery experiments along gradients and across scales; and recognition of the potential for different results among systems driven by key species or weak interactions and degraded versus diverse systems. Importantly, empirical studies will not be sufficient alone, as measured resilience will be relative and context-dependent. Instead we need the development, testing and verification of models that incorporate empirical measurements and identify the positive feedbacks that will drive systems to rapid change.
Despite our present inability to measure ecological resilience, there are still important implications for ecological management with escalating degradative ecological change occurring as alterations in the disturbance regime feedback onto local and regional changes in ecological communities (Folke et al. 2004). The over-arching science question we need to address is how much exploitation, disturbance or stress can a particular ecosystem withstand without the loss of resilience and a range of other ecosystem services and values. In most cases we cannot yet answer these questions with certainty, but frameworks are beginning to be developed to inform managers whether specific ecosystems are likely to exhibit alternative stable states and regime shifts (Suding & Hobbs 2009). There will need to be some adaptation of these frameworks for application to different ecosystems owing to sampling opportunities, differences in spatial structures, connectivity, and functional interactions. Until our ability to predict shifts in resilience improves, management and policy should focus on insurance and capacity maintenance, e.g. managing habitats in light of differences in connectivity and disturbance regimes, creating reserves, and ensuring the viability of key species that drive ecosystem function and enhance biodiversity. Managing to ensure resilience can be used to draw attention to ecological dynamics across scales as well as quantitatively linking the dynamics of ecosystem state and function to societal functions of uses and values. The ensuing dialogue should have benefits for both the development of ecosystem-based management and underpinning research. In particular, as resilience implies that ecological systems do not simply track environmental forcing, the adoption of this term into management will de-emphasize treating environmental stress as a simple dose-dependent problem. Instead it promotes a focus on the need: to recognize ecosystem response and the potential for feedbacks to be broken; and to provide ecological buffers to change.
Acknowledgements
This work was supported by FRST C01X0501 and a Marie Curie International Incoming Fellowship to SFT. Grants from the Academy of Finland (project numbers 110999 and 114076) were given to AN and JN. This paper is dedicated to Sam, resilient to the end.
References
- Airoldi L., Beck M. W.2007Loss, Status and trends for coastal marine habitats of Europe. Oceanogr. Mar. Biol. 45, 345–405 [Google Scholar]
- Airoldi L., Balata D., Beck M. W.2008The gray zone: relationships between habitat loss and marine biodiversity and their applications in conservation. J. Exp. Mar. Biol. Ecol. 366, 8–15 (doi:10.1016/j.jembe.2008.07.034) [Google Scholar]
- Allison G.2004The influence of species diversity and stress intensity on community resistance and resilience. Ecol. Monogr. 74, 117–134 (doi:10.1890/02-0681) [Google Scholar]
- Andersen T., Carstensen J., Hernandez-Garcia E., Duarte C. M.2008Ecological thresholds and regime shifts: approaches and identification. Trends Ecol. Evol. 24, 49–57 (doi:10.1016/j.tree.2008.07.014) [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Thompson A. A.2004Multivariate control charts for ecological and environmental monitoring. Ecol. Appl. 14, 1921–1935 (doi:10.1890/03-5379) [Google Scholar]
- Biggs R., Carpenter S. R., Brock W. A.2009Turning back from the brink: detecting an impending regime shift in time to advert it. Proc. Natl Acad. Sci. USA 106, 826–831 (doi:10.1073/pnas.0811729106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock W. A., Carpenter S. R.2006Variance as a leading indicator of regime shift in ecosystem services. Ecol. Soc. 11, 9 (http://www.ecologyandsociety.org/vol11/iss2/art9/) [Google Scholar]
- Carpenter S. R.2003Regime shifts in lake ecosystems: pattern and variation Excellence in Ecology Series, vol. 15 Oldendorf/Luhe, Germany: Ecology Institute [Google Scholar]
- Carpenter S. R., Brock W. A.2006Rising variance: a leading indicator of ecological transition. Ecol. Lett. 9, 311–318 (doi:10.1111/j.1461-0248.2005.00877.x) [DOI] [PubMed] [Google Scholar]
- Carpenter S., Walker B., Anderies J. M., Abel N.2001From metaphor to measurement: resilience of what to what? Ecosystems 4, 765–781 (doi:10.1007/s10021-001-0045-9) [Google Scholar]
- Carpenter S. R., Westley F., Turner M. G.2005Surrogates for resilience of social–ecological systems. Ecosystems 8, 941–948 (doi:10.1007/s10021-005-0170-y) [Google Scholar]
- Casini M., Hjelm J., Molinero J.-C., Lovgren J., Cardinale M., Bartolino V., Belgrano A., Kornilovs G.2009Trophic cascades promote threshold-like shifts in pelagic marine ecosystems. Proc. Natl Acad. Sci. USA 106, 197–202 (doi:10.1073/pnas.0806649105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collie J. S., Richardson K., Steele J. H.2004Regime shifts: can ecological theory illuminate the mechanisms? Progr. Oceanogr. 60, 281–302 (doi:10.1016/j.pocean.2004.02.013) [Google Scholar]
- Conely D. J., Carstensen J., Aertebjerg G., Christensen P. B., Dalsgaard T., Hansen J. L. S., Josefson A. B.2007Long-term changes and impacts of hypoxia in Danish coastal waters. Ecol. Appl. 17, S165–S184 (doi:10.1890/05-0766.1) [Google Scholar]
- Contamin R., Ellison A. M.2009Indicators of regime shifts in ecological systems: what do we need to know and when to we need to know it? Ecol. Appl. 19, 799–816 (doi:10.1890/08-0109.1) [DOI] [PubMed] [Google Scholar]
- Costanza R., et al. 1997The value of the world's ecosystem services and natural capital. Nature 387, 253–260 (doi:10.1038/387253a0) [Google Scholar]
- Crooks K. R., Sanjayan M.2006Connectivity conservation Cambridge, UK: Cambridge University Press [Google Scholar]
- Cumming G. S., et al. 2005An exploratory framework for the empirical measurement of resilience. Ecosystems 8, 975–987 (doi:10.1007/s10021-005-0129-z) [Google Scholar]
- Dayton P. K.1989Interdecadal variation in an Antarctic sponge and its predators from oceanographic climate shifts. Science 243, 151–160 [DOI] [PubMed] [Google Scholar]
- Dayton P. K., Currie V., Gerrodette T., Keller B. D., Rosenthal R., Ven Tresca D.1984Patch dynamics and stability of some Californian kelp communities. Ecol. Monogr. 54, 253–289 (doi:10.2307/1942498) [Google Scholar]
- Dayton P. K., Tegner M. J., Parnell P. E., Edwards P. B.1992Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecol. Monogr. 62, 421–445 (doi:10.2307/2937118) [Google Scholar]
- Dayton P. K., Tegner M. J., Edwards P. B., Riser K. L.1998Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecol. Appl. 8, 309–322 (doi:10.1890/1051-0761(1998)008[0309:SBGARE]2.0.CO;2) [Google Scholar]
- de Young B., Barange M., Beaugrand G., Harris R., Perry R. I., Scheffer M., Werner F.2008Regime shifts in marine ecosystems: detection, prediction and management. Trends Ecol. Evol. 23, 403–409 [DOI] [PubMed] [Google Scholar]
- Duarte C. M., Conley D., Carstensen J., Sanchez-Comacho M.2009Return to neverland: shifting baselines affect eutrophication restoration targets. Estuaries and Coasts 32, 29–36 (doi:10.1007/s12237-008-9111-2) [Google Scholar]
- Edwards M., Richardson A. J.2004The impact of climate change on the phenology of the plankton community and trophic mismatch. Nature 430, 881–884 (doi:10.1038/nature02808) [DOI] [PubMed] [Google Scholar]
- Fleeger J. W., Carman K. R., Nisbet R. M.2003Indirect effects of contaminants in aquatic ecosystems. Sci. Total Environ. 317, 207–233 (doi:10.1016/S0048-9697(03)00141-4) [DOI] [PubMed] [Google Scholar]
- Folke C., Carpenter S., Walker B., Scheffer M., Elmqvist T., Gunderson L., Holling C. S.2004Regime shifts, resilience and biodiversity in ecosystem management. Annu. Rev. Ecol. Systematics 35, 557–581 (doi:10.1146/annurev.ecolsys.35.021103.105711) [Google Scholar]
- Gunderson L. H.2000Ecological resilience—in theory and application. Annu. Rev. Ecol. Systematics 31, 425–439 (doi:10.1146/annurev.ecolsys.31.1.425) [Google Scholar]
- Guttal V., Jayaprakash C.2008Changing skewness: an early warning signal of regime shifts in ecosystems. Ecol. Lett. 11, 450–460 (doi:10.1111/j.1461-0248.2008.01160.x) [DOI] [PubMed] [Google Scholar]
- Hewitt J. E., Thrush S. F., Dayton P. K., Bonsdorf E.2007The effect of scale on empirical studies of ecology. Am. Nat. 169, 398–408 (doi:10.1086/510925) [DOI] [PubMed] [Google Scholar]
- Holling C. S.1973Resilience and stability of ecological systems. Annu. Rev. Ecol. Systematics 4, 1–23 (doi:10.1146/annurev.es.04.110173.000245) [Google Scholar]
- Holling C. S.2001Understanding the complexity of economic, social and ecological systems. Ecosystems 4, 390–405 (doi:10.1007/s10021-001-0101-5) [Google Scholar]
- Holyoak M., Leibold M. A., Mouquet N., Holt R. D., Hoopes M. F.2005Matacommunities: a framework for large-scale community ecology. In Metacommunities: spatial dynamics and ecological communities (eds Holyoak M., Leibold M. A., Holt R. D.), pp. 1–32 Chicago, IL: Chicago University Press [Google Scholar]
- Houseman G. R., Mittelbach G. G., Reynolds H. L., Gross K. L.2008Perturbations alter community convergence, divergence and formation of multiple community states. Ecology 89, 2172–2180 (doi:10.1890/07-1228.1) [DOI] [PubMed] [Google Scholar]
- Hughes T. P., Bellwood D. R., Folke C., Steneck R. S., Wilson J.2005New paradigms for supporting the resilience of marine ecosystems. Trends Ecol. Evol. 20, 380–386 (doi:10.1016/j.tree.2005.03.022) [DOI] [PubMed] [Google Scholar]
- Johnston E. L., Keough M. J.2005Reduction of pollution impacts through the control of toxicant release must be site- and season-specific. J. Exp. Mar. Biol. Ecol. 320, 9–33 (doi:10.1016/j.jembe.2004.12.024) [Google Scholar]
- Jonsson M., Malmqvist B.2000Ecosystem process rate increases with animal species richness: evidence from leaf eating aquatic insects. Oikos 89, 519–523 (doi:10.1034/j.1600-0706.2000.890311.x) [Google Scholar]
- Litzow M. A., Urban J. D., Laurel B. J.2008Increased spatial variance accompanies reorganization of two continental shelf ecosystems. Ecol. Appl. 18, 1331–1337 (doi:10.1890/07-0998.1) [DOI] [PubMed] [Google Scholar]
- Lohrer A. M., Thrush S. F., Gibbs M. M.2004Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 431, 1092–1095 (doi:10.1038/nature03042) [DOI] [PubMed] [Google Scholar]
- Lotze H. K., et al. 2006Depletion, degradation and recovery potential of estuaries and coastal seas. Science 312, 1806–1809 (doi:10.1126/science.1128035) [DOI] [PubMed] [Google Scholar]
- Ludwig D., Walker B., Holling C. S.1997Sustainability, stability and resilience. Ecol. Soc. 1 (http://www.consecol.org/vol1/iss1/art7/) [Google Scholar]
- Mantua N.2004Methods for detecting regime shifts in large marine ecosystems: a review with approaches applied to North Pacific data. Progr. Oceanogr. 60, 165–182 (doi:10.1016/j.pocean.2004.02.016) [Google Scholar]
- Nichols F. H., Cloern J. E., Luoma S. N., Peterson D. H.1986The modification of an estuary. Science 231, 567–648 (doi:10.1126/science.231.4738.567) [DOI] [PubMed] [Google Scholar]
- Norkko A., Hewitt J. E., Thrush S. F., Funnell G. A.2006aConditional outcomes of facilitation by a habitat-modifying subtidal bivalve. Ecology 87, 226–234 (doi:10.1890/05-0176) [DOI] [PubMed] [Google Scholar]
- Norkko A., Rosenberg R., Thrush S. F., Whitlatch R. B.2006bScale- and intensity-dependent disturbance determines the magnitude of opportunistic response. J. Exp. Mar. Biol. Ecol. 330, 195–207 (doi:10.1016/j.jembe.2005.12.027) [Google Scholar]
- Paine R. T.1980Food webs: linkages, interaction strength and community infrastructure. J. Anim. Ecol. 49, 667–685 [Google Scholar]
- Pascual M., Guichard F.2005Criticality and disturbance in spatial ecological systems. Trends Ecol. Evol. 20, 88–95 (doi:10.1016/j.tree.2004.11.012) [DOI] [PubMed] [Google Scholar]
- Peterson G., Allen C. R., Holling C. S.1998Ecological resilience, biodiversity, and scale. Ecosystems 1, 6–18 (doi:10.1007/s100219900002) [Google Scholar]
- Petersen J. K., Hansen J. W., Laurensen M. B., Clausen P., Carstensen J., Conley D.2008Regime shift in a coastal marine system. Ecol. Appl. 18, 497–510 (doi:10.1890/07-0752.1) [DOI] [PubMed] [Google Scholar]
- Pimm S. L.1991. In The balance of nature: ecological issues in the conservation of species and communities Chicago, IL: The University of Chicago Press [Google Scholar]
- Reid P. C., Holliday N. P., Smyth T. J.2001Pulses in the eastern margin current and warmer water off the north west European shelf linked to North Sea ecosystem changes. Mar. Ecol. Progr. Ser. 215, 283–287 (doi:10.3354/meps215283) [Google Scholar]
- Rietkerk M., Van de Koppel J.2008Regular pattern formation in real ecosystems. Trends Ecol. Evol. 23, 169–175 (doi:10.1016/j.tree.2007.10.013) [DOI] [PubMed] [Google Scholar]
- Rodionov S. N.2005A brief overview of the regime shift detection methods. In Large-scale disturbances (regime shifts) and recovery in aquatic ecosystems: challenges for management toward sustainability (eds Velikova V., Chipev N.). Varna, Bulgaria: UNESCO-ROSTE/BAS Workshop on Regime Shifts; <http://biocore.ecolab.bas.bg/events/past/unesco-ws/> [Google Scholar]
- Rosewell J., Shorrocks B., Edwards K.1990Competition on a divided and ephemeral resource: testing the assumptions. 1 Aggregation. J. Anim. Ecol. 59, 977–1001 [Google Scholar]
- Scheffer M., Carpenter S., Foley J. A., Folke C., Walker B.2001Catastrophic shifts in ecosystems. Nature 413, 591–596 (doi:10.1038/35098000) [DOI] [PubMed] [Google Scholar]
- Schiel D. R.2006Rivets or bolts? When single species count in the function of temperate rocky reef communities. J. Exp. Mar. Biol. Ecol. 338, 233–252 (doi:10.1016/j.jembe.2006.06.023) [Google Scholar]
- Schroder A., Persson L., De Roos A. M.2005Direct experimental evidence for alternative stable states: a review. Oikos 110, 3–19 (doi:10.1111/j.0030-1299.2005.13962.x) [Google Scholar]
- Slocum M. G., Mendelssohn I. A.2008Use of experimental disturbances to assess resilience along a known stress gradient. Ecol. Indicators 8, 181–190 (doi:10.1016/j.ecolind.2007.01.011) [Google Scholar]
- Suding K. N., Hobbs R. J.2009Threshold models in restoration and conservation: a developing framework. Trends Ecol. Evol. 24, 271–279 (doi:10.1016/j.tree.2008.11.012) [DOI] [PubMed] [Google Scholar]
- Sugihara G.1994Nonlinear forecasting for the classification of natural time series. Phil. Trans. R. Soc. Lond. A 348, 477–495 (doi:10.1098/rsta.1994.0106) [Google Scholar]
- Taylor L. R.1961Aggregation, variance, and the mean. Nature 189, 732–735 (doi:10.1038/189732a0) [Google Scholar]
- Thrush S. F., Dayton P. K.2002Disturbance to marine benthic habitats by trawling and dredging—Implications for marine biodiversity. Annu. Rev. Ecol. Systematics 33, 449–473 (doi:10.1146/annurev.ecolsys.33.010802.150515) [Google Scholar]
- Thrush S. F., Whitlatch R. B.2001Recovery dynamics in benthic communities: balancing detail with simplification. In Ecological comparisons of sedimentary shores (ed. Reise K.), pp. 297–316 Berlin, Germany: Springer-Verlag [Google Scholar]
- Thrush S. F., Whitlatch R. B., Pridmore R. D., Hewitt J. E., Cummings V. J., Maskery M.1996Scale-dependent recolonization: the role of sediment stability in a dynamic sandflat habitat. Ecology 77, 2472–2487 (doi:10.2307/2265747) [Google Scholar]
- Thrush S. F., et al. 1997Matching the outcome of small-scale density manipulation experiments with larger scale patterns: an example of bivalve adult/juvenile interactions. J. Exp. Mar. Biol. Ecol. 216, 153–170 (doi:10.1016/S0022-0981(97)00094-4) [Google Scholar]
- Thrush S. F., Hewitt J. E., Cummings V. J., Green M. O., Funnell G. A., Wilkinson M. R.2000The generality of field experiments: interactions between local and broad-scale processes. Ecology 81, 399–415 [Google Scholar]
- Thrush S. F., Gray J. S., Hewitt J. E., Ugland K. I.2006Predicting the effects of habitat homogenization on marine biodiversity. Ecol. Appl. 16, 1636–1642 (doi:10.1890/1051-0761(2006)016[1636:PTEOHH]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Thrush S. F., Coco G., Hewitt J. E.2008aComplex positive connections between functional groups are revealed by neural network analysis of ecological time-series. Am. Nat. 171, 669–677 (doi:10.1086/587069) [DOI] [PubMed] [Google Scholar]
- Thrush S. F., Halliday J., Hewitt J. E., Lohrer A. M.2008bCumulative degradation in estuaries: the effects of habitat, loss fragmentation and community homogenization on resilience. Ecol. Appl. 18, 12–21 (doi:10.1890/07-0436.1) [DOI] [PubMed] [Google Scholar]
- Thrush S. F., Hewitt J. E., Hickey C. W., Kelly S.2008cMultiple stressor effects identified from species abundance distributions: interactions between urban contaminants and species habitat relationships. J. Exp. Mar. Biol. Ecol. 366, 160–168 (doi:10.1016/j.jembe.2008.07.020) [Google Scholar]
- Troell M., Pihl L., Ronnback P., Wennhage H., Soderqvist T. S., Kautsky N.2005Regime shifts and ecosystem services in Swedish coastal soft bottom habitats: when resilience is undesirable. Ecol. Soc. 10 (URL: U606-U618) [Google Scholar]
- Vandermeer J., de la Cerda I. G., Perfecto I., Boucher D., Ruiz J., Kaufman A.2004Multiple basins of attraction in a tropical forest: evidence of nonequilibrum community structure. Ecology 85, 575–579 (doi:10.1890/02-3140) [Google Scholar]
- van Nes E. H., Scheffer M.2007Slow recovery from perturbations as a generic indicator of a nearby catastrophic shift. Am. Nat. 169, 738–747 (doi:10.1086/516845) [DOI] [PubMed] [Google Scholar]
- van Nes E. H., Amaro T., Scheffer M., Duineveld G. C. A.2007Possible mechanisms for a marine benthic regime shift in the North Sea. Mar. Ecol. Progr. Ser. 330, 39–47 (doi:10.3354/meps330039) [Google Scholar]
- Walker B., Kinzig A., Langridge J.1999Plant attribute diversity, resilience and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 2, 95–113 (doi:10.1007/s100219900062) [Google Scholar]
- Ware D. M., Thomson R. E.2005Bottom-up ecosystem trophic dynamics determine fish production in the Northeast Pacific. Science 308, 1280–1284 (doi:10.1126/science.1109049) [DOI] [PubMed] [Google Scholar]
- Warwick R. M., Clarke K. R.1993Increased variability as a symptom of stress in marine communities. J. Exp. Mar. Biol. Ecol. 172, 215–226 (doi:10.1016/0022-0981(93)90098-9) [Google Scholar]
- Warwick R. M., et al. 2002Inter-annual changes in the biodiversity and community structure of the macrobenthos in Tees Bay and the Tees estuary, UK, associated with local and regional environmental events. Mar. Ecol. Progr. Ser. 234, 1–13 (doi:10.3354/meps234001) [Google Scholar]
- Weijerman M., Lindeboom H., Zuur A. F.2005Regime shifts in the ecosystems of the North Sea and Wadden Sea. Mar. Ecol. Progr. Ser. 298, 21–39 (doi:10.3354/meps298021) [Google Scholar]
- Zaldivar J.-M., Strozzi F., Dueri S., Marinov D., Zbilut J. P.2008Characterisation of regime shifts in environmental time series with recurrence quantification analysis. Ecol. Model. 210, 58–70 (doi:10.1016/j.ecolmodel.2007.07.012) [Google Scholar]