Abstract
Sexual selection theory asserts that females are well adapted to sense signals indicating the quality of potential mates. One crucial male quality parameter is functional fertility (i.e. the success of ejaculates in fertilizing eggs). The phenotype-linked fertility hypothesis (PLFH) predicts that functional fertility of males is reflected by phenotypic traits that influence female mate choice. Here, we show for Nasonia vitripennis, a parasitic wasp with haplodiploid sex determination and female-biased sex ratios, that females use olfactory cues to discriminate against sperm-limited males. We found sperm limitation in newly emerged and multiply mated males (seven or more previous matings) as indicated by a higher proportion of sons in the offspring fathered by these males. Sperm limitation correlated with clearly reduced pheromone titres. In behavioural bioassays, females oriented towards higher doses of the synthetic pheromone and were attracted more often to scent marks of males with a full sperm load than to those of sperm-limited males. Our data support the PLFH and suggest that N. vitripennis females are able to decrease the risk of getting constrained to produce suboptimal offspring sex ratios by orienting towards gradients of the male sex pheromone.
Keywords: mate choice, mate assessment pheromone, phenotype-linked fertility hypothesis, sexual selection, local mate competition, sex ratios
1. Introduction
It is well established by sexual selection theory that females can maximize their reproductive success by reacting to signals that indicate the quality of potential mates (Kirkpatrick & Ryan 1991; Andersson 1994; Andersson & Simmons 2006). Male quality is typically advertised by behavioural displays or morphological ornaments but can, in principle, be mediated by any sensory modality including olfaction (Johansson & Jones 2007). Benefits for females resulting from mate choice, which by definition includes ‘all behaviour that restricts a set of potential mates’ (Wiley & Poston 1996), can be direct or indirect. Direct benefits may be obtained, for instance, if the chosen male is sufficiently fertile, free of transferable diseases or able to provide parental care, access to territories or fitness-related resources (Kirkpatrick & Ryan 1991; Andersson 1994). Indirect benefits may arise from genetic traits of the chosen male (e.g. ‘good genes’, heterozygosity or genetic compatibility), leading to an increased fitness of the resulting progeny (Brown 1997; Tregenza & Wedell 2000; Mays & Hill 2004). In many cases, however, male quality is difficult to ascertain and fitness benefits resulting from female preferences for certain male traits have often been inferred indirectly rather than demonstrated experimentally.
Functional fertility (i.e. the success of male ejaculates in fertilizing eggs) is a crucial quality parameter of males providing direct benefits to females. The phenotype-linked fertility hypothesis (PLFH) predicts that functional fertility covaries with the male phenotype (Sheldon 1994), thus enabling females to select mates that are able to transfer a sufficient amount of viable sperm. However, previous studies testing the PLFH have been contradictory. Some studies have provided evidence that preferred male phenotypes correlate with proxy measures of male fertility like testis size or spermatozoon quantity and quality (e.g. Matthews et al. 1997; Skinner & Watt 2007), whereas others failed to show this correlation (e.g. Birkhead & Fletcher 1995; Parker et al. 2006). Furthermore, these traits do not inevitably translate into an increased fertilizing efficiency (Pizzari et al. 2004; Pilastro et al. 2008); thus, actual fecundity benefits for the selective females have only rarely been demonstrated (Wagner & Harper 2003; Malo et al. 2005a,b; Rogers et al. 2008).
Parasitic wasps are ideal models to study functional fertility of males because of their haplodiploid sex determination. Fertilized eggs in these insects develop into diploid females and unfertilized eggs into haploid males (Heimpel & de Boer 2008). Therefore, sperm limitation of a given male can be inferred from an increased number of males in the offspring of the female with whom he has mated (Godfray 1990).
Chemical senses are of particular importance in the sexual communication of insects, and there is increasing evidence that sex pheromones provide not only information on the presence and identity of a potential mate but also its individual quality (Lewis & Austad 1994; Eisner et al. 1996; Svensson 1996; Iyengar et al. 2001; Reusch et al. 2001; Beeler et al. 2002; Rantala et al. 2002; Carazo et al. 2004; Johansson & Jones 2007; Koh et al. 2009). Key criteria for the function of a chemical signal as mate assessment pheromone are as follows. (i) The signal should honestly reflect an individual's quality, and hence has to be costly. Potential costs may include both direct metabolic costs (for instance, when the signalling molecule is synthesized from limited resources) and indirect ecological costs (for instance, when pheromone signalling increases the chance of being discovered by natural enemies or aggressive competitors). (ii) There must be individual variation of the signal depending on the sender's condition or quality. This variation may be represented by differences in the composition of the pheromone blend or simply by the individual pheromone amounts emitted. (iii) Ultimately, it has to be demonstrated that the receiver actually discriminates these differences and is guided by the chemical signal to a mate of higher quality (Johansson & Jones 2007).
Here, we investigate the putative function of a male sex pheromone for mate assessment in the model organism Nasonia vitripennis Walker (Hymenoptera: Pteromalidae), a pupal parasitoid of numerous fly species occurring in nests of hole-breeding birds (Whiting 1967). The mating system of N. vitripennis is characterized by local mate competition (LMC; Hamilton 1967), with single or few females colonizing patchy habitats, leading to a high degree of sibling mating, which is restricted to the natal site. Females of N. vitripennis typically adjust their offspring sex ratio flexibly according to the number of foundresses. In the case of a single foundress, she produces a minimum number of males necessary to inseminate all females in the brood. This strategy increases her reproductive success because competition between her sons is kept to a minimum and only daughters are able to disperse to locate new host patches. With increasing number of foundresses, however, sex ratios shift in favour of males and approach an equal number of both sexes (Werren 1980; Shuker & West 2004; Shuker et al. 2005). As the number of foundresses is typically low, offspring sex ratios in natural habitats are mostly strongly female biased (Molbo & Parker 1996; Grillenberger et al. 2008) and, therefore, females are highly motivated to find a mate with a sufficient sperm load even though virgins are able to produce offspring (Steiner & Ruther 2009a). Males of N. vitripennis are protandrous (i.e. they emerge before the females) and release a mixture of (4R,5R)- and (4R,5S)-5-hydroxy-4-decanolides (HDL) to attract virgin females that emerge shortly after (Ruther et al. 2007). The attractiveness of HDL is synergized by the trace component 4-methylquinazoline (MeQ) being released together with HDL at a ratio of approximately 1 : 100 (MeQ : HDL) (Ruther et al. 2008). Males are promiscuous, whereas most females mate only once in natural populations (Grillenberger et al. 2008). HDL is synthesized in the male rectal papillae (Abdel-latief et al. 2008) and pheromone release occurs via the anal orifice by dabbing movements of the abdominal tip. This behaviour is increasingly shown after copulation, probably to attract and arrest further virgins that may emerge in rapid succession from the hosts (Steiner & Ruther 2009b). This suggests that pheromone titres of males decrease with increasing number of copulations and might reflect the mating history of a male. Furthermore, the pheromone titres of newly emerged males are very low (Ruther et al. 2007), which might correlate with an incomplete sexual maturity. Hence, orientation towards higher pheromone concentrations might help females to avoid those males that are not yet, or no longer, able to transfer a sufficient amount of sperm.
In the present study, we performed behavioural experiments and chemical analyses to test the PLFH in N. vitripennis. Our predictions were that mating history and age are quality parameters of N. vitripennis males that are reflected by the pheromone titres, and that females discriminate olfactorily between males according to these parameters. First, we investigated whether females mated with multiply mated (assumed to be sperm-limited) or newly emerged (assumed to be sexually immature) males are constrained to produce a higher proportion of male offspring compared with control females mated with mature males having a full sperm load. Second, we studied whether male pheromone titres decrease with increasing number of previous matings and are significantly lower in newly emerged males compared with older males. Finally, we tested whether females are attracted to higher doses of the synthetic pheromone and discriminate olfactorily between natural pheromone deposits released by males of different mating histories and age.
2. Material and methods
(a). Insects
Nasonia vitripennis wasps originating from a bird's nest near Hamburg, northern Germany, were reared on freeze-killed puparia of the green bottle fly, Lucilia caesar. Laboratory cultures were kept at 25°C and 60 per cent relative humidity with a daily light : dark cycle of 16 : 8 h. Parasitoids emerged from the host puparia after a development time of 14–15 days. To obtain unmated parasitoids for the experiments, parasitoid pupae were excised from host puparia 1–2 days prior to eclosion and kept singly in 1.5 ml microcentrifuge tubes until emergence. Females used in the olfactometer bioassays were 1–3 days old and unmated. Males were of similar size and either newly emerged or 2 days old. Size was determined by measuring the head width of fully melanized pupae under a stereo microscope with the help of a measuring eyepiece. Only pupae with a medium head width (660–700 µm) were used for the experiments. ‘Newly emerged’ signifies that males were not older than 1 h after eclosion from the exuviae. Two-day-old males with a defined mating history were obtained by offering them successively 1, 3, 5, 7 or 10 virgin females for mating. Couples were kept in observation chambers (0.5 cm high, 7 cm inner diameter) and each copulation was observed under a stereo microscope. After each mating, males were allowed to mark the substrate with the sex pheromone for 10 min. Clean bioassay chambers were used for each successive mating. To make sure that these males differed only with respect to the number of matings, each male was relocated 10 times to clean bioassay chambers irrespective of the number of virgin females offered. Control males without mating experience were obtained by subjecting them to the same procedure without offering virgin females.
(b). Mating history and male functional fertility
In haplodiploid species, the amount of sperm transferred by males to females during copulation can be inferred indirectly from the lifetime sex ratio of the mother (Henter 2004). Females that received an insufficient amount of sperm owing to mating with a sperm-exhausted or immature male are constrained to lay clutches with a higher proportion of males when compared with unconstrained females kept under the same conditions. To investigate the functional fertility of males depending on mating history and age, we determined the lifetime sex ratio of females mated with differently conditioned males: (i) 2-day-old males with 0 (n = 7), 3 (n = 10), 5 (n = 10), 7 (n = 10) or 10 (n = 11) previous matings and (ii) newly emerged versus 2-day-old males with no previous matings (n = 8). Males of different mating histories were obtained as described above. Females of the last mating were transferred to Petri dishes containing 20 host puparia for oviposition. Host puparia were renewed every 2 days until the death of the female, and parasitized hosts were kept under rearing conditions until the next generation emerged. Offspring of individual females were counted and sexed, and lifetime sex ratio was calculated (given as proportion of males in the brood).
(c). Male pheromone titres
Pheromone titres of differently treated males were estimated by solvent extraction and subsequent chemical analysis by coupled gas chromatography–mass spectrometry using the instrumentation and conditions described elsewhere (Ruther et al. 2007). The abdomens of individual freeze-killed males were cut off with a scalpel and transferred to 100 µl microvial inserts filled with 20 µl dichloromethane containing 20 ng µl−1 methyl undecanoate (Sigma-Aldrich, Deisenhofen, Germany) as an internal standard. Each abdomen was extracted for 30 min. The pheromone components (4R,5R)- and (4R,5S)-HDL were quantified by relating peak areas to the internal standard. Pheromone titres of the following groups of males were determined and compared: (i) males with different mating histories (i.e. with 0, 1, 3, 5, 7 or 10 previous matings; n = 8 per group) and (ii) males of different ages (i.e. newly emerged versus 2-day-old males; n = 10 per group).
(d). Female olfactory responses
The behavioural responses of virgin females to natural male pheromone deposits and different doses of synthetic pheromone were tested in a still-air Y-tube olfactometer consisting of translucent polyethylene. The Y-tube was fixed vertically to a piece of white cardboard and illuminated from above with a desk light (60 W, with a distance of 20 cm to the olfactometer). Pheromone sources (natural/synthetic) were allowed to diffuse into the Y-tube for 5 min before the experiment started. Females (n = 40) were then released singly into the olfactometer via the stem (50 mm × 5 mm inner diameter) and allowed to choose one of the two arms (30 mm length × 5 mm inner diameter). Females were considered to have made a choice when they first walked at least 2.5 cm into one of the two arms. Depending on the attractiveness of the stimulus, 23 ± 4 per cent of the females did not make a decision within the observation time of 3 min. These were excluded from statistical analyses.
In a first series of experiments, we tested the response of virgin females to the following naturally deposited pheromone treatments: pheromone deposits of 2-day-old males with 0 versus 7 previous matings, and pheromone deposits of newly emerged versus 2-day-old virgin males (experiments a and b, respectively). In these experiments, the two arms of the Y-tube were capped with removable polyethylene chambers (10 mm length, 5 mm inner diameter) that were closed at the distal end and contained pheromone deposits of single males. Pheromone deposits were obtained by releasing single males together with a virgin female into the removable chamber. After copulation was observed, the female was removed and the males were allowed to deposit pheromones for 10 min. Subsequently, the males were also removed and the two chambers were fixed to the Y-tube. Pheromone chambers marked by males of the two conditions to be compared were always prepared simultaneously.
In a second series of experiments, we determined the attractive threshold dose of the synthetic pheromone (=1 : 1 mixture of (4R,5R)- and (4R,5S)-HDL + MeQ dissolved in dichloromethane at a ratio 100 : 1; Ruther et al. 2008) by testing doses of 40, 30, 20, 10 and 5 ng versus the pure solvent (experiments c–g, respectively). Then we investigated whether females discriminate different doses of the synthetic pheromone by testing 10 versus 20 ng and 10 versus 30 ng (experiments h and i, respectively). In experiments with synthetic pheromone, test and control solutions were applied to a 5 mm diameter filter paper disc and the solvent was allowed to evaporate for 2 min. Then the filter papers of different pheromone doses or control solvent were fixed to the distal end of the Y-tube with Parafilm®.
Preliminary tests showed that females did not discriminate between higher doses of the synthetic pheromone in the Y-tube olfactometer, presumably because of saturation of the small olfactometer volume and the absence of an odour gradient. We therefore tested in experiment (j) the female response to synthetic pheromone at 30 versus 100 ng in an open arena bioassay consisting of a 19-cm-diameter walking arena made of fine gauze with a central 1 cm inlet hole. The arena was bordered by a 1-cm-high acrylic glass ring that was covered by a glass plate. Pheromone-treated filter paper discs were placed in the centre of two virtual circles with a diameter of 3 cm, which were located opposite each other at a distance of 3 cm from the inlet hole. Females were released singly into the arena via the inlet hole and the first choice was recorded when females entered one of the two circles within an observation time of 3 min.
Male and female parasitoids were used only once in the bioassays. Pheromone sources (natural/synthetic) were renewed either after five individuals had been tested or after 10 min at the latest. Preliminary tests revealed no differences between the successively tested females; thus, data were pooled for statistical analysis. Olfactometers were regularly cleaned with ethanol and demineralized water and turned by 180° to avoid biased results owing to side preferences.
(e). Statistical analysis
Total offspring numbers and lifetime sex ratios of females inseminated by males with different mating histories or ages did not meet the assumptions for parametric statistical tests, and were thus compared by a non-parametric Kruskal–Wallis H-test and multiple Mann–Whitney U-tests with sequential Bonferroni correction for individual comparisons. Pheromone titres of males with different mating histories were log transformed and compared by a one-way analysis of variance (ANOVA) followed by least significant difference tests for post hoc comparisons (results show untransformed data). Age dependency of pheromone titres was analysed by a Mann–Whitney U-test because neither original nor log-transformed data were normally distributed. Choices of virgin females in the Y-olfactometer were analysed by a one-tailed binominal test using Graphpad software (available online at www.graphpad.com/quickcalcs/binomial1.cfm). The one-tailed test was used because our hypothesis implied the direction of the difference (attraction of the females to the pheromone and to higher pheromone doses, respectively). All other statistical analyses were done using Statistica 4.5 scientific software (StatSoft, Tulsa, OK, USA).
3. Results
(a). Mating history and male functional fertility
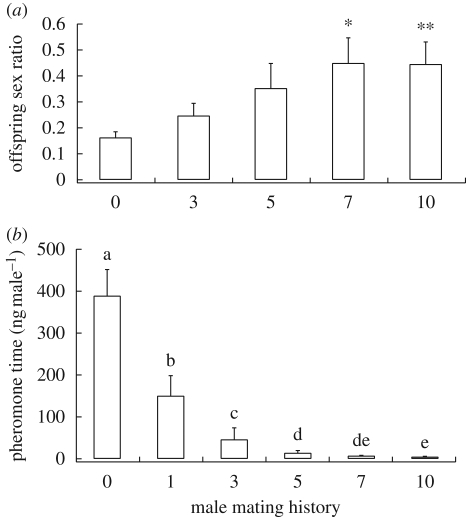
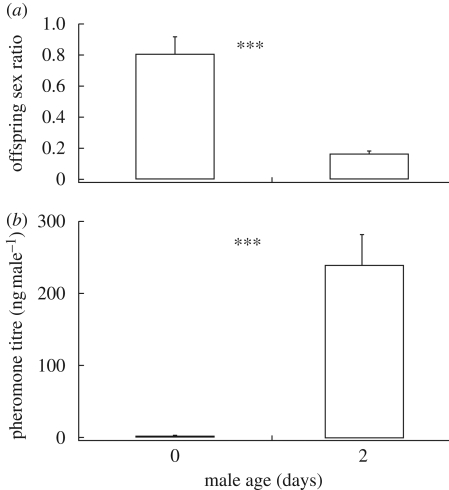
The lifetime sex ratio of the females’ offspring depended on both the mating history (Kruskal–Wallis: H = 10.4412, d.f. = 4, p = 0.0336, figure 1a) and age (Mann–Whitney: Z = 3.1529, d.f. = 1, p = 0.0016, figure 2a) of the males with whom they had mated. Females that had been inseminated by males with 7 (Mann–Whitney: Z = −2.4398, p = 0.0147) or 10 (Mann–Whitney: Z = −3.0340, p = 0.0024) previous matings produced significantly more male offspring (i.e. higher lifetime sex ratios) than control females that had been inseminated by virgin males of similar size and age. Likewise, females that had been inseminated by newly emerged virgin males produced significantly more male offspring than females that had been inseminated by 2-day-old virgin males of similar size. The total number of offspring produced by individual females in our experiments was 590 ± 40 (mean ± s.e.). However, this number was influenced neither by the mating history (Kruskal–Wallis: H = 6.5350, d.f. = 4, p = 0.1626) nor by the age (Mann–Whitney: Z = 0.4629, d.f. = 1, p = 0.6434) of the males with whom they had mated.
Figure 1.
Relationship between the mating history (number of previous copulations) of Nasonia vitripennis males and (a) the lifetime offspring sex ratio (proportion of males) produced by the last mated female, and (b) the pheromone titre. Data are given as means±s.e. Asterisks indicate significant differences between treatments and the control (= 0 previous copulations) at (*) p < 0.05 or (**) p < 0.01 (Kruskal–Wallis H-test and multiple Bonferroni-corrected Mann–Whitney U-tests). Different lowercase letters indicate significant differences at p < 0.05 (one-way ANOVA followed by least significant difference tests).
Figure 2.
Relationship between the age of Nasonia vitripennis males and (a) the lifetime offspring sex ratio (proportion of males) produced by the mated female, and (b) the pheromone titre. Asterisks indicate significant differences at (***) p < 0.001 (Mann–Whitney U-test).
(b). Male pheromone titres
Males' HDL titres were influenced by both their mating history (ANOVA: F5,41 = 41.2995, p < 0.001, figure 1b) and age (Mann–Whitney: Z = − 3.3606, p < 0.001, figure 2b). Males contained almost no HDL upon emergence, but their pheromone titres increased strongly after 2 days. Male pheromone titres were negatively correlated with increasing number of copulations and subsequent bouts of postcopulatory scent marking. Differences between mean pheromone titres of males with different mating histories allowed for a rough estimate of the pheromone doses released during successive mating sequences. Accordingly, males released more than 200 ng after their first mating, a total of about 100 ng between copulations 1 and 3, and about 30 ng between copulations 3 and 5. Between copulations 5 and 7, less than 10 ng was deposited by the males, and after 7 or 10 copulations less than 5 ng was extractable from the male abdomens. However, the typical postcopulatory scent-marking behaviour was shown by all males tested irrespective of the amount of pheromone they contained.
(c). Female olfactory responses
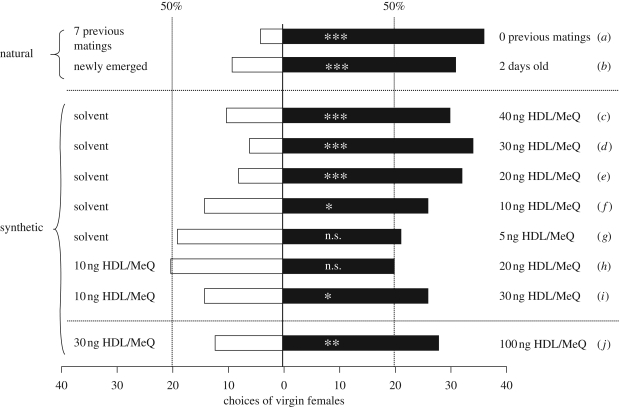
The response of virgin females to natural pheromone deposits in the Y-tube olfactometer was significantly influenced by both mating history and age of the scent-marking males. Females were attracted more often to scent marks of 2-day-old males without previous matings than to those of males that had seven matings prior to the experiment (figure 3a). Furthermore, females were attracted more often to scent marks of 2-day-old males than to those of newly emerged males (figure 3b). When given the choice between different doses of the synthetic pheromone and the pure solvent, females were attracted to pheromone doses down to 10 ng but no longer responded to 5 ng (figure 3c–g). When exposed to different pheromone doses simultaneously, females were not able to discriminate between the threshold dose of 10 and 20 ng, but were attracted more often to the higher dose when increased to 30 ng (figure 3h,i). In the open arena bioassay, females were attracted more often to 100 ng of the synthetic pheromone when tested against 30 ng (figure 3j).
Figure 3.
Behavioural response of virgin Nasonia vitripennis females to natural male pheromone deposits and to different doses of synthetic pheromone in a still-air Y-olfactometer (a–i) and an open arena bioassay (j). Asterisks indicate significant differences at (***) p < 0.001, (**) p < 0.01 and (*) p < 0.05; n.s., not significant (one-tailed binominal test).
4. Discussion
The present study supports the PLFH by clearly demonstrating a relationship between phenotype (pheromone titre) and functional fertility in N. vitripennis males and by showing that females actually use this phenotypic trait to discriminate males according to their sperm load. Lifetime offspring sex ratios of N. vitripennis females were shifted to a higher proportion of males after mating with newly emerged or multiply mated males (seven or more matings). As in haplodiploid species the ability of females to produce daughters depends on the availability of sperm, our data indicate that males are not fully sexually mature upon emergence and multiple mating of mature males results in significant sperm exhaustion. For females, these males are of lesser quality because they constrain them to produce suboptimal lifetime offspring sex ratios, i.e. more sons than necessary or even male offspring only (Godfray 1990). This might cause fitness losses for the mother because her flightless sons are more likely to compete among each other for females (LMC), and only her daughters are able to disperse for reproduction (Hamilton 1967; Werren 1980). Therefore, females are predicted to detect and avoid sperm-limited males prior to copulation. However, because it appears unlikely that females are able to directly assess the sperm load of a potential mate, they use an indirect signal instead.
In the cockroach Nauphoeta cinerea, females use cuticular chemicals to discriminate against multiply mated males during courtship, and even those males that had mere contact with females but did not mate were less preferred (Harris & Moore 2005). In the present study, we did not observe any female rejecting a male of any quality during courtship, suggesting that N. vitripennis females do not perform active mate choice (Parker 1983; Gibson & Langen 1996). Rather, our chemical and behavioural data indicated that females reduce the danger of becoming constrained indirectly by orientating towards higher pheromone concentrations because the sperm limitation of newly emerged and multiply mated males is clearly reflected by reduced pheromone titres. Females were not only attracted to higher doses of the synthetic pheromone, but also to naturally laid scent marks of males with a full sperm load rather than to those originating from sperm-limited males. In fact, owing to putative physiological constraints (young males) and repeated postcopulatory scent marking (multiply mated males), mean pheromone titres of sperm-limited males were below 10 ng, the biologically active threshold dose in our bioassays. This suggests that many of these sperm-limited males are neither able to scent-mark new territories nor to refresh existing ones.
We conclude from our data that the male sex attractant of N. vitripennis meets crucial criteria of a mate assessment pheromone because it varies quantitatively with male quality, and females actually discriminate these differences behaviourally and are therefore more likely to mate with suitable males (Johansson & Jones 2007). An important question is whether the signal is honest because it is costly to produce or maintain. A possible answer to this question can be given by our recent work on the biosynthetic pathway of HDL in N. vitripennis. We found that linoleic acid, but not saturated fatty acids like palmitic and stearic acids, may function as an HDL precursor (Abdel-latief et al. 2008; J. Ruther & L.-A. Garbe 2009, unpublished data). This suggests that N. vitripennis males, like most animals (Wathes et al. 2007 and references therein; but see Borgeson et al. 1991 and Weinert et al. 1993), do not possess the enzymatic prerequisites to synthesize linoleic acid themselves but depend on the uptake of polyunsaturated fatty acids with their nutrition. Therefore, it can be hypothesized that the amount of HDL produced by an N. vitripennis male depends on the availability of the limited resource linoleic acid and therefore might be costly. Taking into account that polyunsaturated fatty acids may influence both the fertility and the number of sperm (Wathes et al. 2007; Estienne et al. 2008), and also that sperm production is costly (Dewsbury 1982), a trade-off might be predicted in N. vitripennis between sperm production, pheromone production and pheromone release. Disadvantaged males might produce more pheromone at the cost of transferable sperm, or release higher pheromone doses at the cost of scent-marking frequency, in order to increase the chance of attracting mates at all. Such a trade-off might be influenced by intrinsic parameters like male size, which hitherto was assumed to be of minor importance for the fitness of N. vitripennis males (Burton-Chellew et al. 2007). In fact, we found a clear size dependence of male pheromone titres, but females did not discriminate between the first scent marks laid by small and large males (data not shown).
Reduction in sperm transfer with successive inseminations has been shown in several other parasitic Hymenoptera (e.g. King 2000; Jacob & Boivin 2004; Bressac et al. 2008; Steiner et al. 2008). The present study revealed considerable sperm exhaustion in N. vitripennis males after seven successive matings, supporting previous reports that males (under artificial conditions) are able to copulate with hundreds of females, with a full amount of sperm being transferred only for a few copulations (Barras 1961; van den Assem 1986). But is the occurrence of sperm-exhausted males also a realistic scenario for N. vitripennis in natural habitats, as studies on other parasitoids indicate (Henter 2004)? We believe that this is the case because of the strongly female-biased sex ratios and the fact that mating chances are not equally distributed among males because of the territoriality of dominant males (Leonard & Boake 2006).
The present study emphasizes that sex pheromones are much more than mere indicators of potential mates, but can also help the responder to avoid the costs associated with mating. The use of the male sex attractant for mate assessment enables female N. vitripennis to minimize search costs and avoid the fitness losses caused by mating with sperm-limited males. Owing to the volatility of the chemical signal, females are able to discriminate males from a distance and thus to reduce the costs of sexual harassment by less desirable males (Gay et al. 2009 and references therein). In fact, after mating, females are no longer attracted to the male signal and orientate towards host odours instead (Ruther et al. 2007; Steiner & Ruther 2009a). Discrimination among males from a distance according to their functional fertility adds another facet to the multitude of mechanisms driving sexual selection.
Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, grant RU 717/10-1). J.R. was supported by a Heisenberg fellowship of the DFG (grant RU 717/7-1). We are grateful to Sophia Bohlke, Emanuel Voigt and Judith Schwartz for technical assistance. Erhard Strohm and two anonymous reviewers gave helpful comments on an earlier version of the manuscript.
References
- Abdel-latief M., Garbe L.-A., Koch M., Ruther J.2008An epoxide hydrolase involved in the biosynthesis of an insect sex attractant and its use to localize the production site. Proc. Natl Acad. Sci. USA 105, 8914–8919 (doi:10.1073/pnas.0801559105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M.1994Sexual selection Princeton, NJ: Princeton University Press [Google Scholar]
- Andersson M., Simmons L. W.2006Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302 (doi:10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- Barras R.1961A quantitative study of the behavior of the male Mormoniella vitripennis towards two constant stimulus situations. Behaviour 18, 288–312 (doi:10.1163/156853961X00178) [Google Scholar]
- Beeler A. E., Rauter C. M., Moore A. J.2002Mate discrimination by females in the burying beetle Nicrophorus orbicollis: the influence of male size on attractiveness to females. Ecol. Entomol. 27, 1–6 (doi:10.1046/j.1365-2311.2002.0371a.x) [Google Scholar]
- Birkhead T. R., Fletcher F.1995Male phenotype and ejaculate quality in the zebra finch Taeniopygia guttata. Proc. R. Soc. Lond. B 262, 329–335 (doi:10.1098/rspb.1995.0213) [DOI] [PubMed] [Google Scholar]
- Borgeson C. E., Kurtti T. J., Munderloh U. G., Blomquist G. J.1991Insect tissues not microorganisms produce linoleic acid in the house cricket and the American cockroach. Experientia 47, 238–241 (doi:10.1007/BF01958146) [DOI] [PubMed] [Google Scholar]
- Bressac C., Damiens D., Chevrier C.2008Sperm stock and mating of males in a parasitoid wasp. J. Exp. Zool. 308B, 1–7 (doi:10.1002/jez.b.21168) [DOI] [PubMed] [Google Scholar]
- Brown J. L.1997A theory of mate choice based on heterozygosity. Behav. Ecol. 8, 60–65 (doi:org/10.1093/beheco/8.1.60) [Google Scholar]
- Burton-Chellew M. N., Sykes E. M., Patterson S., Shuker D. M., West S. A.2007The cost of mating and the relationship between body size and fitness in males of the parasitoid wasp Nasonia vitripennis. Evol. Ecol. Res. 9, 921–934 [Google Scholar]
- Carazo P., Sanchez E., Font E., Desfilis E.2004Chemosensory cues allow male Tenebrio molitor beetles to assess the reproductive status of potential mates. Anim. Behav. 68, 123–129 (doi:10.1016/j.anbehav.2003.10.014) [Google Scholar]
- Dewsbury D. A.1982Ejaculate cost and male choice. Am. Nat. 119, 601–610 (doi:10.1086/283938) [Google Scholar]
- Eisner T., Smedley S. R., Young D. K., Eisner M., Roach B., Meinwald J.1996Chemical basis of courtship in a beetle (Neopyrocjroa flabellate): cantharidin as precopulatory ‘enticing’ agent. Proc. Natl Acad. Sci. USA. 93, 6494–6498 (doi:org/10.1073/pnas.93.13.6494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estienne M. J., Harper A. F., Crawford R. J.2008Dietary supplementation with a source of omega-3 fatty acids increases sperm number and the duration of ejaculation in boars. Theriogenology 70, 70–76 (doi:10.1016/j.theriogenology.2008.02.007) [DOI] [PubMed] [Google Scholar]
- Gay L., Eady P. E., Vasudev R., Hosken D. J., Tregenza T.2009Costly sexual harassment in a beetle. Physiol. Entomol. 34, 86–92 (doi:10.1111/j.1365-3032.2008.00656.x) [Google Scholar]
- Gibson R. M., Langen T. A.1996How do animals choose their mates? Trends Ecol. Evol. 11, 468–470 (doi:10.1016/0169-5347(96)10050-1) [DOI] [PubMed] [Google Scholar]
- Godfray H. C. J.1990The causes and consequences of constrained sex allocation in haplodiploid animals. J. Evol. Biol 3, 3–17 (doi:10.1046/j.1420-9101.1990.3010003.x) [Google Scholar]
- Grillenberger B. K., Koevoets T., Burton-Chellew M. N., Sykes E. M., Shuker D. M., van de Zande L., Bijlsma R., Gadau J., Beukeboom L. W.2008Genetic structure of natural Nasonia vitripennis populations: validating assumptions of sex-ratio theory. Mol. Ecol. 17, 2854–2864 (doi:10.1111/j.1365-294X.2008.03800.x) [DOI] [PubMed] [Google Scholar]
- Hamilton W. D.1967Extraordinary sex ratios. Science 156, 477–488 (doi:10.1126/science.156.3774.477) [DOI] [PubMed] [Google Scholar]
- Harris W. E., Moore J.2005Female mate preference and sexual conflict: females prefer males that have had fewer consorts. Am. Nat. 165, 64–71 (doi:10.1086/429352) [DOI] [PubMed] [Google Scholar]
- Heimpel G. E., de Boer J. G.2008Sex determination in the Hymenoptera. Annu. Rev. Entomol. 53, 209–230 (doi:10.1146/annurev.ento.53.103106.093441) [DOI] [PubMed] [Google Scholar]
- Henter H. J.2004Constrained sex allocation in a parasitoid due to variation in male quality. J. Evol. Biol. 17, 886–896 (doi:10.1111/j.1420-9101.2004.00746.x) [DOI] [PubMed] [Google Scholar]
- Iyengar V. K., Rossini C., Eisner T.2001Precopulatory assessment of male quality in an arctiid moth (Utetheisa ornatrix): hydroxydanaidal is the only criterion of choice. Behav. Ecol. Sociobiol. 49, 283–288 (doi:10.1007/s002650000292) [Google Scholar]
- Jacob S., Boivin G.2004Insemination potential of male Trichogramma evanescens. Entomol. Exp. Appl. 113, 181–186 (doi:10.1111/j.0013-8703.2004.00221.x) [Google Scholar]
- Johansson B. J., Jones T. M.2007The role of chemical communication in mate choice. Biol. Rev. 82, 265–289 (doi:10.1111/j.1469-185X.2007.00009.x) [DOI] [PubMed] [Google Scholar]
- King B. H.2000Sperm depletion and mating behavior in the parasitoid wasp Spalangia cameroni (Hymenoptera: Pteronamlidae). Gt. Lakes Entomol. 33, 117–127 [Google Scholar]
- Kirkpatrick M., Ryan M. J.1991The evolution of mating preferences and the paradox of the lek. Nature 350, 33–38 (doi:10.1038/350033a0) [Google Scholar]
- Koh T. H., Seah K. W., Yap L.-M. Y. L., Li D.2009Pheromone-based female mate choice and its effects on reproductive investment in a spitting spider. Behav. Ecol. Sociobiol. 63, 923–930 (doi:10.1007/s00265-009-0735-4) [Google Scholar]
- Leonard J. E., Boake C. R. B.2006Site-dependent aggression and mating behaviour in three species of Nasonia (Hymenoptera: Pteromalidae). Anim. Behav. 71, 641–647 (doi:10.1016/j.anbehav.2005.07.010) [Google Scholar]
- Lewis S. M., Austad S. N.1994Sexual selection in flour beetles: the relationship between sperm precedence and male olfactory attractiveness. Behav. Ecol. 5, 219–224 (doi:10.1093/beheco/5.2.223) [Google Scholar]
- Malo A. F., Garde J. J., Soler A. J., Garcia A. J., Gomendio M., Roldan E. R. S.2005aMale fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. Biol. Reprod. 72, 822–829 (doi:10.1095/biolreprod.104.036368) [DOI] [PubMed] [Google Scholar]
- Malo A. F., Roldan E. R. S., Garde J. J., Soler A. J., Gomendio M.2005bAntlers honestly advertise sperm production and quality. Proc. R. Soc. B 272, 149–157 (doi:10.1098/rspb.2004.2933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews I. M., Evans J. P., Magurran A. E.1997Male display rate reveals ejaculate characteristics in the Trinidadian guppy Poecilia reticulata. Proc. R. Soc. Lond. B 264, 695–700 (doi:10.1098/rspb.1997.0099) [Google Scholar]
- Mays H. L., Jr, Hill G. E.2004Choosing mates: good genes versus genes that are a good fit. Tends Ecol. Evol. 10, 554–559 (doi:10.1016/j.tree.2004.07.018) [DOI] [PubMed] [Google Scholar]
- Molbo D., Parker E. D.1996Mating structure and sex ratio variation in a natural population of Nasonia vitripennis. Proc. R. Soc. Lond. B. 265, 1703–1709 (doi:10.1098/rspb.1996.0249) [Google Scholar]
- Parker G. A.1983Mate quality and mating decisions. In Mate choice (ed. Bateson P. G. G.), pp. 141–166 Cambridge, UK: Cambridge University Press [Google Scholar]
- Parker T. H., Thompson D., Ligon J. D., Schneider B., Byrn F.2006Does red junglefowl comb size predict sperm swimming speed and motility? Ethol. Ecol. Evol. 18, 53–60 [Google Scholar]
- Pilastro A., Gasparini C., Boschetto C., Evans J. P.2008Colorful male guppies do not provide females with fecundity benefits. Behav. Ecol. 19, 374–381 (doi:10.1093/beheco/arm140) [Google Scholar]
- Pizzari T., Jensen P., Cornwallis C. K.2004A novel test of the phenotype-linked fertility hypothesis reveals independent components of fertility. Proc. R. Soc. Lond. B 271, 51–58 (doi:10.1098/rspb.2003.2577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala M. J., Jokinen I., Kortet R., Vainikka A., Suhonen J.2002Do pheromones reveal male immunocompetence? Proc. R. Soc. Lond. B 269, 1681–1685 (doi:10.1098/rspb.2002.2056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch T. B. H., Haberli M. A., Aeschlimann P. B., Milinski M.2001Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature 414, 300–302 (doi:10.1038/35104547) [DOI] [PubMed] [Google Scholar]
- Rogers D. W., Denniff M., Chapman T., Fowler K., Pomiankowski A.2008Male sexual ornament size is positively associated with reproductive morphology and enhanced fertility in the stalk-eyed fly Teleopsis dalmanni. BMC Evol. Biol. 8, 236 (doi:10.1186/1471-2148-236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruther J., Stahl L. M., Steiner S., Garbe L. A., Tolasch T.2007A male sex pheromone in a parasitic wasp and control of the behavioral response by the female's mating status. J. Exp. Biol. 210, 2163–2169 (doi:10.1242/jeb.02789) [DOI] [PubMed] [Google Scholar]
- Ruther J., Steiner S., Garbe L. A.20084-Methylquinazoline is a minor component of the male sex pheromone in Nasonia vitripennis. J. Chem. Ecol. 34, 1–4 (doi:10.1007/s10886-007-9411-1) [DOI] [PubMed] [Google Scholar]
- Sheldon B. C.1994Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc. R. Soc. Lond. B 257, 25–30 (doi:10.1098/rspb.1994.0089) [Google Scholar]
- Shuker D. M., West S. A.2004Information constraints and the precision of adaptation: sex ratio manipulation in wasps. Proc. Natl Acad. Sci. USA 101, 10 363–10 367 (doi:10.1073/pnas.0308034101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuker D. M., Pen I., Duncan A. B., Reece S. E., West S. A.2005Sex ratios under asymmetrical local mate competition: theory and a test with parasitoid wasps. Am. Nat. 166, 301–316 (doi:10.1086/432562) [DOI] [PubMed] [Google Scholar]
- Skinner A. M. J., Watt P. J.2007Phenotypic correlates of spermatozoon quality in the guppy Poecilia reticulate. Behav. Ecol. 18, 47–52 (doi:10.1093/beheco/arl049) [Google Scholar]
- Steiner S., Henrich N., Ruther J.2008Mating with sperm-depleted males does not increase female mating frequency in the parasitoid Lariophagus distinguendus. Entomol. Exp. Appl. 126, 131–137 (doi:10.1111/j.1570-7458.2007.00641.x) [Google Scholar]
- Steiner S., Ruther J.2009aHow important is sex for females of a haplodiploid species under local mate competition? Behav. Ecol. 20, 570–574 (doi:10.1093/beheco/arp033) [Google Scholar]
- Steiner S., Ruther J.2009bMechanism and behavioral context of male sex pheromone release in Nasonia vitripennis. J. Chem. Ecol. 35, 416–421 (doi:10.1007/s10886-009-9624-6) [DOI] [PubMed] [Google Scholar]
- Svensson M.1996Sexual selection in moths: the role of chemical communication. Biol. Rev. 71, 113–135 (doi:10.1111/j.1469-185X.1996.tb00743.x) [Google Scholar]
- Tregenza T., Wedell N.2000Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 9, 1013–1027 (doi:10.1046/j.1365-294x.2000.00964.x) [DOI] [PubMed] [Google Scholar]
- van den Assem J.1986Mating behaviour in parasitic wasps. In Insect parasitoids (eds Waage J., Greathead D.), pp. 137–167 London, UK: Academic Press [Google Scholar]
- Wagner W. E., Harper C. J.2003Female life span and fertility are increased by the ejaculates of preferred males. Evolution 57, 2054–2066 (doi:10.1554/02-548) [DOI] [PubMed] [Google Scholar]
- Wathes D. C., Abayasekara D. R. E., Aitken R. J.2007Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 77, 190–201 (doi:10.1095/biolreprod.107.060558) [DOI] [PubMed] [Google Scholar]
- Weinert J., Blomquist G. J., Borgeson C. E.1993De novo biosynthesis of linoleic acid in two non-insect invertebrates—the land slug and the garden snail. Experientia 49, 919–921 (doi:10.1007/BF01952610) [Google Scholar]
- Werren J. H.1980Sex ratio adaptions to local mate competition in a parasitic wasp. Science 208, 1157–1159 (doi:10.1126/science.208.4448.1157) [DOI] [PubMed] [Google Scholar]
- Whiting A. R.1967The biology of the parasitic wasp Mormoniella vitripennis. Q. Rev. Biol. 42, 333–406 (doi:org/10.1086/405402) [Google Scholar]
- Wiley R. H., Poston J.1996Perspective: indirect mate choice, competition for mates, and coevolution of the sexes. Evolution 50, 1371–1381 (doi:org/10.2307/2410875) [DOI] [PubMed] [Google Scholar]