Abstract
Apart from growing fungi for nutrition, as seen in the New World Attini, ants cultivate fungi for reinforcement of the walls of their nests or tunnel-shaped runway galleries. These fungi are grown on organic material such as bark, epiphylls or trichomes, and form stable ‘carton structures’. In this study, the carton of the runway galleries built by Azteca brevis (Formicidae, Dolichoderinae) on branches of Tetrathylacium macrophyllum (Flacourtiaceae) is investigated. For the first time, molecular tools are used to address the biodiversity and phylogenetic affinities of fungi involved in tropical ant carton architecture, a previously neglected ant–fungus mutualism.
The A. brevis carton involves a complex association of several fungi. All the isolated fungi were unequivocally placed within the Chaetothyriales by DNA sequence data. Whereas five types of fungal hyphae were morphologically distinguishable, our DNA data showed that more species are involved, applying a phylogenetic species concept based on DNA phylogenies and hyphal morphology. In contrast to the New World Attini with their many-to-one (different ant species—one fungal cultivar) pattern, and temperate Lasius with a one-to-two (one ant species—two mutualists) or many-to-one (different ant species share the same mutualist) system, the A. brevis–fungi association is a one-to-many multi-species network. Vertical fungus transmission has not yet been found, indicating that the A. brevis–fungi interaction is rather generalized.
Keywords: ant fungiculture, Azteca brevis, Chaetothyriales, multi-species network, nest-wall fungi, Tetrathylacium macrophyllum
1. Introduction
Ant–fungus associations in the New World fungus-growing ants (Myrmicinae: Attini) are well-studied examples of mutualism between ants and fungi; the ants involved are obligate agriculturists and cultivate basidiomycetes of the mushroom families Lepiotaceae and Pterulaceae for food (Mueller et al. 1998; Munkacsi et al. 2004). A less well-studied mutualism is the cultivation of fungi for carton constructions seen in Old World Lasius ants (Formicinae) (Elliott 1915; Maschwitz & Hölldobler 1970; Schlick-Steiner et al. 2008), arboricolous Asian ants of the genera Camponotus, Crematogaster, Dolichoderus, Monomorium and Technomyrmex (Weissflog 2001) and the Neotropical genus Allomerus (Dejean et al. 2005). The stabilization of the carton construction was first investigated in detail in nests of Lasius subgenera Dendrolasius and Chthonolasius, and seems to work in that the fungal mycelium ‘grows through the walls of the carton and reinforces them in the same way that steel mesh or rods reinforce the walls of a building’ (Hölldobler & Wilson 1990). The material used for the carton may be soil and shredded wood particles as in Lasius (Elliott 1915; Maschwitz & Hölldobler 1970), or cut trichomes bound together ‘with a compound that they [the ants] regurgitate’ as in Allomerus decemarticulatus (Myrmicinae) nesting obligately in the domatia of Hirtella physophora (Chrysobalanaceae; Dejean et al. 2005).
In nearly all cases, the associated fungi are not further investigated or classified. Dejean et al. (2005) stated that the carton walls of the galleries of Allomerus decemarticulatus are ‘reinforced by the mycelium of a complex of sooty-mould species that has been manipulated by the ants’.
The taxonomic position of the carton wall fungi found in Lasius subgenera Dendrolasius and Chthonolasius was only recently clarified as ascomycetes belonging to the Capnodiales, Chaetothyriales and Venturiaceae (Schlick-Steiner et al. 2008). Interestingly, Schlick-Steiner et al. (2008) found three species of Venturiaceae occurring exclusively and invariably with their respective hosts, and two other species of Chaetothyriales and Capnodiales occurring only occasionally in nests of both subgenera. There is some evidence that the latter two are not mutualists (Schlick-Steiner et al. 2008).
In the present study, the previously unknown carton structure of the runway galleries built by Azteca brevis Forel inhabiting live stems of Tetrathylacium macrophyllum (Flacourtiaceae) was examined. Apart from T. macrophyllum, A. brevis is found on Grias (Lecythidaceae), Licania (Chrysobalanaceae), Myriocarpa (Urticaceae), and Ocotea nicaraguensis (Lauraceae) (Longino 2008). Ants of the genus Azteca are known to subdivide pre-formed cavities with carton constructions into functional units using small platforms and baffles (Wheeler & Bequaert 1929; Longino 1996); A. brevis also builds extensive systems of galleries made of arched tunnels of a black, very crusty carton with small circular holes. The carton galleries and the holes, which are large enough for the workers to pass through, enhance a sophisticated hunting and defence strategy (Schmidt 2001). We demonstrate experimentally that the materials used for the construction of the galleries are chiefly particles found on the branches of the host tree. SEM images show that the particles are densely interwoven and stabilized by fungal hyphae. With the aid of light microscopy and DNA analyses, the phylogenetic affinities of the fungi involved in the carton nest construction of A. brevis are revealed. We can demonstrate for the first time for A. brevis that at least four different fungi coexist in the same carton gallery, which indicates that this mutualism involves more than one fungal partner per host and is a multi-species mutualism in respect not only of the host tree but also of the fungi used to stabilize the carton.
2. Material and methods
(a). Species and study site
Azteca brevis Forel (Formicidae, Dolichoderinae) is a species known only from the Pacific side of Costa Rica, and mostly from the wet forests of the southern Pacific lowlands. It nests in live stems of trees in polydomous colonies (more than one nest) with workers and brood found in many branch tips. The nesting chambers inside the stems are usually connected externally by an extensive system of runway galleries made of a characteristic black, crusty carton. Inside the stems and in the tunnels of the carton galleries A. brevis maintained large populations of an as yet undescribed species (P. Gullan 2004, personal communication) of the coccoid hemipteran genus Cryptostigma. The carton galleries investigated in this study were found on T. macrophyllum Poepp. & Endl. (Flacourtiaceae), a tree of approximately 15–20 m height occurring preferentially on steep slopes near rivers and streams in primary forests (Janzen 1983). It can be colonized by various ant species (Tennant 1989; Schmidt 2001), of which A. brevis is the most frequent inhabitant.
In 2007 and 2008, samples from eight different Tetrathylacium trees (four per year) were taken. Each tree was separated from the others by a walking distance of at least 10 min, therefore it is assumed that each tree was inhabited by a different colony. Pieces of the carton approximately 0.5–1.0 cm long were removed, placed in a paper bag using sterile forceps and dried at 30°C. Carton specimens from three different branches were sampled from each tree and ant colony, and each sample kept in a separate paper bag.
Due to contamination of the samples with Aspergillus and Penicillium, fungal isolates could only be grown successfully from three of the eight trees. CR07/2 and CR07/3 were collected in 2007 along the ‘waterfall trail’, while CR08/2 was collected in 2008 along the ‘ocelot trail’, both of which are within lowland primary rain forest near the Biological Research Station La Gamba (08°42′46″ N, 83°12′90″ W, 70 m.a.s.l.) in the vicinity of the Parque Nacional Piedras Blancas on the southern Pacific slope of Costa Rica. Voucher specimens of plants and ants were deposited at the Museo Nacional in San José, Costa Rica and in the Herbarium of the University of Vienna (WU Schaber 2860, Mayer 07-2, 07-3, 08-2).
(b). SEM and light microscopy (LM) investigation of carton nest structure and nest material
Carton nests were sputter coated in high vacuum mode at 15 kV and the inner and outer side investigated in a Phillips XL30 environmental scanning electron microscope. For light microscopy, freshly collected specimens were placed on a microscope slide, moistened with water and examined and documented without staining in a Zeiss AxioImager A1 compound microscope equipped with a Zeiss AxioCam ICc3 digital camera.
(c). Field experiments
To test whether particles from the branches’ surfaces are used for the construction of the carton, the following experiment was conducted. Ten easily accessible trees occupied by A. brevis were randomly selected. On two branches per tree, the carton tunnel was locally destroyed and a bright blue textile fibre adhesive tape (TESA, 19 mm width) was mounted (n = 20, total number of taped branches), placing the sticky tape on the bark surface of the tree. One week after mounting the tapes, the adhesive tapes were examined to determine whether the ants had removed fibres from the tape and incorporated the particles in their carton structure.
(d). Fungal cultures
Small parts of the dried carton tunnels were soaked in a drop of sterile water on a sterile microscope slide for approximately 5 min, and carefully broken up after soaking using fine forceps and preparation needles. The resulting mycelial suspension was pipetted onto a 2 per cent malt extract agar plate (MEA) containing antibiotics (0.5% penicillin and 0.5% streptomycin), and spread by gently rotating the plates. The plates were incubated at room temperature and visually checked at least daily under a dissecting microscope. Owing to the rapid growth of contaminating fungi (mainly Aspergillus and Penicillium spp.), especially during the initial phase of incubation, fast-growing mycelia with hyaline hyphae were immediately cut out from the plate. Upon germination, the hyphal fragments were examined under the microscope to ensure they originated from hyphae building the carton structure and transferred to a 2 per cent MEA plate. Water mounts of the mycelium of the cultures were examined with the light microscope to determine hyphal morphology and the presence of dispersal units (conidia).
(e). DNA extraction, amplification and sequencing
For DNA extraction, mycelium was grown in 1.5 ml reaction tubes containing 1 ml 2 per cent malt extract solution at room temperature for 14–25 days depending on growth rate. During incubation, the tubes were gently shaken on a horizontal shaker at 30 r.p.m. and regularly vortexed to avoid agglutination of the mycelia. After incubation, the tubes were centrifuged for 10 min at 13 000 r.p.m. to pellet the mycelium, the supernatant was removed, the pellet washed once with 3 × CTAB extraction buffer (75 mM Tris pH 8, 0.17 M sorbitol, 2 M sodium chloride, 15 mM EDTA, 3% CTAB, 4% PVP-40) and immediately processed for DNA extraction or stored at −20°C.
The mycelia were ground using sterile quartz sand and a micropestle fitting the tube. The DNA was extracted with the modified CTAB method described in Riethmüller et al. (2002), but using a high salt 3 × CTAB buffer (see above) with 1 per cent mercaptoethanol and two chloroform–isoamyl alcohol (24 : 1) extraction steps. After precipitation, the pellet was washed twice with 70 per cent aqueous ethanol, dried in a vacuum centrifuge, and the pellet dissolved in 50 µl 1 × TE buffer. One µl RNAse (5 mg/ml) was added, followed by incubation at 37°C for 60 min.
PCR was performed in 20 µl volumes: 1 µl of template DNA, 18 µl of 1.1 × ReddyMix PCR Master Mix (ABgene, Epsom, UK) and 0.25 µM forward and reverse primers. A ca 1.1 kb fragment of the nuclear small subunit ribosomal DNA was amplified using the primers NS1 and NS4 (White et al. 1990) or nssu1088 (Kauff & Lutzoni 2002). A 1.5–1.9 kb fragment containing the 5′ end of the small subunit, the complete ITS1-5.8S-ITS2 and the variable D1 and D2 domains of the large subunit of the nuclear ribosomal DNA were amplified with primers V9G (de Hoog & Gerrits van den Ende 1998) or ITS5 (White et al. 1990) and LR5 (Vilgalys & Hester 1990). The PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994): 20 µl PCR reactions were digested with 10 U Exonuclease I (Fermentas, St. Leon-Rot, BRD) and 2 U Calf Intestine Alkaline Phosphatase (Fermentas) for 30 min at 37°C, followed by an enzyme deactivation step at 85°C for 15 min. DNA was sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington) with the same primers as for PCR; in addition, fITS5.8Sr (TGCGTTCAAARATTCGATG), fITS5.8Sf (CAACAACGGATCTCTTGGYTC), ITS4 (White et al. 1990), LR0R (Rehner & Samuels 1994) and LR3 (Vilgalys & Hester 1990) were used as internal primers for the long SSU-ITS-LSU fragment. Sequences were analysed with an automated DNA sequencer (3130xl Genetic Analyzer, Applied Biosystems).
(f). Analysis of sequence data
For the phylogenetic analyses of the nuSSU rDNA (SSU), the nuLSU rDNA (LSU) and the complete ITS1-5.8S rDNA-ITS2 (ITS) regions, a representative sample of most similar sequences were selected from GenBank after a BLAST search; representative sequences from other lineages were also added. Sequence alignments were produced with Muscle v. 3.6 (Edgar 2004). After exclusion of leading/trailing gap regions and large insertions in some sequences, 938 characters remained across 35 sequences in the nuLSU rDNA alignment. One thousand and sixty six characters across 34 sequences of the nuSSU rDNA alignment and 765 characters across 95 sequences of the complete ITS alignment were included in the subsequent phylogenetic analyses. The resulting alignments were checked and refined using BioEdit v. 7.0.9.0 (Hall 1999).
Maximum parsimony (MP) analyses of the data matrices were performed with PAUP* v. 4.0 b10 (Swofford 2002), using 1000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, COLLAPSE=MAXBRLEN, steepest descent option not in effect, and limiting the number of rearrangements per replicate to 500 000 in the ITS dataset). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data. Bootstrap analysis with 1000 replicates was performed in the same way, but using 10 rounds of random sequence addition and subsequent branch swapping during each bootstrap replicate.
For Bayesian analyses, the program MrBayes (v. 3.1.2; Huelsenbeck & Ronquist 2001) was used. According to the nucleotide substitution models suggested by Modeltest 3.6 (Posada & Crandall 1998), the general time reversible model was implemented for the three (LSU, SSU, ITS) datasets, assuming a proportion of invariant sites with the remaining sites having substitution rates drawn from a gamma distribution (GTR+I+Γ). Three parallel runs of four incrementally heated simultaneous Markov chains were performed over 1 million generations from which every 100th tree was sampled in each run. Trees saved before the cold chain reached the stationary stage were discarded as burn-in (300 for LSU, 500 for SSU, 1000 for ITS). A 90 per cent majority rule consensus of the remaining trees was computed, giving estimates for the probabilities that groups are monophyletic given the sequence data (posterior probabilities).
3. Results
(a). Carton structure and construction
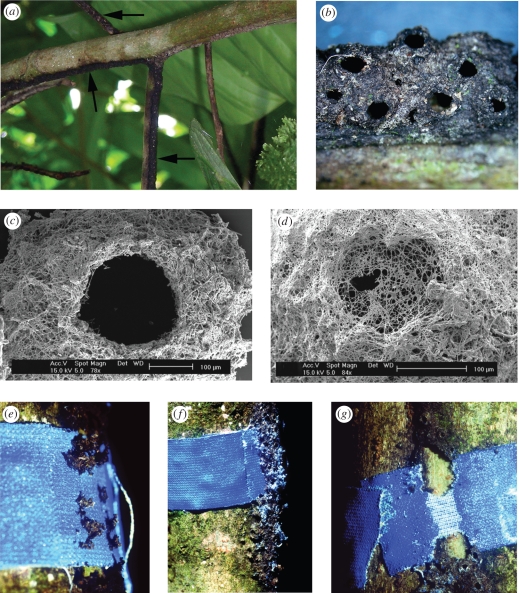
Extensive carton galleries were seen to cover the natural cavities of T. macrophyllum formed through partial pith degeneration of the secondary branches. The tissue surrounding the cavity was scarified on one side of the branch, with a slit making the cavity easily accessible to ants. A. brevis occupied all the domatium chambers available within its territory and excavated the remaining pith inside the living stem to form domatia with a maximum length of more than 1 m (Schmidt 2001). The carton galleries take the form of arched tunnels, ca 3 mm broad and 2 mm high, which run along the lower sides of branches (figure 1a). To construct the gallery, A. brevis workers regurgitate a pulp to construct two rows of lateral pillars spaced 1 mm apart along the lower surface of young branches. Adjacent pillars are then connected by an arch. The galleries contain holes, each surrounded by a funnel-shaped ring with an inner diameter of 0.9 (±0.2) mm (figure 1b,c). Newly added construction material was a light brown colour which, after a few days, turned black and had a distinctive tar-like odour. Even newly produced pillars were observed to be structurally stable, since those located on the lateral surface of the branch resisted heavy rains during the construction period. SEM investigations of the carton structure showed the fibre particles to be woven into a dense net of fungal hyphae. Although it is difficult to quantify the absolute ratio, detailed LM investigations revealed that, upon fungal colonization, the carton structure consists mainly of fungal biomass. Few Cryptostigma sp. coccids were tended in the carton galleries, the majority being maintained in the domatia of the hollow branches.
Figure 1.
The carton tunnels built by Azteca brevis ants. (a) Branches of Tetrathylacium macrophyllum with the black carton tunnels on the lower side. (b) The galleries contain numerous holes with an inner diameter of ca 0.9 mm. (c) SEM picture of a hole in an colonized part of the gallery. (d) A hole overgrown by hyphae in an abandoned part of the gallery. (e–g) The textile fibre tape experiment shows that A. brevis uses fibres from the tape to reconstruct the destroyed gallery (see text for further details).
(b). Carton material
When a textile fibre tape was placed around branches with a carton runway (n = 20), A. brevis workers reconstructed the destroyed carton gallery over the textile fibre tape within one week (figure 1e,f). After removing the newly built carton, only a minority of the tapes was intact or only slightly removed. In 85 per cent of the cases, A. brevis removed much or all of the tape (figure 1g). On all taped branches, the reconstructed part of the carton gallery was spotted with bright blue fibre particles, which seemed to be the main source of construction material in this area (figure 1f). On branches where the tape was entirely removed, the fibres could be found incorporated into parts of the gallery up to 1 m away from where the tape was originally placed.
(c). Mycelium description
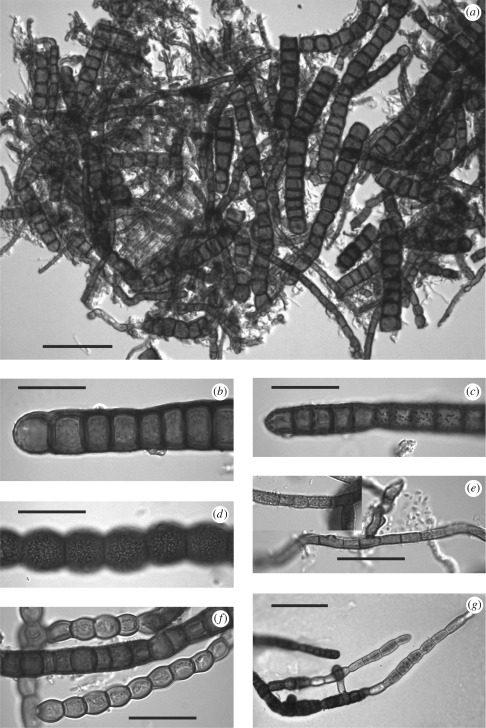
The fungal mycelium mainly consists of tightly interwoven, ramifying, mostly moniliform fuscous hyphae (figure 2a). SEM and LM investigation showed that the mycelia are composed of complex associations of different types of hyphae. The following hyphal types could be distinguished morphologically: (i) rarely branched, slightly moniliform hyphae, 9–17 µm in diameter, composed of regular, dull to dark brown, isodiametric or shorter than wide cells with smooth-to-slightly verrucose walls (figure 2b); (ii) non-moniliform to slightly moniliform hyphae, frequently branched at right angles, sometimes but not always constricted at the septa, 5–11 µm in diameter, composed of distinctly red-brown, isodiametric to elongated cells with slightly to strongly verrucose walls (figure 2c,f,g); (iii) strongly moniliform hyphae, 9–16 µm in diameter, composed of distinctly globose, dark reddish brown cells with strongly reticulate-verrucose walls (figure 2d); (iv) non-moniliform, frequently branched hyphae, 2.5–5 µm in diameter, composed of brown, distinctly elongated, smooth to verrucose cells (figure 2e); and (v) strongly moniliform hyphae frequently branched at right angles, 5–8 µm in diameter, strongly constricted at septa, composed of light reddish brown, globose to subglobose cells with smooth cell walls (figure 2f). Hyphal types 1 and 2 predominated in all samples examined, while types 3–5 were less frequent. No sporulation was observed on the natural substrate.
Figure 2.
Light microscopy of the various morphological types of fungi colonizing the tunnels. (a) Overview of a squash mount, showing fungal hyphae of types 1, 2 and 5. (b) Hyphal type 1; note cells being as broad as or broader than long and the dull, dark brown, smooth cell walls. (c) Hyphal type 2; note the cells as long as or longer than broad and the distinctly reddish brown, verrucose cell wall. (d) Hyphal type 3; note the dark reddish brown globose cells with reticulate-verrucose ornamentation. (e) Hyphal type 4; note the comparatively thin, brown, elongated cells. (f) Hyphal types 2 (centre) and 5 (above and below); note the strongly moniliform, subglobose cells and the brown, smooth cell wall of type 5. (g) Germinating hyphae of type 2. Bars: a, G 50 µm, b–f, 20 µm.
It is evident that the hyphae are trimmed to avoid disorganized growth and the rims of the pores in particular indicate that these are cut by the ants (figure 1c). Pores in abandoned parts of the gallery become closed by a membrane-like mycelium of thin and poorly septate hyphae (figure 1d). Although trimmed, the fungi do not seem to be eaten by the ants. Microscopical inspection of 10 crushed worker heads per tree revealed no hyphal fragments in the contents of their buccal pocket. The mycelium is epiphytic and does not invade the host plant.
(d). Isolation of fungi in pure culture
Pure cultures could be obtained from hyphal types 1, 2, 4 and 5, while type 3 failed to grow. Few cultures per sample could be obtained from the mycelial suspensions. This may be because of sub-optimal preservation of the material after collecting in the field (high humidity and temperature) as well as the slow germination and growth of the fungi. In all attempts at isolation, fast-growing fungal contaminants (mostly from the genera Aspergillus and Penicillium) had to be continually removed to avoid rapid overgrowth of the agar plate. All cultures produced dark brown to blackish slow-growing mycelia with abundant aerial mycelium. The hyphae were similar to those observed on the tunnels, although there were some differences in pigmentation, surface ornamentation and hyphal diameter; such differences are commonly observed in fungi grown on artificial media. With the exception of culture CR07/2-2, the mycelia did not produce conidia or fruiting bodies in pure culture.
(e). Phylogenetic affiliation and classification of the fungi
Except for one isolate (CR07/2-2), which may be assigned morphologically to the anamorph genus Cladophialophora, the cultures did not sporulate in pure culture and could not therefore be formally classified. However, their phylogenetic affiliation could be clarified unequivocally with DNA data.
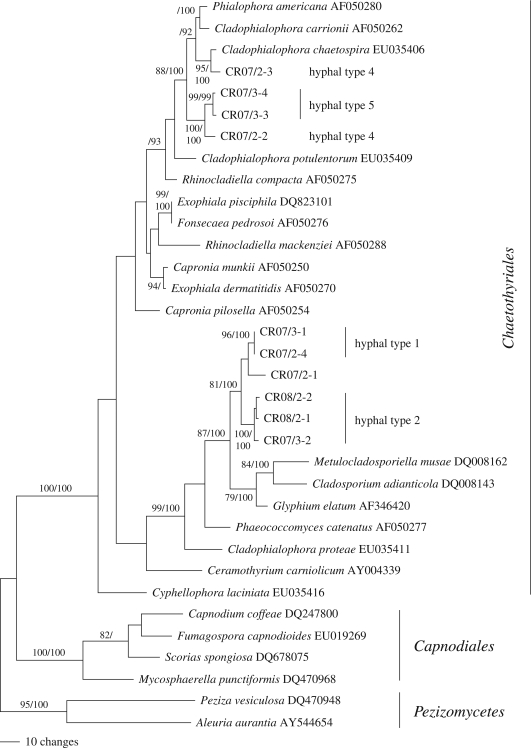
The nuLSU, the nuSSU rDNA and the ITS1-5.8S rDNA-ITS2 matrices contained 218, 116 and 354 parsimony-informative characters, respectively. The maximum parsimony analysis of the nuLSU rDNA revealed a single most parsimonious tree of score 800, which is shown in figure 3. Analysis of the nuSSU rDNA data revealed four equally most parsimonious trees of score 351 (figure S1 in the electronic supplementary material). Maximum parsimony analysis of the complete ITS1-5.8S rDNA-ITS2 matrix resulted in 16 712 trees of score 1765, one of which is shown as a phylogram in figure S2 in the electronic supplementary material. Bayesian analyses revealed trees fully compatible with the MP trees. The nuLSU, nuSSU and ITS1-5.8S rDNA-ITS2 data matrices and the corresponding trees have been deposited at TreeBASE (http://www.treebase.org/treebase/) as S2423.
Figure 3.
Phylogram showing the single most parsimonious tree of 800 steps revealed by an MP analysis of 938 characters of the nuLSU rDNA alignment of representative sequences of Chaetothyriales, Capnodiales and Pezizomycetes (outgroup), demonstrating the phylogenetic affinities of the fungi isolated from Azteca carton tunnels. MP bootstrap and Bayesian posterior probability values above 70 per cent and 90 per cent, respectively, are given above or below the branches. Numbers following taxon names denote GenBank accession numbers. If known, the corresponding hyphal types are given for the isolates from Azteca tunnels.
The molecular investigations of both SSU and LSU rDNA sequence alignments placed all isolates unequivocally into the Chaetothyriales (Ascomycetes) with high internal support (figure S3, figure S1 in the electronic supplementary material). In both datasets, the fungal isolates formed three distinct clades within the Chaetothyriales, which corroborated the ITS rDNA tree (figure S2 in the electronic supplementary material).
Applying a phylogenetic species concept (Donoghue 1985) to the DNA phylogenies and hyphal morphology, the isolated cultures belonged to at least six species (species 1: CR07/2-3 with hyphal type 4; species 2 (Cladophialophora sp.): CR07/2-2 with hyphal type 4; species 3: CR07/3-3 and CR07/3-4 with hyphal type 5; species 4: CR07/2-4 and CR07/3-1 with hyphal type 1; species 5: CR07/2-1 with undefined hyphal type; species 6: CR07/3-2, CR08/2-1 and CR08/2-2 with hyphal type 2). Including the uncultured hyphal type 3, at least seven species are therefore present on Azteca carton nests.
4. Discussion
This is the first investigation using molecular tools that addresses the biodiversity as well as the phylogenetic affinities of fungi involved in the construction of ant carton tunnels in the tropics. While the microscopic investigations of the tunnel material alone clearly showed that the colonized carton material involves a complex association of several morphological types of fungal hyphae (figure 2), the isolation in pure culture and subsequent molecular analyses of fungal cultures allowed a more detailed evaluation of systematic affinities and biodiversity of the fungi involved. Remarkably, despite showing quite distinct morphological features in culture, all the isolated fungi were placed unequivocally within the Chaetothyriales in the molecular analyses (figure S3, figures S1 and S2 in the electronic supplementary material). This order currently contains two families (Chaetothyriaceae and Herpotrichiellaceae) and is ecologically unusual. Its members are primarily saprotrophic, and numerous species are extremophiles colonizing nutrient-poor substrates like rocks and living leaves; others are plant parasites or important animal and human pathogens causing chromoblastomycoses and phaeohyphomycoses (Cannon & Kirk 2007). Apart from the important pathogens, however, little is known in detail about the overall biodiversity and ecology, and the order is in need of detailed taxonomic revision.
The DNA data for the different isolates indicate that numerous related species are involved in the construction of the carton tunnels. While five primary types of fungal hyphae could be distinguished morphologically, the cultures isolated during the present study belonged to at least 6 species using a phylogenetic species concept. Sequence divergence of ITS (see branch lengths in figure S2 in the electronic supplementary material) and cultural characteristics indicate that an even higher species number (up to 9) may be involved, but this needs additional investigation including more isolates and sequence data. Hyphal type 4 may comprise several species, as similar hyphae showing little morphological differentiation are commonly observed in various lineages of fungi. Only the cultures of hyphal type 1 (CR07/2-4, CR07/3-1), the most common hyphae on the carton, clearly form a single species, since their ITS-LSU sequences are identical.
The species isolated from different samples only overlapped to a minor extent; this may be largely owing to the few samples investigated and the methodological difficulties involved in obtaining pure cultures. From the current data, we predict that the full spectrum of fungi present on the ant tunnels has not yet been isolated. However, the two most important hyphal types 1 and 2 were grown in culture several times each.
The simultaneous presence of several species involved in the construction of the carton tunnels suggests a widely generalized relationship between Azteca and their fungal symbionts. This is a marked difference between Azteca and the leaf-cutting ants of the genera Atta and Acromyrmex which cultivate a single biological species of fungus (Mikheyev et al. 2006, 2007; Schultz & Brady 2008). It is also in contrast to Lasius, which show a one-to-two (one ant species with two fungal mutualists) or many-to-one (different ant species share the same fungal mutualist) pattern (Schlick-Steiner et al. 2008). The A. brevis–fungi association is a one-to-many multi-species network.
There are also further differences between Azteca and Attini. There is no indication that Azteca uses its fungal symbionts for nutrition. Contrary to previous authors in the early twentieth century (Emery 1899; Lagerheim 1900; Ferdinandsen & Winge 1908), Bailey (1920) stated that there is no indication that ants are fungivorous, other than the Attini, even though many other ants are closely associated with fungi. Detailed observations confirmed that neither Lasius fuliginosus nor other ants with fungal carton structures seem to eat the hyphae (Maschwitz & Hölldobler 1970; Weissflog 2001). Asian Technomyrmex sp. colonies kept only with nest material rich in fungi and water died from starvation, whereas those kept with a honey solution survived (Weissflog 2001). The unsuitability of the fungi growing in and on the carton tunnels for consumption is evident from the morphology of the hyphae, which are thick-walled, darkly pigmented and therefore difficult to digest. It is, however, clear that A. brevis workers constantly groom the hyphae to prevent the carton tunnels and entrance holes from being overgrown, as happens in abandoned or infrequently visited parts (figure 1c,d). The main role of the fungi appears to be, therefore, to increase the stability of the building material used for the construction of the carton tunnels rather than to provide nutrition. Such reinforcement is important for superficial structures exposed to heavy rain in tropical climates.
Azteca brevis ants were not particular in their choice of material used for the carton construction, as our experiments with adhesive tape showed. It can include bark from the host tree, shredded epiphylls, small particles of epiphytes and even shredded fibres of adhesive tape. It is not yet known whether the fungi are nourished with carbohydrates (e.g. of the exudates of Cryptostigma coccids found in the tunnels), as in Lasius fuliginosus (Maschwitz & Hölldobler 1970), or with the ants’ faeces, as in tropical Technomyrmex sp. and Crematogaster sp. ants (Weissflog 2001). However, the microscopic investigations provide evidence that their main source of carbon is the organic particles of the carton structure itself, which is subsequently replaced by the fungal hyphae. Though the substrate seems to be suitable for many fungi, the manner of management and maintenance by the ants is likely to determine the fungal composition.
The use of fungi to stabilize carton nest structures has long been known for some temperate species of Lasius (Lagerheim 1900; Elliott 1915; Maschwitz & Hölldobler 1970), and has recently been investigated in detail by Schlick-Steiner et al. (2008). For palaeotropic arboricolous ants, Weissflog (2001) found that 70 per cent of old Technomyrmex nests and 50 per cent of Monomorium nests were almost entirely built from fungi, but unfortunately the fungi were not identified. Similarly, in some Crematogaster, Camponotus and Dolichoderus species, fungal hyphae seem to be used for nest stabilization (Weissflog 2001), thus indicating that this is a characteristic that has evolved more than once independently.
For a long time, it was thought that the carton nest fungus cultivated by Lasius belonged to a single species (for ‘pure cultures’, see Elliott 1915), with the two subgenera culturing one each (Elliott 1915; Maschwitz & Hölldobler 1970). Schlick-Steiner et al. (2008) recently isolated several species from the carton structures and found a different pattern of ant-to-fungus specificity. In subgenus Dendrolasius, a one-to-two specifity was observed, whereas Chthonolasius displayed a many-to-one specifity, as found in the Attini (Mikheyev et al. 2006). In addition, non-mutualistic fungal species are present at lower frequencies that are apparently controlled by the ants to protect their mutualists. The occurrence of a one-to-many system occurring on the same carton material, as in A. brevis, has not previously been proved. A remarkable difference between the mutualistic fungi of Lasius and Azteca concerns, however, their systematic affiliation. Whereas the mutualistic fungi grown by Lasius belong to or near the Venturiaceae, all fungi isolated from the Azteca carton tunnels cluster within the Chaetothyriales. Among others, important habitats for Chaetothyriales are plant leaf surfaces, where they apparently grow saprotrophically, a niche which is especially prominent in the tropics. The preparation of the infrabuccal pocket, a filtering structure within the oral cavity, did not reveal conidia or hyphal fragments. However, as only 10 workers per tree were investigated, this evidence has to be considered cautiously. In future studies, a higher proportion of the colony, including young queens before their nuptial flight, will need to be investigated to determine whether they take hyphal fragments from their home nest to new nest sites. We assume that the fungi from the Azteca tunnels originate from the surface mycobiota of the leaves or bark that provides the construction material. Epifoliar fungi were found on approximately 29 per cent of the plant species in the canopy of a Panamanian rainforest (Gilbert et al. 2007) and are an abundant component of tropical forest communities. Presumably most—if not all—of the carton wall fungi were taken into culture by the ants, and the transmission of the carton fungi may be horizontal. In the Attini, vertical transmission of the hyphae was found (von Ihering 1898; Huber 1905), with frequent horizontal transmission between ant species and recombination between cultivars in different nests (Bot et al. 2001; Green et al. 2002; Mikheyev et al. 2006, 2007). In Lasius, the transmission is also vertical and seems sometimes to be augmented by horizontal transmission (Schlick-Steiner et al. 2008). However, as little is known about the biodiversity and ecology of plant surface mycobiota in general, and from the current study area in particular, additional investigations are needed to test whether the fungi occur without the ants or whether the ant–fungus association is more specific, with at least some of the fungi being confined to the carton tunnels. The stabilization of the carton walls can be provided by several fungal species, and a multi-species system may have the advantage of increased stability under variable environmental conditions.
Multi-species networks often occur in mutualisms between free-living organisms and there is strong evidence that the degree of specificity tends to be strikingly asymmetrical (Bascompte et al. 2003, 2006; Guimarães et al. 2006). In the A. brevis–fungi association, we do not yet know which side of the interaction is the more specialized.
Acknowledgements
We thank J. Longino and P. Gullan for their identification of A. brevis and the Cryptostigma coccids inhabiting T. macrophyllum, respectively. M. R. Schmidt is thanked for performing the experiments with the Tesa tape in the field and the photos of figure 1e–g. We also thank C. J. Dixon for improving the English of our manuscript.
References
- Bailey I. W.1920Some relations between ants and fungi. Ecology 1, 174–189 (doi:10.2307/1929134) [Google Scholar]
- Bascompte J., Jordano P., Melián C. J., Olesen J. M.2003The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA 100, 9383–9387 (doi:10.1073/pnas.1633576100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J., Jordano P., Olesen J. M.2006Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312, 431–433 (doi:10.1126/science.1123412) [DOI] [PubMed] [Google Scholar]
- Bot A. N. M., Rehner S. A., Boomsma J. J.2001Partial incompatibility between ants and symbiotic fungi in two sympatric species of Acromyrmex leaf-cutter ants. Evolution 55, 1980–1991 (doi:10.1111/j.0014-3820.2001.tb01315.x) [DOI] [PubMed] [Google Scholar]
- Cannon P. F., Kirk P. M.2007Fungal families of the world Wallingford, UK: CABI International [Google Scholar]
- de Hoog G. S., Gerrits van den Ende A. H. G.1998Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41, 183–189 (doi:10.1111/j.1439-0507.1998.tb00321.x) [DOI] [PubMed] [Google Scholar]
- Dejean A., Solano P. J., Ayroles J., Corbara B., Orivel J.2005Insect behaviour: arboreal ants build traps to capture prey. Nature 434, 973 (doi:10.1038/434973a) [DOI] [PubMed] [Google Scholar]
- Donoghue M. J.1985A critique of the biological species concept and recommendations for a phylogenetic alternative. Bryologist 88, 172–181 (doi:10.2307/3243026) [Google Scholar]
- Edgar R. C.2004MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 32, 1792–1797 (doi:10.1093/nar/gkh340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. S. B.1915Fungi in the nests of ants. Trans. Brit. Mycol. Soc. 5, 138–142 [Google Scholar]
- Emery M. C.1899Végétarianisme chez les fourmis. Arch. Sci. Phys. Nat. 8, 488–490 [Google Scholar]
- Ferdinandsen C., Winge O.1908Fungi from the Danish West Indies collected 1905–1906. Bot. Tidskr. 29, 1–25 [Google Scholar]
- Gilbert G. S., Reynolds D. R., Bethancourt A.2007The patchiness of epifoliar fungi in tropical forests: host range, host abundance, and environment. Ecology 88, 575–581 (doi:10.1890/05-1170) [DOI] [PubMed] [Google Scholar]
- Green A. M., Mueller U. G., Adams M. M.2002Extensive exchange of fungal cultivars between sympatric species of fungus-growing ants. Mol. Ecol. 11, 191–195 (doi:10.1046/j.1365-294X.2002.01433.x) [DOI] [PubMed] [Google Scholar]
- Guimarães P. R., Rico-Gray V., Furtado dos Reis S., Thompson J. N.2006Asymmetries in specialization in ant–plant networks. Proc. R. Soc. B 273, 2041–2047 (doi:10.1098/rspb.2006.3548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. A.1999BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 41, 95–98 [Google Scholar]
- Hölldobler B., Wilson E. O.1990The fungus growers. Ant–fungus symbioses outside the Attini. In The Ants, p. 607 Cambridge, MA: Belknap University Press [Google Scholar]
- Huber J.1905Über die Koloniegründung bei Atta sexdens. Biol. Centbl. 25, 606–619, 625–635 [Google Scholar]
- Huelsenbeck J. P., Ronquist F.2001MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- Janzen D. H. (ed.) 1983Costa Rican natural history Chicago, IL: The University of Chicago Press [Google Scholar]
- Kauff F., Lutzoni F.2002Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Mol. Phyl. Evol. 25, 138–156 (doi:10.1016/S1055-7903(02)00214-2) [DOI] [PubMed] [Google Scholar]
- Lagerheim G.1900Über Lasius fuliginosus und seine Pilzzucht. Entomol. Tidskr. 21, 17–29 [Google Scholar]
- Longino J. T.1996Taxonomic characterization of some live-stem inhabiting Azteca (Hymenoptera: Formicidae) in Costa Rica, with special reference to the ants of Cordia (Boraginaceae) and Triplaris (Polygonaceae). J. Hym. Res. 5, 131–156 [Google Scholar]
- Longino J. T.2008The ants of Costa Rica. http://academic.evergreen.edu/projects/ants/AntsofCostaRica.html
- Maschwitz U., Hölldobler B.1970Der Kartonnestbau bei Lasius fuliginosus Latr. (Hym. Formicidae). Z. vergl. Physiol. 66, 176–189 (doi:10.1007/BF00297777) [Google Scholar]
- Mikheyev A. S., Mueller U. G., Abbot P.2006Cryptic sex and many-to-one coevolution in the fungus-growing ant symbiosis. Proc. Natl Acad. Sci. USA 103, 10 702–10 706 (doi:10.1073/pnas.0601441103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheyev A. S., Mueller U. G., Boomsma J. J.2007Population genetic signatures of diffuse co-evolution between leaf-cutting ants and their cultivar fungi. Mol. Ecol. 16, 209–216 (doi:10.1111/j.1365-294X.2006.03134.x) [DOI] [PubMed] [Google Scholar]
- Mueller U. G., Rehner S. A., Schultz T. R.1998The evolution of agriculture in ants. Science 281, 2034–2038 (doi:10.1126/science.281.5385.2034) [DOI] [PubMed] [Google Scholar]
- Munkacsi A. B., Pan J. J., Villesen P., Mueller U. G., Blackwell M., McLaughlin D. J.2004Convergent coevolution in the domestication of coral mushrooms by fungus-growing ants. Proc. R. Soc. Lond. B 271, 1777–1782 (doi:10.1098/rspb2004.2759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D., Crandall K. A.1998Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818 (doi:10.1093/bioinformatics/14.9.817) [DOI] [PubMed] [Google Scholar]
- Rehner S. A., Samuels G. J.1994Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 98, 625–634 (doi:10.1016/S0953-7562(09)80409-7) [Google Scholar]
- Riethmüller A., Voglmayr H., Göker M., Weiß M., Oberwinkler F.2002Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia 94, 834–849 (doi:10.2307/3761698) [DOI] [PubMed] [Google Scholar]
- Schlick-Steiner B. C., Steiner F. M., Konrad H., Seifert B., Christian E., Moder K., Stauffer C., Crozier R. H.2008Specificity and transmission mosaic of ant nest wall fungi. Proc. Natl Acad. Sci. USA 105, 941–944 (doi:10.1073/pnas.0708320105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. R.2001Interactions between Tetrathylacium macrophyllum (Flacourtiaceae) and its live-stem inhabiting ants. Austria: University of Vienna [Google Scholar]
- Schultz T. R., Brady S. G.2008Major evolutionary transitions in ant agriculture. Proc. Natl Acad. Sci. USA 105, 5435–5440 (doi:10.1073/pnas.0711024105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D. L.2002PAUP*: phylogenetic analysis using parsimony (*and other methods), Version 4.0b10. Sunderland, MA: Sinauer Associates [Google Scholar]
- Tennant (Alonso) L. E.1989A new ant–plant, Tetrathylacium costaricense. Symposium: interactions between ants and plants, p. 27 Oxford [Google Scholar]
- Vilgalys R., Hester M.1990Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ihering H.1898Die Anlagen neuer Colonien und Pilzgärten bei Atta sexdens. Zool. Anz 21, 238–245 [Google Scholar]
- White T. J., Bruns T., Lee S., Taylor J.1990Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols: a guide to methods and applications (eds Innis M. A., Gelfand D. H., Sninsky J. J., White T. J.), pp. 315–322 San Diego, CA: Academic Press [Google Scholar]
- Weissflog A.2001Freinestbau von Ameisen (Hymenoptera, Formicidae) in der Kronenregion feuchttropischer Wälder Südostasiens. Bestandsaufnahme und Phänologie, Ethoökologie und funktionelle Analyse des Nestbaus Main, Germany: J. W. Goethe University Frankfurt am Main [Google Scholar]
- Werle E., Schneider C., Renner M., Völker M., Fiehn W.1994Convenient single-step, one tube purification of PCR products for direct sequencing. Nucl. Acids Res. 22, 4354–4355 (doi:10.1093/nar/22.20.4354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler W. M., Bequaert J. C.1929Amazonian myrmecophytes and their ants. Zool. Anz. 82, 10–39 [Google Scholar]