Abstract
Little is known about how fishes and other non-calcifying marine organisms will respond to the increased levels of dissolved CO2 and reduced sea water pH that are predicted to occur over the coming century. We reared eggs and larvae of the orange clownfish, Amphiprion percula, in sea water simulating a range of ocean acidification scenarios for the next 50–100 years (current day, 550, 750 and 1030 ppm atmospheric CO2). CO2 acidification had no detectable effect on embryonic duration, egg survival and size at hatching. In contrast, CO2 acidification tended to increase the growth rate of larvae. By the time of settlement (11 days post-hatching), larvae from some parental pairs were 15 to 18 per cent longer and 47 to 52 per cent heavier in acidified water compared with controls. Larvae from other parents were unaffected by CO2 acidification. Elevated CO2 and reduced pH had no effect on the maximum swimming speed of settlement-stage larvae. There was, however, a weak positive relationship between length and swimming speed. Large size is usually considered to be advantageous for larvae and newly settled juveniles. Consequently, these results suggest that levels of ocean acidification likely to be experienced in the near future might not, in isolation, significantly disadvantage the growth and performance of larvae from benthic-spawning marine fishes.
Keywords: ocean acidification, climate change, hypercapnia, growth rate, critical swimming speed, coral reef fish
1. Introduction
Approximately 30 per cent of the additional carbon dioxide (CO2) released into the atmosphere during the past 200 years from human activities has been absorbed by the oceans (Sabine et al. 2004). Additional CO2 reacts with water to form carbonic acid, which through a series of reactions leads to a decline in pH and a shift in the carbonate–bicarbonate ion balance (Feely et al. 2004), a process known as ocean acidification. Global ocean pH is estimated to have dropped 0.1 U since pre-industrial times and is predicted to drop another 0.3–0.4 U by 2100 owing to existing and future emission of CO2 into the atmosphere (Caldeira & Wickett 2005; Royal Society 2005; Meehl et al. 2007). A decline of this magnitude would make the ocean more acidic than at any time in the past 650 000 years (Feely et al. 2004) and the rate of decline approximately 100 times faster than at any time during the same period (Royal Society 2005; Hoegh-Guldberg et al. 2007; Fabry et al. 2008). It is widely recognized that reduced carbonate ion saturation states that accompany ocean acidification could have significant impacts for many calcifying organisms, especially corals and other invertebrates that precipitate aragonite skeletons (Feely et al. 2004; Orr et al. 2005; Kleypas et al. 2006; Hoegh-Guldberg et al. 2007; Anthony et al. 2008). In contrast, the range of impacts that ocean acidification will have on other marine organisms remains poorly understood (Fabry et al. 2008; Munday et al. 2008).
Increased levels of dissolved CO2 not only acidify the ocean, they also act to decrease the pH of animal tissue (Pörtner et al. 2004). When exposed to high levels of CO2 (hypercapnia) and low pH, many organisms can regulate their acid–base balance by intra- and extracellular bicarbonate buffering and active ion transport (Pörtner et al. 2005). However, these mechanisms may have long-term consequences for individual performance due to their energetic cost, or because they affect the function of other physiological processes (e.g. Michaelidis et al. 2005, 2007). Alternatively, incomplete regulation of acid–base balance can directly affect the efficiency of cellular activities, with potential long-term effects for growth and reproduction (Pörtner et al. 2005). Much of the past research on hypercapnia has been conducted at CO2 concentrations too high to be considered relevant for predicting the effects of ocean acidification; however, several recent studies have shown that levels of CO2-induced acidification that could occur within the next 100 years can affect the growth and development of some marine invertebrates (e.g. Kurihara & Shirayama 2004; Shirayama & Thornton 2005; Havenhand et al. 2008). Consequently, there is concern that continued increases in atmospheric CO2 over the next century could have significant impacts on a wide range of marine species, not just those with calcified skeletons (Fabry et al. 2008; Widdicombe & Spicer 2008).
In general, fishes appear to be relatively tolerant to mild increases in CO2 and decreases in pH (Ishimatsu et al. 2005, 2008). Fish control their tissue pH by bicarbonate buffering and the exchange of ions, mostly across the gills, and small changes in internal or external pH can readily be compensated (Heisler 1989; Claiborne et al. 2002). Although these compensatory mechanisms are not detrimental in the short term, ultimately they might have some physiological consequences, especially for species or life stages with high metabolic demands (Pörtner & Farrell 2008). Fish embryos and young larvae are usually more sensitive to pH changes than are juveniles and adults (Brown & Sadler 1989) and, thus, significant effects of ocean acidification are most likely to be detected in these early life stages.
Although ocean acidification could lead to reduced individual performance due to the effects of hypercapnia and low pH on cellular function, Dockray et al. (1998) found that rainbow trout exposed to water treated with an acid (H2SO4) had higher growth rates and higher energy conversion efficiency than fish in control water. Other experiments revealed that acid-exposed fish were able to compensate for additional branchial ion losses (a problem for freshwater fishes) with increased intake of dietary salts, and that an increased appetite could potentially explain the increased growth rates at low pH (Morgan et al. 2001). Currently, it is unknown whether levels of ocean acidification that could occur over the next 100 years will have negative effects on the early life-history traits of marine fishes (Ishimatsu et al. 2008; Munday et al. 2008), or whether CO2-induced acidification of sea water could potentially have a similar positive effect on growth rate to that observed in acid-exposed freshwater fishes.
We tested the effects of CO2-induced ocean acidification on the embryonic and larval life histories of a tropical marine fish, the orange clownfish Amphiprion percula. We used a range of treatments relevant to predicted future atmospheric CO2 concentrations (current day, 550, 750 and 1030 ppm). The upper levels of our treatments (750 and 1030 ppm CO2) were based on the IPCC A2-SRES emission trajectory, in which atmospheric CO2 concentrations are predicted to range between 730 and 1020 ppm in the year 2100 and ocean pH is predicted to decline by 0.3–0.4 U between 2000 and 2100 (Meehl et al. 2007). We also included a more conservative treatment (550 ppm) to represent atmospheric CO2 concentrations that would be reached by mid-century on the A2-SRES emission trajectory. This treatment is also relevant to proposed strategies to stabilize atmospheric CO2 at approximately 550 ppm (Stern 2006; Raupach et al. 2007). First, to test whether elevated CO2 and reduced pH affected the development of clownfish embryos, eggs were reared from the day of laying until hatching in control sea water and in sea water where the pH had been reduced 0.35 units by equilibration with approximately 1030 ppm CO2. The effect of CO2 acidification on embryonic duration, embryonic survival, size at hatching and energy provisioning (yolk size) was examined. Clutches of larvae were then reared to the end of their pelagic phase in sea water aerated with 390 (current-day control), 550, 750 or 1030 ppm of CO2 in air. Length and weight of larvae at the end of the pelagic phase were compared between the control and treatments.
Finally, the maximum swimming speed of the settlement-stage larvae from each of the treatments was tested in a swim chamber to determine whether exposure to acidification affected individual performance. Strong swimming ability is thought to be ecologically important to larval reef fishes because it can assist with the location of food, avoidance of predators and the ability to find suitable settlement habitat at the end of the pelagic phase (Leis & McCormick 2002; Fisher et al. 2005). Therefore, this method provides a relevant test of the effects of CO2 acidification on individual performance.
2. Material and methods
(a). Study species
Clownfish were chosen as the model species because they belong to one of the most abundant and diverse families of coral reef fishes (the damselfishes: Pomacentridae) and because they are one of the few coral reef fishes that can be reliably reared through the pelagic larval stage in captivity. Clownfish form breeding pairs living on sea anemones. The female lays clutches of eggs on a hard surface near the anemone and the male cares for the eggs until hatching. Egg care includes fanning the eggs to increase oxygenation, removal of dead eggs and protection from predators (Fautin & Allen 1992). After hatching, larvae have a pelagic larval duration of 11–12 days (Almany et al. 2007). At the end of the larval stage, clownfish larvae settle to anemones where they usually become part of a social queue waiting to attain breeding status (Buston 2004).
(b). Breeding and egg stage
Clownfish were reared in a 70 000 l recirculating sea water system at James Cook University's experimental marine aquarium facility. Brood stock were wild-caught pairs of A. percula from the Great Barrier Reef, Australia, which had been kept at the experimental facility for approximately 2 years. Breeding pairs were kept in separate 70 l aquariums supplied with a continuous flow of filtered sea water at 18 l h−1. Water temperature was maintained at 30°C ± 0.6 (s.d.). Adults were fed twice daily to satiation with INVE Aquaculture Nutrition 12/20 pellets. Half a terracotta pot on the bottom of each aquarium served as a shelter and breeding site. The pot was checked each morning and evening for the presence of eggs.
Each new egg clutch was photographed with an underwater camera and the date of laying recorded. Egg clutches were reared with their parents in either control sea water or sea water where the pH had been reduced 0.35 units by dissolving additional CO2 (see below). On the evening of hatching (6–8 days), each egg clutch was photographed again. The appearance of the embryos identified their readiness to hatch. The pot with eggs attached was then transferred to a hatching tank where all viable eggs usually hatched within a 1–2 h period immediately after dark. After hatching, 20 haphazardly selected larvae were collected for the measurement of physical attributes. Sampled larvae were euthanized with an overdose of clove oil and preserved in 4 per cent phosphate-buffered formaldehyde solution.
The average pH of unmanipulated sea water in the parental breeding area was 8.15 ± 0.07 (s.d.). Treatment sea water was adjusted to a pH of 7.8 ± 0.05 using an automated CO2 injection system. A pH controller (Tunze Aquarientechnik, Germany) was attached to each aquarium to maintain pH at the desired level. The pH controller was connected to a laboratory-grade pH probe in the aquarium and to an electronic solenoid connected to a cylinder of CO2. The solenoid injected bubbles of CO2 into a diffuser (Red Sea Reactor 500) at the bottom of the aquarium whenever the pH of the aquarium sea water rose above the set point. The diffuser rapidly dissolved CO2 into the sea water and also served as a vigorous stirrer. The equivalent atmospheric concentration of CO2 in the water using this method was estimated to be approximately 1030 ppm (Munday et al. 2009a). All water returned to a storage tank where it was degassed by stirring, filtered and any additional nutrients removed in a 1000 l algal bio-remediation tank. Partial exchanges with freshly collected sea water occurred weekly. Oxygen levels were measured daily (WTW Oxi340i electrode) and were always above 90 per cent saturation.
(c). Larval stage
Larvae were reared from hatching to the end of their pelagic stage (11 days post-hatching) in tanks aerated with 396 ± 28 (s.d.; control), 538 ± 54, 744 ± 47 or 1024 ± 64 ppm CO2 in air (figure 1). The previous experiment revealed that the size of newly hatched larvae was not affected by exposure to approximately 1030 ppm CO2 during the egg stage. Consequently, in this experiment, newly hatched larvae from egg clutches that had not been exposed to acidification were divided equally into the four different CO2 treatments. Four groups of newly hatched larvae, each from a different parent, were divided this way and reared to the end of the pelagic stage. This design had the advantage of allowing any differences in growth performance between clutches to be factored into the experiment and subsequent analysis.
Figure 1.
Schematic illustration of the experimental system used to simulate future ocean acidification environments for rearing larval reef fishes. Four separate lines delivered air with approximately 390 (current day), 550, 750 or 1030 ppm CO2. For simplicity, only one of the lines is shown here. Each CO2 line aerated four replicate aquariums.
We used a semiclosed rearing system where each 60 l rearing tank had no water flow during the day and was then flushed at night with clean, filtered sea water that had been aerated all day with the same concentration of CO2-enriched air. This cycle ensured that larvae could feed ad libitum throughout daylight hours and that any unconsumed food was removed each night. Larvae were fed rotifers (Brachionus sp.) at five individuals per millilitre each morning for the first 3 days. Artemia nauplii were added at one individual per millilitre each morning from day 3. The ratio of Artemia nauplii to rotifers was increased each day until larvae were only fed five Artemia nauplii per millilitre from 8 days post-hatching. Non-living Nannochloropsis algal paste was added to the tanks each morning for the first 5 days to feed the rotifers. Water temperature was maintained at 30°C ± 0.5 using electric heaters. Oxygen levels were always above 90 per cent saturation. A summer light cycle of 13 h light/11 h dark was simulated with fluorescent lights.
The concentration of CO2 delivered to tanks in each treatment was controlled by a scientific-grade pressure regulator and precision needle valve, and measured continuously with an in-line infrared CO2 probe (Vaisala GMT222) linked to a computer (figure 1). The CO2-enriched air was continuously bubbled at 1.5 l m−1 through an ultra-fine aeration stone in each tank. Each rearing tank was sealed with a perspex lid to maintain the appropriate CO2 atmosphere above the water surface. The pH of treatment sea water was measured daily with a portable meter (Hach HQ11D) calibrated with fresh buffers (Merck). The average pH of control sea water in the larval rearing area and in tanks aerated with CO2-enriched air is shown in table 1.
Table 1.
Mean ± s.e. CO2 concentrations and pH of sea water in the larval-rearing experiment.
| CO2 (ppm) | pH |
|---|---|
| 396 ± 28 | 8.06 ± 0.08 |
| 538 ± 54 | 7.94 ± 0.11 |
| 744 ± 47 | 7.88 ± 0.15 |
| 1024 ± 64 | 7.84 ± 0.10 |
Larvae exhibited settlement behaviour (attraction to the side of the rearing tank) on day 11 post-hatching. The swimming performance of a random selection of larvae was tested in a purpose-built swim chamber (see below). All the larvae were then euthanized and fixed in 4 per cent phosphate-buffered formaldehyde solution in preparation for the measurement of physical attributes.
(d). Critical swimming speed
The standard U-crit method for measuring maximum swimming speed was used to test whether exposure to CO2-acidified sea water affected the swimming performance of settlement-stage clownfish. This method measures maximum short-term swimming speed that is likely to be ecologically important to larvae and which are often correlated with other measures of swimming performance (Fisher et al. 2005). Eleven-day-old larvae were swum in a 5-channel swim chamber specifically designed for larval fishes (Fisher et al. 2000). At the start of each trial, a single fish was placed in each channel of the chamber and the lid sealed. Water flow was increased to a starting speed of 8 cm s−1. The water speed in the chamber was then increased by 1.95 cm s−1 every 2 min and the maximum swim time of each fish recorded. Critical swimming speed for each fish was calculated as U-crit=U + (t/t*i Ui), where U is the penultimate speed, Ui is the velocity increment, t is the time swum in the final velocity increment and ti is the time interval for each velocity increment (2 min). Water temperature was maintained at 30 ± 0.5°C.
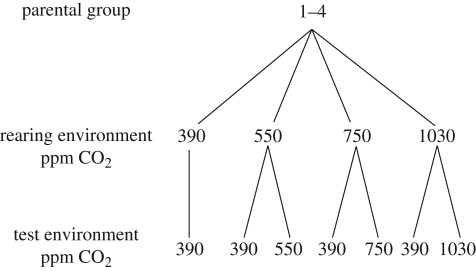
For each of the four parental groups, 10–20 larvae from each CO2 treatment were swum in acidified water (same level as the rearing environment) and in control water (figure 2). This design enabled us to test whether larval swimming speed was affected by rearing in CO2-acidified water and also whether exposure to CO2-treated water during maximum aerobic performance affected swimming speed independently of being reared in CO2-acidified water during the larval phase.
Figure 2.
Experimental design used to test the effect of CO2 acidification on the swimming performance of settlement-stage larvae.
(e). Measurement of physical attributes
Larvae were removed from the fixative within 24 h of preserving, blotted dry and then photographed in a lateral position under a stereomicroscope. Day 11 fish were weighed to the nearest milligram before being photographed. Standard length (SL) and yolk area (for newly hatched larvae) were estimated for each fish from the digital photograph using image analysis (Optimas 6.5, Media Cybernetics). All attributes were estimated three times and an average of the three values was calculated (estimates varied by less than 2%).
(f). Data analysis
Embryonic duration was calculated for each clutch and compared between control and acidified water with a t-test. The proportion of eggs that survived to hatching was calculated by viewing high-resolution digital photographs of the egg clutches and counting the number of eggs present on the day of laying versus the number present immediately before hatching. Any eggs that did not hatch were subtracted from the count of eggs present immediately before hatching. Proportional survivorship of eggs was then compared between control and acidified water with a t-test.
Two-way ANOVA (factors: parental group and treatment) was used to compare SL and yolk area at hatching among different breeding pairs that had egg clutches reared in both control and acidified water. Two-way ANOVA (factors: parental group and treatment) was also used to compare SL and weight of settlement-stage larvae that had been reared in different CO2-acidification treatments (control and three levels of elevated CO2). Tukey's HSD post hoc tests were then used to detect significant differences between means, and specifically to compare the average length and weight of larvae from each parental group in control water with each level of CO2 acidification.
A nested ANOVA was used to test whether CO2 acidification affected larval swimming performance. In this analysis, parental group and CO2 treatments were considered to be fixed factors. The test water used in the swim chamber (control or treatment water) was nested within the CO2-rearing treatments. Because swimming speed is often correlated with length in fish, we first regressed swimming speed on SL and then used the residuals in the analysis. Swimming speed data were square root transformed to linearize the relationship with SL and improve the distribution of residuals in the analysis.
3. Results
Four focal breeding pairs produced a total of 15 egg clutches (2–5 clutches each) during the experimental period. Embryonic duration was always 6–7 days, except for one clutch with a duration of 8 days. Embryonic duration did not differ between control (n = 8) and acidified water (n = 7; T13 = 0.54, p = 0.6). Egg survivorship was highly variable, but did not differ between control and acidified water (T13 = 0.53, p = 0.6). On average, 65 per cent of eggs in a clutch survived to hatching.
Replicate egg clutches from three of the breeding pairs were reared in control and acidified water. Size at hatching differed among parents (F2,103 = 17.9, p < 0.001), but did not differ between eggs reared in acidified water (3.33 mm ± 0.03 s.e.) compared with controls (3.30 mm ± 0.03 s.e.; F1,103 = 0.96, p = 0.3). There was no interaction for size at hatching between parents and acidification.
Yolk area of newly hatched larvae differed among parents (F2,103 = 33.9, p < 0.001). Yolk size also differed between larvae from acidified water compared with controls (F1,103 = 23.9, p < 0.001). Yolk size was approximately 6 per cent lower for larvae from eggs reared in water acidified with 1030 ppm CO2 (0.303 mm2 ± 0.008 s.e.) compared with controls (0.324 mm2 ± 0.006 s.e.). There was no interaction for yolk area between parents and acidification.
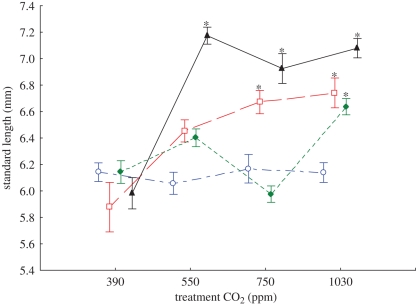
Four groups of newly hatched larvae, each from a different parent, were split into different CO2 treatments and reared to the end of their pelagic phase (11 days post-hatching). There was significant group * treatment effect (F9,326 = 11.33, p < 0.001) for length at the end of the pelagic phase. There was no difference in the length of larvae from the different parental groups that had been reared in control water (396 ppm CO2; figure 3). Larvae that had been reared in CO2-acidificied water tended to be longer than controls; however, the treatments affected parental groups differently. The length of larvae from one parental group (Group 1) was unaffected by any of the elevated CO2 treatments (figure 3). Larvae from the other three parental groups were longer in CO2-acidified water compared with controls (figure 3). For these three groups, larvae were 15 to 18 per cent longer when reared in water acidified with 1030 ppm CO2 compared with controls. However, the sensitivity to lower levels of CO2 differed between groups (figure 3). Larvae from Group 4 were longer at all levels of elevated CO2 (550, 750 and 1030 ppm). Larvae from Group 2 were longer at 750 and 1030 ppm, but not 550 ppm CO2. Larvae from Group 3 were only larger at 1030 ppm CO2.
Figure 3.
Mean ± s.e. SL of settlement-stage A. percula larvae from four parental pairs when reared in sea water treated with approximately 390 (current day), 550, 750 and 1030 ppm CO2. Newly hatched clutches were divided equally among the four CO2 treatments and reared until the end of the pelagic larval stage. Each clutch is coded with a different symbol and colour. Asterisks show mean values that are different from controls (390 ppm CO2) for each group. Group 1, open blue circle; Group 2, open red square; Group 3, filled green diamond; Group 4, filled black triangle.
There was also a significant group * treatment effect (F9,326 = 11.33, p < 0.001) for weight at the end of the pelagic phase. There was no difference in the weight of larvae from different parental groups when reared in control water (396 ppm CO2; figure 4). However, elevated CO2 treatments affected parental groups differently. The weight of larvae from two parental groups (Group 1 and Group 2) was unaffected by any of the elevated CO2 treatments (figure 4). Larvae from the other two parental groups were heavier in all the elevated CO2 treatments (figure 4). For these two groups larvae were 47 to 52 per cent heavier when reared in water acidified with 1030 ppm CO2 compared with controls.
Figure 4.
Mean ± s.e. weight of settlement-stage A. percula larvae from each of the four parental pairs when reared in sea water treated with approximately 390 (current day), 550, 750 and 1030 ppm CO2. Newly hatched clutches were divided equally among the four CO2 treatments and reared until the end of the pelagic larval stage. Each clutch is coded with a different symbol and colour. Asterisks show mean values that are different from controls (390 ppm CO2) for each group. Group 1, open blue circle; Group 2, open red square; Group 3, filled green diamond; Group 4, filled black triangle.
Critical swimming speed was tested for 250 individual fish. There was a weak, marginally significant relationship between maximum swimming and SL (R2 = 0.015, p = 0.05). Length-corrected swimming speed differed between parental groups (F3,240 = 8.87, p < 0.001), but was not affected by either CO2 acidification during rearing (F3,240 = 0.34, p = 0.8) or by swimming in either control or CO2-acidified water in the chamber (F3,240 = 1.11, p = 0.3). Average U-crit swimming speed was 18.43 cm s−1 ± 0.4 (s.e.); however, the mean U-crit of Group 2 (22.55 ± 1.12) was significantly higher than the other three groups (17.2 ± 0.64–18.04 ± 0.5).
4. Discussion
Although many recent studies have demonstrated that ocean acidification could significantly affect calcifying organisms (Orr et al. 2005; Kleypas et al. 2006; Anthony et al. 2008; Kuffner et al. 2008), very little is known about how marine fishes and other non-calcifying animals will respond to the levels of dissolved CO2 and sea water pH that could occur in the near future (Ishimatsu et al. 2005; Munday et al. 2008). The most common prediction is that ocean acidification could affect individual performance (e.g. development, growth, survival, swimming ability), especially during the early life history. Contrary to expectations, we found that CO2-induced acidification up to the maximum values likely to be experienced over the next 100 years had no noticeable effect on embryonic duration, egg survivorship and size at hatching for A. percula, and tended to have a positive effect on the length and weight of larvae. Furthermore, maximum swimming performance of settlement-stage larvae was unaffected by CO2 acidification. Importantly, our results suggest that near-future levels of ocean acidification might not, by themselves, have a significant negative impact on early life-history traits of A. percula, and possibly other benthic-spawning marine fishes.
In our experiments, the duration of the embryonic stage, egg survival and size at hatching were unaffected by acidification with approximately 1030 ppm CO2. There was a slight decline in yolk size of newly hatched larvae, but the effect size was small (6% reduction in yolk area), and experiments at a higher level of CO2 (approx. 1600 ppm) found no reduction in yolk area (P. L. Munday 2008, unpublished data). Overall, these results suggest that the egg stage of A. percula is relatively tolerant to elevated CO2 and low pH. These results contrast with studies on invertebrates where acidification with approximately 1000 ppm CO2 has been shown to increase embryonic duration (Ellis et al. 2009) and reduce embryonic development and viability of some species (reviewed by Kurihara 2008). There are several possible reasons for the different outcomes observed here for a benthic-spawning fish compared with a range of marine invertebrates. First, the effects of elevated CO2 on physiological processes appear to be greater for some marine invertebrates than for most fishes, possibly because fishes have a greater capacity for acid–base regulation than most invertebrates (Pörtner et al. 2004; Widdicombe & Spicer 2008). Some invertebrates are unable to compensate fully for disturbances in their acid–base balance when exposed to elevated CO2 and this can lead to metabolic depression and reduced growth (Kurihara & Shirayama 2004; Pörtner et al. 2004; Michaelidis et al. 2005, 2007; Miles et al. 2007). In contrast, most shallow-water fish tested to date appear to compensate fully their acid–base balance within several days of exposure to mild hypercapnia (Michaelidis et al. 2007; Ishimatsu et al. 2008). The well-developed capacity for acid–base regulation in fishes may explain why exposure to approximately 1030 ppm CO2 and a pH of 7.8 had little obvious effect on the life-history traits of A. percula embryos.
Another important difference is that many invertebrates are broadcast spawners where the eggs are released directly into the ocean. In contrast, A. percula is a benthic spawner and the eggs are retained on the reef for approximately one week after laying. The pH of reef water can vary substantially throughout the day, sometimes reaching levels below 8.0 in the early morning due to accumulated respiration of reef organisms in shallow water overnight (Ohde & van Woesik 1999; Kuffner et al. 2008). The eggs of benthic spawners might be adapted to such variation in ambient CO2 and pH levels and this may increase their tolerance to mild hypercapnia. The majority of small reef fish are demersal spawners (Munday & Jones 1998) and perhaps, like A. percula, they might be more tolerant to CO2 and pH fluctuations than are the eggs of pelagic spawners (Munday et al. 2008). Eggs and larvae from a range of different benthic- and pelagic-spawning fishes need to be reared in relevant acidified conditions to test this hypothesis.
The most unexpected result of our study was that larvae from some parental groups reared in CO2-acidified water were longer and heavier at the end of their pelagic phase than larvae from the same clutch reared in control water. This effect was most marked at the highest CO2 treatment (1030 ppm), where larvae from three of the four groups were 15 to 18 per cent longer than controls and larvae from two of the four groups were 47 to 52 per cent heavier than controls. Enhanced growth performance in acidified water could be achieved either by increased energy intake or reduced energy expenditure. Acid-exposed rainbow trout exhibited increased appetite to compensate for greater branchial ion loss at low pH and the associated increased energy intake increased growth rate (Morgan et al. 2001). Although increased flux of ions is less likely to be a problem for marine fishes, because they must actively excrete excess ions to maintain their osmotic balance, it is still possible that elevated CO2 and low pH stimulated appetite and dietary intake by larvae. Munday et al. (2009a) demonstrated that exposure to approximately 1030 ppm CO2 increased the attraction of larval fishes to a range of olfactory stimuli. If gustatory senses are similarly stimulated by elevated CO2, it is possible that feeding activity of some larvae could be enhanced in acidified water.
Alternatively, larvae in some groups may have simply reduced their activity levels in acidified water, thereby reducing total energy expenditure. It is also possible that ionic regulatory processes may have operated more effectively in acidified water, leading to improved energy conversion. There were no obvious differences in feeding rate or activity levels of the larvae among treatments in our experiment, and the mechanisms of acid–base regulation in marine fishes are still not fully understood, especially for larval-stage fishes (Claiborne et al. 2002); therefore, each of these hypotheses requires further testing to determine the mechanism responsible for increased growth of larvae in some groups, but not others. However, the strikingly different responses to CO2 acidification we observed among groups is more consistent with a behavioural effect on feeding rate or activity levels than it is with a physiological effect associated with acid–base regulation, which might be expected to operate similarly among individuals in all groups.
Whatever the mechanism responsible for increased growth in acidified water, large size and rapid growth appear to be advantageous for larval fish and newly settled juveniles (Searcy & Sponaugle 2000; Bergenius et al. 2002; Meekan et al. 2003) because they offer competitive advantages and reduce the time individuals spend in highly vulnerable small-size classes (Jones & McCormick 2002). Therefore, larvae from some groups were potentially at a selective advantage when exposed to elevated CO2 and low pH. While individuals in some groups were larger under acidified conditions, other groups were not affected by acidification at all. Even among groups where there was a positive effect of acidification on length at settlement, there was considerable variation among groups in the responses to lower concentrations of CO2, with some groups only affected at the higher concentrations (750–1030 ppm). These differences suggest that either parental genotype or non-genetic interclutch variation resulting from parental effects on the offspring (Green 2008) had a significant influence on the responses of larvae to acidified conditions. Variation in the responses of larvae to ocean acidification is particularly important because it could be beneficial in helping species adapt to future ocean acidification (Skelly et al. 2007).
Most important is that none of the groups exhibited a negative response to the acidification treatments. Consequently, our results suggest that levels of CO2-induced acidification predicted to occur over the next century are unlikely to have an adverse effect on the early life-history development of A. percula. This interpretation is further supported by tests of critical swimming performance in acidified and control water. Maximum short-term swimming speed (U-crit) of settlement-stage larvae was unaffected by rearing in CO2-acidified water, or by swimming the larvae in CO2-acidified water compared with control water. Critical swimming speed is thought to be an ecologically relevant test of larval performance because strong swimming ability helps individuals locate and capture food, avoid predators and reach suitable adult habitat at the end of the pelagic phase (Leis & McCormick 2002; Fisher et al. 2005). Critical swimming speed is also a standard test of physiological performance because fish are swum up to their maximum capacity at set time intervals. Neither raw data nor size-corrected data showed any evidence of reduced swimming performance in acidified conditions and the average U-crit swimming speed for A. percula (17.2–22.55 cm s−1) was similar to that recorded for another anemonefish, Amphiprion melanopus (14.6–22.5 cm s−1; Green & Fisher 2004). This suggests that the physiological performance of larval A. percula is unlikely to be affected by CO2 levels that will occur in shallow oceans over the next 50–100 years. There was a weak positive effect of length on maximum swimming speed; therefore, larger larvae from acidified water might be expected to have a slight advantage in swimming performance. This effect was not evident in the analysis, probably because of the very small effect of body size compared with the much larger variation in swimming performance among individuals of similar sizes.
Although we found that acidification increased the length and weight of larvae from some groups, we were not able to assess the potential effects on larval survivorship owing to the logistics of our rearing technique. Furthermore, levels of survival in the laboratory may not provide meaningful information about survival in nature because the usual agents of mortality, such as predators, are absent. Nevertheless, it is possible that the mechanisms responsible for increasing the growth of larvae might somehow affect their susceptibility to predators, and future studies should examine this possibility.
Average sea surface temperatures will increase because of global warming at the same time that ocean pH is declining because of the increased absorption of CO2. Temperature is known to influence developmental rates, growth and survival of reef fishes, especially during embryonic and larval stages (Munday et al. 2008). In general, increased temperature tends to decrease embryonic and larval duration and increase larval growth rate (e.g. Green & Fisher 2004; Sponaugle et al. 2007; Munday et al. 2009b). Small increases in temperature may also reduce the survival rate of embryos (Gagliano et al. 2007). This means that complex interactions between the effects of temperature and acidification will ultimately determine the consequences of climate change for the early life histories of marine fishes. The results of this study suggest that levels of ocean acidification that could occur in the near future are unlikely, in isolation, to have sharply negative effects on early life-history traits of a marine fish. Further research is now needed to determine how temperature and CO2-induced acidification interact to affect both early life-history and adults stages of a wide range of non-calcifying marine organisms.
Acknowledgements
Staff at James Cook University's Marine Aquarium Facility provided excellent logistical support. Thanks to Mark McCormick and three anonymous reviewers for helpful comments on the manuscript. This work was funded by an ARC Center of Excellence grant to P.L.M. The research accomplished in this project was conducted under ethics approval numbers A1335 and A1355, and followed all guidelines for the country in which it took place.
References
- Almany G. R., Berumen M. L., Thorrold S. R., Planes S., Jones G. P.2007Local replenishment of coral reef fish populations in a marine reserve. Science 316, 742–744 (doi:10.1126/science.1140597) [DOI] [PubMed] [Google Scholar]
- Anthony K. R. N., Kline D. I., Diaz-Pulido G., Hoegh-Guldberg O.2008Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. USA 105, 17 442–17 446 (doi:10.1073/pnas.0804478105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenius M. A. J., Meekan M. G., Robertson D. R., McCormick M. I.2002Larval growth predicts the recruitment success of a coral reef fish. Oecologia 131, 521–525 (doi:10.1007/s00442-002-0918-4) [DOI] [PubMed] [Google Scholar]
- Brown D. J. A., Sadler K.1989Fish survival in acid waters. In Acid toxicity and aquatic animals (eds Morris R., Taylor E. W., Brown D. J. A., Brown J. A.), pp. 31–44 Cambridge, UK: Cambridge University Press [Google Scholar]
- Buston P. M.2004Territory inheritance in clownfishes. Proc. R. Soc. Lond. B 271, S252–S254 (doi:10.1098/rsbl.2003.0156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira K., Wickett M. E.2005Anthropogenic carbon and ocean pH. J. Geophys. Res. 110, C09S04 (doi:10.1029/2004JC002671) [DOI] [PubMed] [Google Scholar]
- Claiborne J. B., Edwards S. L., Morrison-Shetlar A. I.2002Acid–base regulation in fishes: cellular and molecular mechanisms. J. Exp. Zool. 293, 302–319 (doi:10.1002/jez.10125) [DOI] [PubMed] [Google Scholar]
- Dockray J. J., Morgan I. J., Reid S. D., Wood C. M.1998Responses of juvenile rainbow trout, under food limitation, to chronic low pH and elevated summer temperatures, alone and in combination. J. Fish. Biol. 52, 62–82 (doi:10.1111/j.1095-8649.1998.tb01553.x) [Google Scholar]
- Ellis R. P., Bersey J., Rundle S. D., Hall-Spencer J. M., Spicer J. L.2009Subtle but significant effects of CO2 acidified seawater on embryos of the intertidal snail Littorina obusata. Aquat. Biol. 5, 41–48 (doi:10.3354/ab00118) [Google Scholar]
- Fabry V. J., Seibel B. A., Feely R. A., Orr J. C.2008Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 (doi:10.1093/icesjms/fsn048) [Google Scholar]
- Fautin D. C., Allen G. R.1992Field guide to anemonefishes and their host sea anemones Perth, Western Australia: Western Australian Museum [Google Scholar]
- Feely R. A., Sabine C. L., Lee K., Berelson W., Kleypas J., Fabry V. J., Millero F. J.2004Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (doi:10.1126/science.1097329) [DOI] [PubMed] [Google Scholar]
- Fisher R., Bellwood D. R., Job S. D.2000Development of swimming abilities in reef fish larvae. Mar. Ecol. Prog. Ser. 202, 163–173 (doi:10.3354/meps202163) [Google Scholar]
- Fisher R., Leis J. M., Clark D. L., Wilson S. K.2005Critical swimming speeds of late-stage coral reef fish larvae: variation within species, among species and between locations. Mar. Biol. 147, 1201–1212 (doi:10.1007/s00227-005-0001-x) [Google Scholar]
- Gagliano M., McCormick M. I., Meekan M. G.2007Temperature-induced shifts in selective pressure at a critical developmental transition. Oecologia 152, 219–225 (doi:10.1007/s00442-006-0647-1) [DOI] [PubMed] [Google Scholar]
- Green B. S.2008Maternal effects in fish. Adv. Mar. Biol. 54, 1–105 (doi:10.1016/S0065-2881(08)00001-1) [DOI] [PubMed] [Google Scholar]
- Green B. S., Fisher R.2004Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J. Exp. Mar. Biol. Ecol. 299, 115–132 (doi:10.1016/j.jembe.2003.09.001) [Google Scholar]
- Havenhand J. N., Buttler F.-R., Thorndyke M. C., Williamson J. E.2008Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 18, R651–R652 (doi:10.1016/j.cub.2008.06.015) [DOI] [PubMed] [Google Scholar]
- Heisler N.1989Acid–base regulation in fishes. 1. Mechanisms. In Acid toxicity and aquatic animals (eds Morris R., Taylor E. W., Brown D. J. A., Brown J. A.), pp. 85–96 Cambridge, UK: Cambridge University Press [Google Scholar]
- Hoegh-Guldberg O., et al. 2007Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- Ishimatsu A., Hayashi M., Lee K.-S., Kikkawa T., Kita J.2005Physiological effects on fishes in a high-CO2 world. J. Geophys. Res. 110, C09S09 (doi:10.1029/2004JC002564) [Google Scholar]
- Ishimatsu A., Hayashi M., Kikkawa T.2008Fishes in high CO2, acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–302 (doi:10.3354/meps07823) [Google Scholar]
- Jones G. P., McCormick M. I.2002Numerical and energetic processes in the ecology of coral reef fishes. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P. F.), pp. 221–238 San Diego, CA: Academic Press [Google Scholar]
- Kleypas J. A., Feely R. A., Fabry V. J., Langdon C., Sabine C. L., Robbins L. L.2006Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research. Workshop held on 18–20 April 2005, St Petersburg, FL Sponsored by NSF, NOAA and the US Geological Survey [Google Scholar]
- Kuffner I. B., Andersson A. J., Jokiel P. L., Rodgers K. S., MacKenzie F. T.2008Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 1, 114–117 (doi:10.1038/ngeo100) [Google Scholar]
- Kurihara H.2008Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 373, 275–284 (doi:10.3354/meps07802) [Google Scholar]
- Kurihara H., Shirayama Y.2004Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 274, 161–169 (doi:10.3354/meps274161) [Google Scholar]
- Leis J. M., McCormick M. I.2002The biology, behaviour and ecology of the pelagic, larval stage of coral reef fishes. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale P. F.), pp. 171–199 San Diego, CA: Academic Press [Google Scholar]
- Meehl G. A., et al. 2007Global climate projections. In Climate change 2007: the physical science basis (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.), pp. 686–688 Cambridge, UK: Cambridge University Press [Google Scholar]
- Meekan M. G., Carleton J. H., McKinnon A. D., Flynn K., Furnas M.2003What determines the growth of tropical reef fish larvae in the plankton: food or temperature? Mar. Ecol. Prog. Ser. 256, 193–204 (doi:10.3354/meps256193) [Google Scholar]
- Michaelidis B., Ouzounis C., Paleras A., Pörtner H. O.2005Effects of long-term moderate hypercapnia on acid–base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser 293, 109–118 (doi:10.3354/meps293109) [Google Scholar]
- Michaelidis B., Spring A., Pörtner H. O.2007Effects of long-term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata. Mar. Biol. 150, 1417–1429 (doi:10.1007/s00227-006-0436-8) [Google Scholar]
- Miles H., Widdicombe S., Spicer J. I., Hall-Spencer J.2007Effects of anthropogenic seawater acidification on acid–base balance in the sea urchin Psammechinus miliaris. Mar. Pollut. Bull. 54, 89–96 (doi:10.1016/j.marpolbul.2006.09.021) [DOI] [PubMed] [Google Scholar]
- Morgan I. J., McDonald D. G., Wood C. M.2001The cost of living for freshwater fish in a warmer, more polluted world. Global Change Biol. 7, 345–355 (doi:10.1046/j.1365-2486.2001.00424.x) [Google Scholar]
- Munday P. L., Jones G. P.1998The ecological implications of small body size among coral-reef fishes. Oceanogr. Mar. Biol. Annu. Rev. 36, 373–411 [Google Scholar]
- Munday P. L., Jones G. P., Pratchett M. S., Williams A. J.2008Climate change and the future for coral reef fishes. Fish Fish. 9, 261–285 (doi:org/10.1111/j.1467-2979.2008.00281.x) [Google Scholar]
- Munday P. L., Dixson D. L., Donelson J. M., Jones G. P., Pratchett M. S., Devitsina G. V., Døving K. B.2009aOcean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852 (doi:10.1073/pnas.0809996106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday P. L., Leis J. M., Lough J. M., Paris C. B., Kingsford M. J., Berumen M. L., Lambrechts J.2009bClimate change and coral reef connectivity. Coral Reefs 28, 379–395 (doi:10.1007/s00338-008-0461-9) [Google Scholar]
- Ohde S., van Woesik R.1999Carbon dioxide flux and metabolic processes of a coral reef, Okinawa. Bull. Mar. Sci. 65, 559–576 [Google Scholar]
- Orr J. C., et al. 2005Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (doi:10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- Pörtner H. O., Farrell A. P.2008Physiology and climate change. Science 322, 690–691 (doi:10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- Pörtner H. O., Langenbuch M., Reipschläger A.2004Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718 (doi:10.1007/s10872-004-5763-0) [Google Scholar]
- Pörtner H. O., Langenbuch M., Michaelidis B.2005Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: from Earth history to global change. J. Geophys. Res. 110, C09S10 (doi:10.1029/2004JC002561) [Google Scholar]
- Raupach M. B., Marland G., Ciais P., Le Quere C., Canadell J. G., Klepper G., Field C. B.2007Global and regional drivers of accelerating CO2 emissions. Proc. Natl Acad. Sci. USA 104, 10 288–10 293 (doi:10.1073/pnas.0700609104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal Society 2005Ocean acidification due to increasing atmospheric carbon dioxide London, UK: The Royal Society [Google Scholar]
- Sabine C. L., et al. 2004The oceanic sink for anthropogenic CO2. Science 305, 367–371 (doi:10.1126/science.1097403) [DOI] [PubMed] [Google Scholar]
- Searcy S. P., Sponaugle S.2000Variable larval growth in a coral reef fish. Mar. Ecol. Prog. Ser. 206, 213–226 (doi:10.3354/meps206213) [Google Scholar]
- Shirayama Y., Thornton H.2005Effect of increased atmospheric CO2 on shallow water marine benthos. J. Geophys. Res. 110, C09S08 (doi:10.1029/2004JC002618) [Google Scholar]
- Skelly D. K., Joseph L. N., Possingham H. P., Freidenburg L. K., Farrugia T. J., Kinnison M. T., Hendry A. P.2007Evolutionary responses to climate change. Conserv. Biol. 21, 1353–1355 (doi:10.1111/j.1523-1739.2007.00764.x) [DOI] [PubMed] [Google Scholar]
- Sponaugle S., Grorud-Colvert K., Pinkard D.2007Temperature-mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida Keys. Mar. Ecol. Prog. Ser. 308, 1–15 (doi:10.3354/meps308001) [Google Scholar]
- Stern N.2006Stern review on the economics of climate change Cambridge, UK: Cambridge University Press [Google Scholar]
- Widdicombe S., Spicer J. I.2008Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J Exp. Mar. Biol. Ecol. 366, 187–197 (doi:10.1016/j.jembe.2008.07.024) [Google Scholar]