Abstract
The cognitive challenges that social animals face depend on species differences in social organization and may affect mosaic brain evolution. We asked whether the relative size of functionally distinct brain regions corresponds to species differences in social behaviour among paper wasps (Hymenoptera: Vespidae). We measured the volumes of targeted brain regions in eight species of paper wasps. We found species variation in functionally distinct brain regions, which was especially strong in queens. Queens from species with open-comb nests had larger central processing regions dedicated to vision (mushroom body (MB) calyx collars) than those with enclosed nests. Queens from advanced eusocial species (swarm founders), who rely on pheromones in several contexts, had larger antennal lobes than primitively eusocial independent founders. Queens from species with morphologically distinct castes had augmented central processing regions dedicated to antennal input (MB lips) relative to caste monomorphic species. Intraspecific caste differences also varied with mode of colony founding. Independent-founding queens had larger MB collars than their workers. Conversely, workers in swarm-founding species with decentralized colony regulation had larger MB calyx collars and optic lobes than their queens. Our results suggest that brain organization is affected by evolutionary transitions in social interactions and is related to the environmental stimuli group members face.
Keywords: social behaviour, brain organization, caste differences, tissue allocation
1. Introduction
Neural tissue is expensive. Both production and maintenance costs of neural tissue are high relative to many other tissues (Niven et al. 2003; Nawroth et al. 2007; Niven 2007). The anatomical organization of brains often indicates differences in relative investment among functionally distinct brain regions (Hampton & Shettleworth 1996; Clayton 1998; Bingman 2004). Brain structure covaries with behavioural differences, suggesting that brain tissue allocation reflects species-typical cognitive challenges (Barton & Harvey 2000; Gronenberg 2001). Sociality poses a number of substantial cognitive challenges to animals, including spatial learning of nest/home sites, group mate discrimination and assessment of relative rank (Anderson 1998; Capaldi et al. 1999; Tibbetts & Lindsay 2008). At the individual level, social roles within groups correspond to differences in brain volume and activity (Withers et al. 1993; Øyvind et al. 1999; Groh et al. 2006; Molina & O'Donnell 2007, 2008).
The social intelligence hypothesis was developed to explain how the evolution of sociality could impact brain evolution (Shultz & Dunbar 2006; Dunbar & Shultz 2007). This hypothesis assumes that sociality imposes novel cognitive challenges (e.g. group mate recognition) to a species in addition to those faced by its solitary ancestors. Social species often have enlarged brains and/or larger specific brain regions than their non-social relatives (Croney & Newberry 2007; Silk 2007). For example, comparative studies of neuroanatomy in Hymenoptera suggested that some brain neuropils were larger in social species than in closely related solitary species (Howse 1974).
The goal of our study was to extend the comparative analysis of brain structure evolution beyond social–solitary species comparisons. The origin of sociality within a clade is an important transition, but there can be further changes in behaviour as a more complex social structure evolves (Bourke 1999; Anderson & McShea 2001; Jeanne 2003). The modification of behaviour in social lineages can include changes in group size, modes of communication and the degree of division of labour among group members (Jeanne 2003; Hölldobler & Wilson 2009). We propose that evolutionary transitions in group structure and behaviour within social taxa can select for additional changes in brain architecture. We predicted that species variation in cognitive challenges would explain differences in brain architecture (Farris & Roberts 2005; Farris 2008).
We used social paper wasps (Vespidae) as subjects. Paper wasp phylogeny is well characterized at the genus level (Carpenter 1991; Arévalo et al. 2004). Paper wasps exhibit two distinct grades of social organization, which are associated with colony initiation behaviour: independent-founding (IF) species and swarm-founding (SF) species (Jeanne 1991, 2003; Smith et al. 2002). IF and SF differ in colony size: minimum mature colony sizes of SF barely overlap the maximum mature colony sizes of IF (Jeanne 1991, 2003). Division of labour is more complex in SF, with greater worker partitioning of tasks, worker team organization and well-developed temporal polyethism (O'Donnell & Jeanne 1992; Jeanne 2003). Within the IF/SF grades of social structure, there is further variation in nest architecture, colony size and mechanisms of caste determination (Jeanne 1991; O'Donnell 1998a; Noll et al. 2004). We extended previous comparisons of IF and SF brain plasticity (O'Donnell et al. 2007; Molina & O'Donnell 2007, 2008) by increasing the number of genera sampled and by accounting for the effects of phylogeny in the analysis (Felsenstein 1985; Martins & Hansen 1996, 1997).
We measured the size (volume) of anatomically discrete brain regions as an indicator of investment in brain tissue. We measured both peripheral and central processing brain regions allocated to two major sensory organs: the compound eyes (vision) and the antennae (chemosensation and tactility). The central processing tissues were in the mushroom bodies (MBs), neuropils located within the insect forebrain. Peripheral sensory processing centres of the insect brain—the optic and antennal lobes (ALs)—innervate different MB calyx subregions: the lip primarily processes olfactory and tactile input from the ALs, whereas the MB calyx collar receives visual information from the optic lobes (OLs; Farris 2005; Fahrbach 2006).
We assessed whether the volume of targeted brain regions was associated with three behavioural and caste covariates. We list the cognitive challenges individuals face and predicted patterns of brain investment (see also electronic supplementary material).
(a). Mode of colony founding
Unlike IF, SF species coordinate movement to new nest sites with pheromone trails (Naumann 1975; Jeanne 1981, 1991). Only SF queens employ pheromones to suppress reproductive development in nest mates (West-Eberhard 1977; Landolt et al. 1998; Jeanne 2003). We predicted that SF would have greater investment in olfactory processing brain regions than IF because of an increased dependence on olfactory communication (pheromones).
We expected the brain structure of IF and SF to differ further because of the dramatic changes in colony organization that accompanied the evolution of SF from IF ancestors (Smith et al. 2002; Jeanne 2003). Principal among these changes is a shift from centralized, hierarchical control of division of labour in IF to relatively decentralized control in SF (Bourke 1999; Beshers & Fewell 2001). As insect colony organization evolves towards decentralization, the cognitive demands on any given colony member may actually decrease. In centralized societies (e.g. IF), the queen regulates colony activity by interacting directly with nestmates (Reeve 1991). IF queens may therefore be capable of individual recognition and associative memory, and queens may assess colony needs as well as the reproductive abilities of nestmates. IF worker behaviour is linked to their dominance rank and workers are plastic in their task performance (Reeve 1991; Premnath et al. 1996; Molina & O'Donnell 2009). In contrast, SF workers specialize on a set of tasks (O'Donnell & Jeanne 1990; Jeanne 2003). SF wasp workers may use only the information necessary for performing specific tasks (Jeanne 1991; Gordon 1996; Chittka et al. 1999), and higher order cognitive processing may be less crucial. IF bumble-bee workers have relatively larger MBs than SF honeybee workers (Howse 1974; Mares et al. 2005). We predicted that brain structure would vary with social complexity in paper wasps: MB calyx volume should be smaller in SF species, whose colony organization is more similar to that of honeybees.
(b). Nest architecture
In both solitary and social insects, ambient light intensities affect the development of visual processing brain regions (Barth & Heisenberg 1997; Gronenberg & Liebig 1999; Julian & Gronenberg 2002). All IF and some SF species build open-comb nests where the nest surface is minimally sheltered from ambient light. In contrast, most SF species either nest in cavities or cover their brood combs with an envelope, reducing light levels on the brood comb (Jeanne 1975; Wenzel 1991). SF species with open nests may use visual cues more heavily than SF species with enclosed nests (Hunt et al. 1995; Nascimento & Tannure-Nascimento 2005; Greiner 2006; Warrant 2008). We predicted that brains from species with open nests would have significantly larger visual processing regions (e.g. MB calyx collar, OL). Because they are largely nestbound (O'Donnell 1998a; Strassmann 2001), queens are especially likely to experience only on-nest light environments. We predicted that queens in closed-nest species would have the most reduced visual processing regions.
(c). Degree of morphological caste differentiation
We compared females of both worker and queen castes for each subject species. As noted above, including queens is important because the effects of variation in colony environment, such as nest architecture (e.g. open versus closed nests), may be most strongly reflected in queen brain structure. Furthermore, increased physiological specialization on egg laying by queens leads to a reduction in their behavioural repertoires (Richards 1978; Anderson & McShea 2001; Jeanne 2003). Fewer cognitive demands may permit reductions in queens’ neural tissue investment.
In addition to our comparative analysis of caste differences, we measured intraspecific patterns of queen/worker brain structure differences. Paper wasp species differ in the degree of queen/worker caste differences and in the developmental mechanisms of caste determination (Keller 1993; O'Donnell 1998a; Hunt et al. 2003; Noll et al. 2004; Deshpande et al. 2006). Morphologically distinct paper wasp queens are often smaller than their workers in some body regions, particularly head capsule size, but typically have larger abdomens (Jeanne et al. 1995; O'Donnell 1998a; Noll et al. 2004). We predicted that caste differences in brain structure would be most dramatic for species with morphologically distinct queens and workers.
In SF, workers depart the nest to forage, relying on cues from multiple sensory modalities (Raveret-Richter & Jeanne 1991; Weiss et al. 2004; McPheron & Mills 2007; Richter 2007). Nestbound SF queens, like ant and honeybee queens, may rely predominantly on olfactory cues, including brood pheromones and other queens’ pheromones (Landolt et al. 1998; Jeanne 2003). We expected queens in species with closed nests to have significantly reduced brain regions dedicated to visual processing relative to their workers.
2. Methods
(a). Study sites and subject collection
We collected subject nests from 20 June to 20 July 2004, 27 June to 24 July 2005 and 27 July to 7 August 2006 in Monteverde (10°18′ N, 84°49′ W) and near Cañas in the Guanacaste Province, Costa Rica (10°26′ N, 85°07′ W), and from 6 June to 23 June 2007 at the Tiputini Biodiversity Station and Yasuni National Park in Ecuador (0°38′ S, 76°08′ W; see electronic supplementary material for more details). Nests were located on the eaves of buildings or in vegetation. Wasps were collected and stored in an aldehyde-based fixative (Prefer, Anatech Ltd).
(b). Ovary dissections and identifying caste
We dissected the ovaries from each female's gaster and photographed them under a dissecting microscope at ×10 magnification using a digital camera. We used the mean area of the two largest oocytes as an index of ovary development (Keeping 2000, 2002). Within each colony, we assigned caste based on ovary development: we labelled females with filamentous oocytes as workers and females with the largest oocytes as queens. We used analyses of morphometric data from Noll et al. (2004) to determine whether each species had morphologically distinct queen/worker castes (see electronic supplementary material).
(c). Neuroanatomical measurements
For each nest, we collected neuroanatomical data from one to four adult females per caste (see the electronic supplementary material for further information on histology, tissue preparation and quantification).
We measured the volume of the following brain subregions: the OL (medulla and lobula), the AL (only the glomeruli) and the MB calyx (lip and collar + basal ring; see electronic supplementary material; Gronenberg 2001; O'Donnell et al. 2004, 2007; Molina & O'Donnell 2007, 2008). The cell body regions and fibres between the subregions of quantified neuropils were not included in analyses. The MB calyx collar and basal ring (BR) were grouped because boundaries between these subdivisions were ambiguous for some species, whereas boundaries between the lip and collar were always distinct.
(d). Statistical analyses
We tested whether neuroanatomy corresponded to nest architecture (open versus enclosed nests), mode of colony founding (IF/SF) and the presence/absence of morphologically distinct castes (see electronic supplementary material; Wenzel 1991; Jeanne 2003; Noll et al. 2004). As in previous studies, we used volume ratios as response variables (Withers et al. 1993; O'Donnell et al. 2004, 2007; Molina & O'Donnell 2007, 2008). We calculated ratios of each brain region to the sum of all quantified brain regions (total quantified volume was the sum of the OL, AL, the MB calyx and lobes and the central complex; Ehmer & Hoy 2000; Ehmer et al. 2001; Ehmer & Gronenberg 2004). We used volume ratios because body size may influence absolute volumetric measurements (Wehner et al. 2007).
For interspecific comparisons, we used Phylip software (http://evolution.genetics.washington.edu/phylip.html) to estimate phylogenetic branch lengths from mitochondrial cytochrome oxidase subunit I (COI) sequences (sequence divergence: Arévalo et al. 2004). For the intraspecific comparisons of caste differences in brain region volume, we used general linear models/multiple regression methods (SPSS Inc., SPSS 15.0, 2007). Unless otherwise noted, all significant relationships shown are the results of partial correlation analysis after accounting for colony effects.
3. Results
(a). Overall species differences
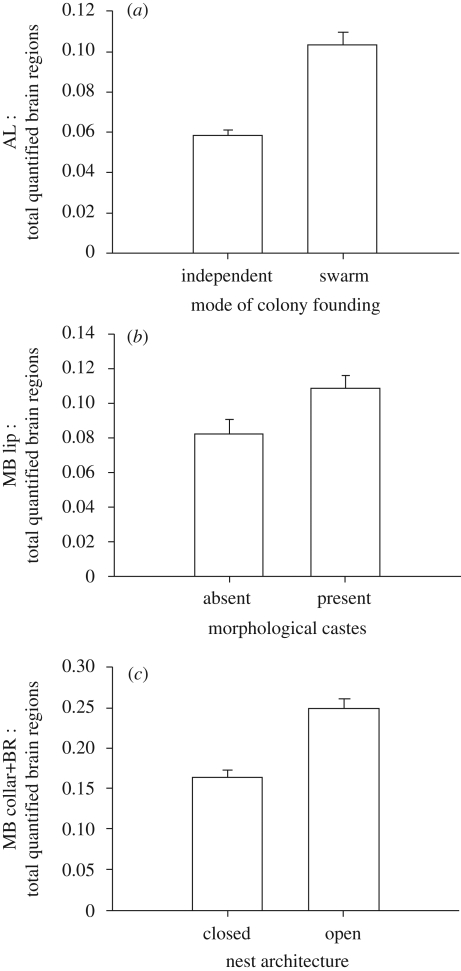
When queen and worker castes were analysed together, neuroanatomical brain regions associated with olfactory processing did not covary with nest architecture (see electronic supplementary material). SF species had significantly larger ALs than IF species (phylogenetic GLC multiple regression analysis, t7 = 3.00, p = 0.02; figure 1a), and species with morphological castes had larger MB calyx lips than caste monomorphic species (phylogenetic GLC multiple regression analysis, t4 = 4.00, p = 0.02; figure 1b). None of the covariates—nest architecture, colony founding, nor caste differences—were significantly related to the size of visual processing regions (the MB calyx collar + BR and the OLs).
Figure 1.
Bar graphs depicting relationships between brain organization and behavioural and caste covariates. (a) Mode of colony founding: bar graph representing means and s.e. of AL volume relative to the total volume of quantified brain regions in IF (n = 11: 4Q, 7W) and SF species (n = 54: 29Q, 25W). (b) Bar graph representing means and s.e. of the volume of the MB calyx lips relative to total quantified brain volume in species with (n = 24: 12Q, 12W) and without (n = 41: 21Q, 20W) morphological castes. (c) Bar graph representing means and s.e. of MB calyx collar + BR volume relative to total quantified brain volume for queens in species with open (n = 10 queens) and closed (n = 23 queens) nests.
(b). Caste-related species differences
Variation among queens appeared to account for species differences in brain structure. SF queens had larger ALs than IF queens (phylogenetic GLC multiple regression analysis, t7 = 5.00, p = 0.002). Queens from species with morphologically distinct castes had larger MB calyx lips than queens from monomorphic species (phylogenetic GLC multiple regression analysis, t7 = 4.00, p = 0.02). Furthermore, queens in species with open nests had larger MB calyx collar + BR values than queens in species with enclosed nests (GLS multiple regression analysis, t4 = −3.50, p = 0.02; figure 1c). When analysed separately, species differences among workers were weaker or absent (see electronic supplementary material).
(c). Intraspecific caste differences
In both of the IF species, which have open nests, queens had greater MB calyx volume dedicated to visual processing than workers (MB calyx collar + BR: Mischocyttarus mastigophorus, F1,6 = 6.21, p = 0.04; Polistes instabilis, F1,2 = 26.64, p = 0.04). In contrast, SF queens in some species with enclosed nests had decreased brain volume dedicated to visual processing relative to their workers (Agelaia xanthopus, OL: F1,6 = 10.12, p = 0.02; Protopolybia exigua, MB calyx collar + BR: F1,7 = 8.27, p = 0.02). In both the IF species, queens had more brain volume dedicated to olfaction than their nestmates (M. mastigophorus, AL: F1,6 = 7.28, p = 0.04; P. instabilis, MB calyx lip: F1,2 = 27.83, p = 0.03).
4. Discussion
We found that species differences in paper wasp brain structure correspond to variation in mode of colony founding, strength of queen/worker caste differences and nest architecture. The patterns further suggest that there is differential investment in modality-specific brain regions.
(a). Mode of colony founding
SF paper wasps had larger peripheral olfactory processing regions (ALs). Olfactory communication becomes increasingly important in social interactions during the evolution of advanced eusocial species (paper wasps, honeybees and ants; Van der Vecht 1959; Darchen 1976; Richards 1978; Le Conte & Hefetz 2008). Only SF paper wasps are known to use trail pheromones to communicate the location of new nest sites (Jeanne 1991; Smith et al. 2002). A greater proportion of SF species may employ alarm pheromones to indicate danger to the nest than IF species (Landolt et al. 1998), and at least some swarm founders rely on queen pheromones to regulate interactions with workers (Naumann 1975; West-Eberhard 1977; Forsyth 1978).
Our prediction of reduced central processing capacity in SF workers, as indicated by MB calyx volume, was not supported. We did not find significant differences in central processing regions between IF and SF species.
(b). Nest architecture: open versus closed nests
Greater constant exposure to light (i.e. open comb nests) was positively linked to larger visual processing regions (MB calyx collars + basal rings). Our data suggest that differential exposure to visual stimuli (e.g. light levels) correlates most strongly with differences in queen brain structure. Foraging away from the nest may provide similar exposure to visual stimuli for workers living in open and closed nests, but queens generally remain nest-bound. Furthermore, IF may use visual cues in dominance interactions (Jeanne 1972; Tibbetts 2002; Tibbetts & Dale 2007).
(c). Caste differences
Queens accounted for most of the species differences we documented: brain structure corresponded more strongly with mode of colony founding and nest architecture in queens than in workers. We suggest that queen brain structures are more diverse because queen behavioural roles vary more widely across species than worker roles. IF queens directly police their nestmates’ reproduction and control or eliminate worker-laid eggs (Gamboa et al. 1990; Reeve 1991; Premnath et al. 1996). IF queens often forage for building materials, while SF queens apparently never do so (O'Donnell 1998b,c). SF queens, in contrast, rarely interact physically with nestmates and may rely on broadcast chemical signals (queen pheromones; Naumann 1975; West-Eberhard 1977; Forsyth 1978). The reduction in SF queen behaviour to on-nest activity means markedly less exposure to environmental stimuli, especially for those living in closed nests, and a greater reliance on information within the nest (pheromones). The greater investment in olfactory processing regions (ALs) in SF species parallels this increased reliance on olfactory information on the nest. Smaller visual processing areas (MB collars) in queens living in closed nests also suggest that queen brain structure reflects the information most relevant for a nest-bound lifestyle.
We did not find evidence for the expected pattern of stronger queen/worker differences in species with morphological castes. Both the IF species we examined, but not most of the SF species, showed augmented olfactory processing neural tissue in queens relative to their workers. One possible explanation for the lack of queen/worker differences in SF is that the evolution of increased reliance on pheromonal communication (e.g. recruitment signals, trail pheromones) has impacted both castes (Landolt et al. 1998; Smith et al. 2002).
(d). Conclusion
Our species and caste comparisons suggest that paper wasps can provide an excellent venue for further comparative investigation in expanding the social intelligence hypothesis. The diversity of paper wasp social structures allowed us to test the effects of the species-specific cognitive challenges that each species faces. Paper wasp brain structure did not merely change with the evolutionary origin of sociality in Vespidae (Howse 1974). Wasp brain architecture mirrors the complex evolution of caste role specialization and social interactions in the Polistinae. Our data emphasize the importance of interpreting social interactions in terms of the sensory information being utilized and the cognitive demands they impose (Farris 2008). Our data provide a basis for further behavioural studies on these species to elucidate these cognitive demands. Our study generates predictions for relationships between sociality and brain structure in other social insects as well as in social vertebrates. For example, the mode of communication used in social interactions may correlate with greater investment in modality-specific brain regions. The need to discriminate between individual group mates, and to track individual identity across multiple interactions, may select for greater investment in higher-order brain regions. We believe that these cognitive demands, which can include higher-order processes such as sensory integration, learning and memory, underlie the positive relationships between brain size and sociality seen in many taxa (Howse 1974; Shultz & Dunbar 2006; Croney & Newberry 2007; Silk 2007).
More detailed analyses of the neural structure of brain regions, such as quantifying the number of sensory neurons in the antennae or the glomerular organization of ALs, would be useful in elucidating the developmental basis of the brain volume differences we measured. Although brain tissue dedicated to vision differed with nest architecture and light environments, we found evidence for changes in peripheral visual processing (OLs) in some species and central visual processing (MB collar + BR) in others. We cannot assess whether and how these brain regions might interact during development (Molina & O'Donnell 2008). It remains to be determined when central versus peripheral processing capacities will respond evolutionarily to changes in species light environments and social parameters (e.g. communication).
Acknowledgements
Two anonymous reviewers, M. Colon, C. Frederick, K. Helem, A. Kumar and T. Soare commented on the manuscript. W. Wood assisted with specimen collection in Ecuador, and G. Onore was a generous host in Ecuador. J. Carpenter, J. Felsenstein and E. Martins made suggestions on phylogenetic analysis. Field research was conducted under permits from the Republics of Costa Rica and Ecuador. Thanks to the Organization for Tropical Studies (Costa Rica), the Catholic University (Quito, Ecuador) and the University of San Francisco (Quito, Ecuador) for assistance in obtaining permits. Research was supported by the Society for Comparative and Integrative Biology (FGST) to Y.M. and by NSF grant IBN-0347315 to S.O'D. Additional support was provided by NSF while S.O'D. was working at the foundation. Any opinions, findings and the conclusions or recommendations are those of the authors and do not necessarily reflect the views of NSF.
References
- Anderson J. R.1998Social stimuli and social rewards in primate learning and recognition. Behav. Process. 42, 159–175 (doi:10.1016/S0376-6357(97)00074-0) [DOI] [PubMed] [Google Scholar]
- Anderson C., McShea D. W.2001Individual versus social complexity, with particular reference to ant colonies. Biol. Rev. 76, 211–237 (doi:10.1017/S1464793101005656) [DOI] [PubMed] [Google Scholar]
- Arévalo E., Zhu Y., Carpenter J. M., Strassmann J. E.2004The phylogeny of the social wasp subfamily Polistinae: evidence from microsatellite flanking sequences, mitochondrial COI sequence, and morphological characters. BMC Evol. Biol. 4, 1–16 (doi:10.1186/1471-2148-4-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth M., Heisenberg M.1997Vision affects mushroom bodies and central complex in Drosophila melanogaster. Learn. Mem. 4, 219–229 (doi:10.1101/lm.4.2.219) [DOI] [PubMed] [Google Scholar]
- Barton R. A., Harvey P. H.2000Mosaic evolution of brain structure in mammals. Nature 405, 1055–1058 (doi:10.1038/35016580) [DOI] [PubMed] [Google Scholar]
- Beshers S. N., Fewell J. H.2001Models of division of labor in social insects. Ann. Rev. Entomol. 46, 413–440 (doi:10.1146/annurev.ento.46.1.413) [DOI] [PubMed] [Google Scholar]
- Bingman V. P.2004The importance of comparative studies and ecological validity for understanding hippocampal structure and cognitive function. Hippocampus 2, 213–219 (doi:10.1002/hipo.450020302) [DOI] [PubMed] [Google Scholar]
- Bourke A. F. G.1999Colony size, social complexity and reproductive conflict in social insects. J. Evol. Biol. 12, 245–257 (doi:10.1046/j.1420-9101.1999.00028.x) [Google Scholar]
- Capaldi E. A., Robinson G. E., Fahrbach S. E.1999Neuroethology of spatial learning: the birds and the bees. Ann. Rev. Psychol. 50, 651–682 (doi:10.1146/annurev.psych.50.1.651) [DOI] [PubMed] [Google Scholar]
- Carpenter J. M.1991Phylogenetic relationships and the origin of social behavior in the Vespinidae. In The social biology of wasps (eds Ross K. G., Matthews R. W.), pp. 7–32 Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- Chittka L., Thomson J. D., Waser N. M.1999Flower constancy, insect psychology and plant evolution. Naturwissenschaften 86, 361–377 (doi:10.1007/s001140050636) [Google Scholar]
- Clayton N. S.1998Memory and the hippocampus in food-storing birds: a comparative approach. Neuropharmacology 37, 441–452 (doi:10.1016/S0028-3908(98)00037-9) [DOI] [PubMed] [Google Scholar]
- Croney C. C., Newberry R. C.2007Group size and cognitive processes. Appl. Anim. Behav. Sci. 103, 215–228 (doi:10.1016/j.applanim.2006.05.023) [Google Scholar]
- Darchen R.1976La formation d'une nouvelle colonie de Polybiodes tabidus. Fab. C R Acad. Sci. Ser. D 282, 457–459 [Google Scholar]
- Deshpande S. A., Sumana A., Surbeck M., Gadagkar R.2006Wasp who would be queen: a comparative study of two primitively eusocial species. Curr. Sci. 91, 332–336 [Google Scholar]
- Dunbar R. I. M., Shultz S.2007Evolution in the social brain. Science 317, 1344–1347 (doi:10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- Ehmer B., Gronenberg W.2004Mushroom body volumes and visual interneurons in ants: comparison between sexes and castes. J. Comp. Neurol. 469, 198–213 (doi:10.1002/cne.11014) [DOI] [PubMed] [Google Scholar]
- Ehmer B., Hoy R.2000Mushroom bodies of vespid wasps. J. Comp. Neurol. 416, 93–100 (doi:10.1002/(SICI)1096-9861(20000103)416:1<93::AID-CNE7>3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- Ehmer B., Reeve H. K., Hoy R. R.2001Comparison of brain volumes between single and multiple foundresses in the paper wasp Polistes dominulus. Brain Behav. Evol. 57, 161–168 (doi:10.1159/000047234) [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E.2006Organization of the mushroom bodies of the insect brain. Ann. Rev. Entomol. 51, 209–232 (doi:10.1146/annurev.ento.51.110104.150954) [DOI] [PubMed] [Google Scholar]
- Farris S. M.2005Evolution of insect mushroom bodies: Old clues, new insights. Arthropod Struct. Dev. 34, 211–234 (doi:10.1016/j.asd.2005.01.008) [Google Scholar]
- Farris S. M.2008Evolutionary convergence of higher brain centers spanning the protosome–deuterostome boundary. Brain Behav. Evol. 72, 106–122 (doi:10.1159/000151471) [DOI] [PubMed] [Google Scholar]
- Farris S. M., Roberts N. S.2005Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proc. Natl Acad. Sci. USA 102, 17 394–17 399 (doi:10.1073/pnas.0508430102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J.1985Phylogenies and the comparative method. Am. Nat. 125, 1–15 (doi:10.1086/284325) [Google Scholar]
- Forsyth A. B.1978Studies on the behavioral ecology of polygynous social wasps Cambridge, MA: Harvard University [Google Scholar]
- Gamboa G. J., Wacker T. L., Scope J. A., Cornell T. J., Shellman-Reeve J.1990The mechanism of queen regulation of foraging in paper wasps (Polistes fuscatus, Hymenoptera: Vespidae). Ethology 85, 335–343 [Google Scholar]
- Gordon D. M.1996The organization of work in social insect colonies. Nature 380, 121–124 (doi:10.1038/380121a0) [Google Scholar]
- Greiner B.2006Visual adaptations in the night-active wasp Apoica pallens. J. Comp. Neurol. 495, 255–262 (doi:10.1002/cne.20882) [DOI] [PubMed] [Google Scholar]
- Groh C., Ahrens D., Rossler W.2006Environment and age-dependent plasticity of synaptic complexes in the mushroom bodies of honey bee queens. Brain Behav. Evol. 68, 1–14 (doi:10.1159/000092309) [DOI] [PubMed] [Google Scholar]
- Gronenberg W.2001Subdivisions of the hymenopteran mushroom body calyces by their afferent supply. J. Comp. Neurol. 435, 47–489 (doi:10.1002/cne.1045) [DOI] [PubMed] [Google Scholar]
- Gronenberg W., Liebig J.1999Smaller brains and optic lobes in reproductive workers of the ant Harpegnathos. Naturwissenschaften 86, 343–345 (doi:10.1007/s001140050631) [Google Scholar]
- Hampton R. R., Shettleworth S. J.1996Hippocampus and memory in a food-storing and in a nonstoring bird species. Behav. Neurosci. 110, 946–964 (doi:10.1037/0735-7044.110.5.946) [DOI] [PubMed] [Google Scholar]
- Hölldobler B., Wilson E. O.2009The superorganism: the beauty, elegance and strangeness of insect societies USA: W.W. Norton & Co [Google Scholar]
- Howse P. E.1974Design and function of the insect brain. In Experimental analysis of insect behavior (ed. Browne L. B.), pp. 180–195 Berlin, Germany: Springer [Google Scholar]
- Hunt J. H., Jeanne R. L., Keeping M. G.1995Observations on Apoica pallens, a nocturnal neotropical social wasp (Hymenoptera: Vespidae, Polistinae, Epiponini). Insect Soc. 42, 223–236 (doi:10.1007/BF01240417) [Google Scholar]
- Hunt J. H., Buck N. A., Wheeler D. E.2003Storage proteins in vespid wasps: characterization, developmental pattern and occurrence in adults. J. Insect Physiol. 49, 785–794 (doi:10.1016/S0022-1910(03)00115-X) [DOI] [PubMed] [Google Scholar]
- Jeanne R. L.1972Social biology of the Neotropical wasp: Mischocyttarus drewseni. Bull. Mus. Comp. Zool. Harvard Univ. 144, 63–150 [Google Scholar]
- Jeanne R. L.1975Adaptiveness of social wasp nest architecture. Q. Rev. Biol. 50, 267–287 (doi:10.1086/408564) [Google Scholar]
- Jeanne R. L.1981Chemical communication during swarm emigration in the social wasp Polybia sericea (Olivier). Anim. Behav. 29, 102–113 (doi:10.1016/S0003-3472(81)80157-1) [Google Scholar]
- Jeanne R. L.1991The swarm-founding Polistinae. In The social biology of wasps (eds Ross K. G., Matthews R. W.), pp. 191–231 Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- Jeanne R. L.2003Social complexity in the Hymenoptera, with special attention to wasps. In Genes, behaviors and evolution of social insects (eds Kitkuchi T., Azuma N., Higashi S.), pp. 81–131 Sapporo, Japan: Hokkaido University Press [Google Scholar]
- Jeanne R. L., Graf C. A., Yandell B. S.1995Non-size-based morphological castes in a social insect. Naturwissenschaften 82, 296–298 (doi:10.1007/BF01134530) [Google Scholar]
- Julian G. E., Gronenberg W.2002Reduction of brain volume correlates with behavioral changes in queen ants. Brain Behav. Evol. 60, 152–164 (doi:10.1159/000065936) [DOI] [PubMed] [Google Scholar]
- Keeping M. G.2000Morphological physiological variability and differentiation of reproductive roles among foundresses of the primitively eusocial wasp Belongaster petiolata (Deeger) (hymenoptera: Vespidae). Insect Soc. 47, 147–154 (doi:10.1007/PL00001693) [Google Scholar]
- Keeping M. G.2002Reproductive and worker castes in the primitive eusocial wasp Belongaster petiolata (DeGeer) (Hymenoptera: Vespidae): evidence for pre-imaginal determination. J. Insect Physiol. 48, 867–879 (doi:10.1016/S0022-1910(02)00156-7) [DOI] [PubMed] [Google Scholar]
- Keller L.1993Queen number and sociality in insects New York, NY: Oxford University Press [Google Scholar]
- Landolt P. J., Jeanne R. L., Reed H. C.1998Chemical communication in social wasps. In Pheromone communication in social insects (eds Vander Meer R. K., Breed M., Winston M., Espelie C.), pp. 216–235 Boulder, CO: Westview Press [Google Scholar]
- Le Conte Y., Hefetz A.2008Primer pheromones in social Hymenoptera. Ann. Rev. Entomol. 53, 523–542 (doi:10.1146/annurev.ento.52.110405.091434) [DOI] [PubMed] [Google Scholar]
- Mares S., Ash L., Gronenberg W.2005Brain allometry in bumblebee and honey bee workers. Brain Behav. Evol. 66, 50–61 (doi:10.1159/000085047) [DOI] [PubMed] [Google Scholar]
- Martins E. P., Hansen T. F.1996Translating between microevolutionary process and macroevolutionary patterns: the correlation structure of interspecific data. Evolution 50, 1404–1417 (doi:10.2307/2410878) [DOI] [PubMed] [Google Scholar]
- Martins E. P., Hansen T. F.1997Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 (doi:10.1086/286013) [Google Scholar]
- McPheron L. J., Mills N. J.2007Discrimination learning of color-odor compounds in a paper wasp (Hymenoptera: Vespidae: Pompilinae: Mischocyttarus flavitarsis). Entomol. Gen. 29, 125–134 [Google Scholar]
- Molina Y., O'Donnell S.2007Mushroom body volume is related to social aggression and ovary development in the paperwasp Polistes instabilis. Brain Behav. Evol. 70, 137–144 (doi:10.1159/000102975) [DOI] [PubMed] [Google Scholar]
- Molina Y., O'Donnell S.2008Age, sex, and dominance-related mushroom body plasticity in the paperwasp Mischocyttarus mastigophorus. Dev. Neurobiol. 68, 950–959 (doi:10.1002/dneu.20633) [DOI] [PubMed] [Google Scholar]
- Molina Y., O'Donnell S.2009Worker reproductive competition affects division of labour in a primitively social paperwasp. Insectes Soc. 56, 14–20 (doi:10.1007/s00040-008-1027-0) [Google Scholar]
- Nascimento F. S., Tannure-Nascimento I. C.2005Foraging patterns in a nocturnal swarm-foudning wasp, Apoica flavissima van der Vecht (Hymenoptera: Vespidae). Neotrop. Entomol. 34, 177–182 [Google Scholar]
- Naumann M. G.1975Swarming behavior: evidence for communication in social wasps. Science 189, 642–644 (doi:10.1126/science.1162347) [DOI] [PubMed] [Google Scholar]
- Nawroth J. C., Greer C. A., Chen W. R., Laughlin S. B., Shepherd G. M.2007An energy budget for the olfactory glomerulus. J. Neurosci. 27, 9790–9800 (doi:10.1523/JNEUROSCI.1415-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven J. E.2007Brains, islands and evolution: breaking all the rules. Trends Ecol. Evol. 22, 57–59 (doi:10.1016/j.tree.2006.11.009) [DOI] [PubMed] [Google Scholar]
- Niven J. E., Vähäsöyrinki M., Juusola M.2003Shaker K+ channels are predicted to reduce the metabolic cost of neural information in Drosophila photoreceptors. Proc. R. Soc. Lond. B 270, S58–S61 (doi:10.1098/rsbl.2003.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll F. B., Wenzel J. W., Zucchi R.2004Evolution of caste in neotropical swarm-founding wasps. Am. Mus. Novit. 3467, 1–24 (doi:10.1206/0003-0082(2004)467<0001:EOCINW>2.0.CO;2) [Google Scholar]
- O'Donnell S.1998aReproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Ann. Rev. Entomol. 43, 323–346 (doi:10.1146/annurev.ento.43.1.323) [DOI] [PubMed] [Google Scholar]
- O'Donnell S.1998bDominance and polyethism in the eusocial wasp Mischocyttarus mastigophorus (Hymenoptera: Vespidae). Behav. Ecol. Sociobiol. 43, 327–331 (doi:10.1007/s002650050498) [Google Scholar]
- O'Donnell S.1998cEffects of experimental forager removals on division of labour in the primitively eusocial wasp Polistes instabilis (Hymenoptera: Vespidae). Behaviour 135, 173–193 [Google Scholar]
- O'Donnell S., Jeanne R. L.1990Forager specialization and the control of nest repair in Polybia occidentalis Olivier (Hymenoptera: Vespidae). Behav. Ecol. Sociobiol. 27, 359–364 (doi:10.1007/BF00164007) [Google Scholar]
- O'Donnell S., Jeanne R. L.1992Life-long patterns of forager behaviour in a tropical swarm-founding wasp: effects of specialization and activity level on longevity. Anim. Behav. 44, 1021–1027 (doi:10.1016/S0003-3472(05)80314-8) [Google Scholar]
- O'Donnell S., Donlan N. A., Jones T. A.2004Mushroom body structural plasticity is associated with temporal polyethism in eusocial wasp workers. Neurosci. Lett. 356, 159–162 (doi:10.1016/j.neulet.2003.11.053) [DOI] [PubMed] [Google Scholar]
- O'Donnell S., Donlan N. A., Jones T. A.2007Organizational and dominance-associated differences in mushroom body organization in the paper wasp, Mischocyttarus mastigophorus. J. Neurobiol. 67, 39–46 (doi:10.1002/neu.20324) [DOI] [PubMed] [Google Scholar]
- Øyvind Ø., Charmaine A. H., Winberg S.1999Short-term effects of fights for social dominance and establishment of dominant–subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav. Evol. 54, 263–275 (doi:10.1159/000006627) [DOI] [PubMed] [Google Scholar]
- Premnath S., Sinha A., Gadagkar R.1996Dominance relationship in the establishment of a reproductive division of labor in a primitively eusocial wasp (Ropalidia marginata). Behav. Ecol. Sociobiol. 39, 125–132 (doi:10.1007/s002650050274) [Google Scholar]
- Raveret-Richter M., Jeanne R. L.1991Hunting behavior, prey capture, and ant aviodance in the tropical social wasp Polybia sericea (Hymenoptera: Vespidae). Insect. Soc. 38, 139–148 (doi:10.1007/BF01240964) [Google Scholar]
- Reeve H. K.1991Polistes. The social biology of wasps (eds Ross K. G., Matthews R. W.), pp. 99–148 Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- Richards O. W.1978The Australian social wasps. Aust. J. Zool. S61, 1–132 [Google Scholar]
- Richter M. R.2007Social wasp (Hymenoptera: Vespidae) foraging behavior. Annu. Rev. Entomol. 45, 121–150 (doi:10.1146/annurev.ento.45.1.121) [DOI] [PubMed] [Google Scholar]
- Shultz S., Dunbar R. I. M.2006Both social and ecological factors predict ungulate brain size. Proc. R. Soc. B 273, 207–215 (doi:10.1098/rspb.2005.3283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J. B.2007The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559 (doi:10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. R., O'Donnell S., Jeanne R. L.2002Evolution of swarm communication in eusocial wasps (Hymenoptera: Vespidae). J. Insect Behav. 16, 751–764 (doi:10.1023/A:1021119322398) [Google Scholar]
- Strassmann J. E.2001The rarity of multiple matings in Hymenoptera. Insect. Soc. 48, 1–13 (doi:10.1007/PL00001737) [Google Scholar]
- Tibbetts E. A.2002Visual signals of individual identity in the paper wasp Polistes fuscatus. Proc. R. Soc. Lond. B 269, 1423–1428 (doi:10.1098/rspb.2002.2031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts E. A., Dale J.2007Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537 (doi:10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- Tibbetts E. A., Lindsay R.2008Visual signals of status and rival assessment in Polistes dominulus paper wasps. Biol. Lett. 4, 237–239 (doi:10.1098/rsbl.2008.0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Vecht J.1959Notes on Oriental Vespinae including some species from China and Japan. Zool. Meded. Rijks. Mus. Nat. Hist. Leiden 34, 205–232 [Google Scholar]
- Warrant E. J.2008Seeing in the dark: vision and visual behaviour in nocturnal bees and wasps. J. Exp. Biol. 211, 1737–1746 (doi:10.1242/jeb.015396) [DOI] [PubMed] [Google Scholar]
- Wehner R., Fukushi T., Isler K.2007On being small: brain allometry in ants. Brain Behav. Evol. 69, 220–228 (doi:10.1159/000097057) [DOI] [PubMed] [Google Scholar]
- Weiss M. R., Wilson E. E., Castellanos I.2004Predatory wasps learn to overcome the shelter defences of their larval prey. Anim. Behav. 68, 45–54 (doi:10.1016/j.anbehav.2003.07.010) [Google Scholar]
- Wenzel J. W.1991Evolution of nest architecture. In The social biology of wasps (eds Ross K. G., Matthews R. W.), pp. 480–519 Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- West-Eberhard M. J.1977The establishment of reproductive dominance in social wasp colonies. In Proc. 8th Int. Congr. of IUSSI, Wageningen, The Netherlands, pp. 223–227 [Google Scholar]
- Withers G. S., Fahrbach S. E., Robinson G. E.1993Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364, 238–240 (doi:10.1038/364238a0) [DOI] [PubMed] [Google Scholar]