Abstract
The family Amphipithecidae is one of the two fossil primate taxa from Asia that appear to be early members of the anthropoid clade. Ganlea megacanina, gen. et sp. nov., is a new amphipithecid from the late middle Eocene Pondaung Formation of central Myanmar. The holotype of Ganlea is distinctive in having a relatively enormous lower canine showing heavy apical wear, indicating an important functional role of the lower canine in food preparation and ingestion. A phylogenetic analysis of amphipithecid relationships suggests that Ganlea is the sister taxon of Myanmarpithecus, a relatively small-bodied taxon that has often, but not always, been included in Amphipithecidae. Pondaungia is the sister taxon of the Ganlea + Myanmarpithecus clade. All three Pondaung amphipithecid genera are monophyletic with respect to Siamopithecus, which is the most basal amphipithecid currently known. The inclusion of Myanmarpithecus in Amphipithecidae diminishes the likelihood that amphipithecids are specially related to adapiform primates. Extremely heavy apical wear has been documented on the lower canines of all three genera of Burmese amphipithecids. This distinctive wear pattern suggests that Burmese amphipithecids were an endemic radiation of hard object feeders that may have been ecological analogues of living New World pitheciin monkeys.
Keywords: Amphipithecidae, Eocene, Myanmar, anthropoid origins
1. Introduction
Since their initial description in the early twentieth century, amphipithecid primates have figured prominently in discussions of anthropoid origins (Pilgrim 1927; Colbert 1937; Szalay 1970, 1972; Simons 1971; Ba Maw et al. 1979; Ciochon et al. 1985). Thanks to renewed field efforts in the Eocene Pondaung Formation of Myanmar, a great deal of additional amphipithecid material has been recovered and described during the past decade (Jaeger et al. 1998, 2004; Chaimanee et al. 2000a; Gunnell et al. 2002; Shigehara et al. 2002; Marivaux et al. 2003; Takai & Shigehara 2004). This newfound abundance of amphipithecid fossils has so far failed to quell the longstanding debate regarding their phylogenetic affinities. A majority of recent workers support anthropoid affinities for amphipithecids (Jaeger et al. 1998, 2004; Chaimanee et al. 2000a; Beard 2002, 2004; Marivaux et al. 2003, 2008; Beard et al. 2005, 2007; Bajpai et al. 2008; Rose et al. 2009). Others maintain that dental and gnathic similarities between amphipithecids and early anthropoids merely reflect convergent adaptations to similar diets, thereby obscuring what they regard to be the adapiform affinities of the former group (Ciochon & Holroyd 1994; Ciochon & Gunnell 2002, 2004; Gunnell et al. 2002, 2008).
Much of the ongoing disagreement about the higher-level relationships of amphipithecids can be attributed to the nature of their fossil record. Amphipithecids are documented primarily on the basis of teeth and jaws from the Pondaung Formation, although the group is also known from the latest Eocene of peninsular Thailand and the Oligocene of central Pakistan (Chaimanee et al. 1997, 2000b; Marivaux et al. 2005). Several cranial and postcranial fossils from the Pondaung Formation have been allocated to Amphipithecidae, but none of these specimens was found in direct association with diagnostic amphipithecid dental remains, rendering all of them controversial to a greater or lesser extent. Particularly problematic in this regard are a partial skeleton of a large-bodied primate (NMMP 20) and two cranial fragments (NMMP 19 and NMMP 27) that have been referred to the Amphipithecidae (Ciochon et al. 2001; Gunnell et al. 2002; Takai et al. 2003). The purported amphipithecid affinities of the NMMP 20 partial skeleton conflict with the anatomy of an isolated primate astragalus from the Pondaung Formation, which bears diagnostic anthropoid traits (Marivaux et al. 2003). We regard the NMMP 20 partial skeleton as that of a large-bodied sivaladapid adapiform, rendering it irrelevant to discussions of amphipithecid relationships (Beard et al. 2007; Marivaux et al. 2008). Similarly, the Pondaung cranial fragments do not appear to pertain to any primate and are unlikely to be mammalian (Beard et al. 2005).
The lower-level systematics of amphipithecids from Myanmar is also in a state of flux. The first two genera that were proposed, Pondaungia and Amphipithecus, are now considered to be synonymous by some experts (Jaeger et al. 2004), a view that is endorsed here. Questions remain regarding the number of valid species of Pondaungia, an issue that is complicated by the possibility of a high level of sexual dimorphism in this group. More problematic is Myanmarpithecus, another fossil primate from the Pondaung Formation that was initially described as a probable basal anthropoid of uncertain taxonomic affinities (Takai et al. 2001). Subsequent authors have regarded Myanmarpithecus as either an omomyid (Ciochon & Gunnell 2002; Gunnell et al. 2008) or another member of the Amphipithecidae (Kay et al. 2004a,b; Marivaux et al. 2005). Here, we describe a new genus of Amphipithecidae from the Pondaung Formation and reassess the phylogenetic and paleobiological affinities of this group in light of this new taxon.
2. Systematic palaeontology
Class Mammalia Linnaeus, 1758
Order Primates Linnaeus, 1758
Suborder Haplorhini Pocock, 1918
Infraorder Anthropoidea Mivart, 1864
Family Amphipithecidae Godinot, 1994
Ganlea megacanina, gen. et sp. nov.
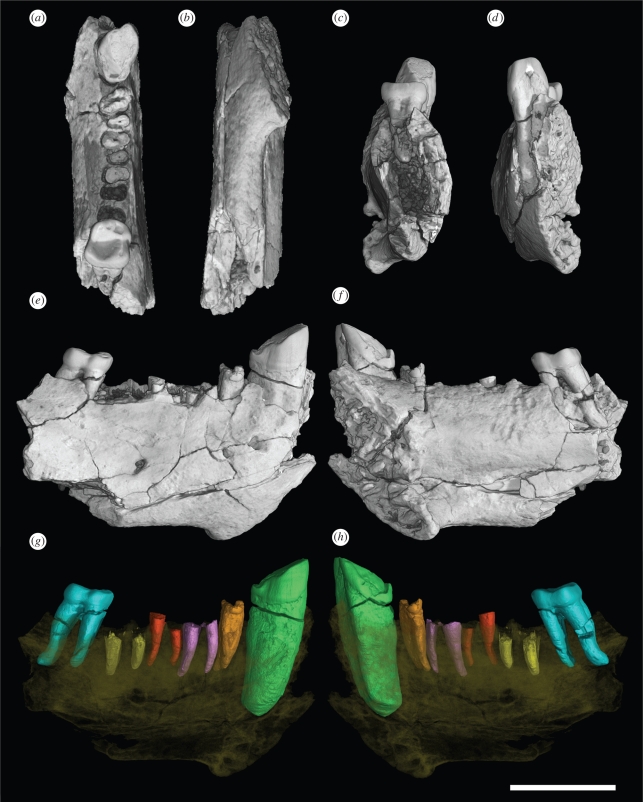
Holotype: NMMP 70, a right dentary preserving the crowns of C1 and M2 and the roots or alveoli for P2–M1 (figure 1). Partial alveoli for I1–2 are also preserved.
Figure 1.
Ganlea megacanina, gen. et sp. nov.: holotype right dentary (NMMP 70) preserving the crowns of C1 and M2 and the roots or alveoli for P2–M1. Partial alveoli for I1–2 are also preserved. The specimen is presented by 3D rendering in (a) occlusal, (b) inferior, (c) distal, (d) mesial, (e) buccal and (f) lingual views. Images (a–f) have been generated from 3D data obtained by X-ray synchrotron microtomography (SR-µCT) on the beamline ID19 at the European Synchrotron Radiation Facility (ESRF, Grenoble, France). Images g and h are 3D rendering of NMMP 70 showing the colour-coded crowns and roots of individual teeth, which have been virtually delimited by manual segmentation. Scale bar equals 1 cm.
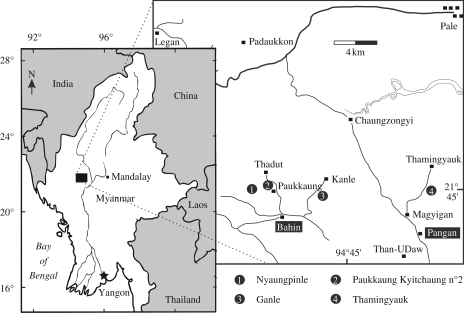
Type locality: Ganle kyitchaung, GPS coordinates = 21°44′04.6″ N, 94°43′25.0″ E (figure 2).
Figure 2.
Location map for the fossiliferous localities in the Bahin (Nyaungpinle, Paukkaung Kyitchaung 2, Ganle) and Pangan (Thamingyauk) areas of central Myanmar.
Age and distribution: Late middle Eocene Pondaung Formation, Myanmar.
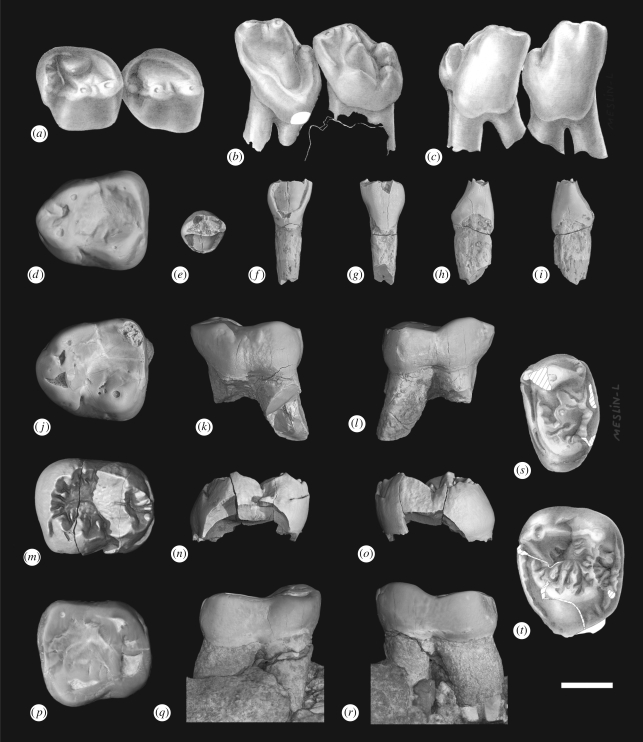
Hypodigm: The holotype; NMMP 69, an isolated left M1 from Nyaungpinle (figure 3d); NMMP 71, an isolated left I2 from Nyaungpinle (figure 3e–i); NMMP 72, an isolated right M2 from Nyaungpinle (figure 3m–o); NMMP 73, an isolated left M1 from Thamingyauk (figure 3j–l); NMMP 74, a right dentary preserving P3–4 from Thamingyauk (figure 3a–c); NMMP 75, an isolated left M1 or M2 from Paukkaung kyitchaung 2 (figure 3t); and NMMP 76, an isolated left P4 from Paukkaung kyitchaung 2 (figure 3s).
Figure 3.
Ganlea megacanina, gen. et sp. nov.: (a–c) right dentary fragment bearing P3–4 (NMMP 74) in (a) occlusal, (b) lingual and (c) buccal views; (d) left M1 (NMMP 69) in occlusal view; (e–i) left lower incisor (NMMP 71) in (e) occlusal, (f) lingual, (g) labial, (h) mesial and (i) distal views; (j–l) left M1 (NMMP 73) in (j) occlusal, (k) buccal and (l) lingual views; (m–o) right M2 (NMMP 72) in (m) occlusal, (n) buccal and (o) lingual views; (p–r) right M2 of the holotype (NMMP 70) in (p) occlusal, (q) buccal and (r) lingual views; (s) left P4 (NMMP 76) in occlusal view; (t) left M1 or M2 (NMMP 75) in occlusal view. Scale bar equals 2 mm. The 3D renderings (d–r) have been obtained by X-ray SR-µCT. Original art (drawings a–c, s–t) by Laurence Meslin, copyright CNRS-Meslin.
Diagnosis: Amphipithecid primate smaller than Pondaungia and Siamopithecus but larger than Myanmarpithecus. Symphyseal region of dentary less vertical in orientation than in Pondaungia and Siamopithecus. C1, at least in male individuals, and P2 larger relative to molar size than in other amphipithecids. C1 further differs from that of Pondaungia and Siamopithecus in having a small, distobuccal tubercle or heel. P2–4 relatively larger and less compressed mesiodistally than in Pondaungia. P3–4 with elevated and mesially oriented preprotocristid, in contrast to the condition in other amphipithecids. P3 further differs from that of Myanmarpithecus and Pondaungia in lacking a metaconid and having a reduced talonid heel. Lower molars differ from those of Bugtipithecus in lacking a prominent lingual notch at the base of the postvallid. M1–2 without hypoconulid, in contrast to Siamopithecus.
Etymology: The generic name derives from the village of Ganle, situated near the type locality. The trivial name refers to the relatively massive canine preserved in the holotype.
Description of the holotype: The dentary is robustly constructed, measuring 14.15 mm in depth and 6.75 mm in width just behind the symphysis. The symphysis is unfused, but its surface is characterized by a highly rugose pattern of alternating ridges, pits and valleys that would have interlocked with corresponding structures on the opposite side. As it is preserved, the greatest vertical height of the symphysis is 18.05 mm. It extends inferiorly and posteriorly to a point below the junction of the mesial and distal roots of P3. The inclination of the main axis of the symphysis in relation to a horizontal line through the toothrow is 53°. The planum alveolare slopes at roughly 21°. The pit for the genioglossus muscle is notable for its depth, and it lies above a stoutly constructed inferior transverse torus. The lateral side of the dentary bears dual mental foramina anteriorly, and a third emissary foramen below the mesial root of M1. The anteriormost mental foramen lies beneath the interstitial junction between C1 and P2. It is significantly larger than the other mental foramen, which lies beneath the junction between P2 and P3.
Based on roots and/or alveoli preserved in the holotype, the lower dental formula of Ganlea was 2-1-3-3. The lower incisors are documented by partial alveoli located mesial to the lower canine crown. Based on the radius of curvature of the preserved lingual parts of these alveoli, it appears that I2 would have been slightly larger than I1. Both incisors were tiny (the estimated mesiodistal dimensions of the lower incisor roots are 0.85 mm for I1 and 1.10 mm for I2) and oriented almost vertically within the dentary. Given the narrow space available between the symphyseal surface and the lower canine crown, it is clear that the anterior lower dentition of Ganlea megacanina was tightly spaced. Indeed, a distinctive, vertically oriented groove on the mesial side of the canine root and the basal part of its crown is appropriate in position to have served as an embrasure for the adjacent I2.
The lower canine is massive (length, 5.90 mm; width, 4.30 mm), being absolutely larger in terms of both mesiodistal length and buccolingual width than the lower canine of NMMP 24, a dentary of Pondaungia that pertains to an animal that was clearly much larger than Ganlea megacanina (Gunnell et al. 2002; Jaeger et al. 2004). The apex of the canine crown has been worn nearly flat, making it impossible to estimate the unworn height of the canine crown. From the enamel–dentine junction on the buccal side of the canine to the wear facet near its apex, the crown measures 4 mm in height. The level of the enamel–dentine junction is not uniform about the circumference of the canine crown. Rather, this junction extends apically on either side of the mesial embrasure groove for I2 noted earlier. In occlusal view the canine is roughly ovoid in outline, with a gently convex buccal surface and a nearly flat lingual surface. These surfaces are separated by the vertically oriented groove mesially and by a small heel or distension of the crown distally. The latter structure was probably characterized by a small tubercle in the unworn condition, but this cuspule (if it actually existed) has been nearly obliterated by the apical wear on the canine noted previously. Similarly obscured by apical wear, there appears to have been a distal crest that would have connected the apex of the canine crown with the distal heel or tubercle. A modest cingulid, which becomes more marked distally, adorns the lingual side of the canine crown, but no similar structure exists on the buccal side of the tooth.
P2–4 are documented in the holotype solely by their roots and aveoli. The crowns of P3–4 are preserved in a referred specimen, and these are described in the following section. We estimate that the combined mesiodistal length of P2–4 would have been roughly 9.25 mm. As is the case in other amphipithecids (and eosimiids), P2 is single-rooted while P3–4 are double-rooted. The single root of P2 is strongly compressed mesiodistally, so that its buccolingual width greatly exceeds its mesiodistal length. This condition also occurs in other amphipithecids, but it contrasts with the presumably more primitive condition found in eosimiids, in which the alvelous or root for P2 is roughly circular in cross section. High-resolution synchrotron images reveal that the P2 root in the holotype of Ganlea was particularly stout and asymmetrical in cross section. Although Myanmarpithecus and Pondaungia share the strong mesiodistal compression of P2 that occurs in Ganlea, the latter taxon is unique among amphipithecids in having a P2 root that is broader buccolingually than the corresponding roots of M1–2. Both P3–4 show the typical amphipithecid pattern of having the mesial root located farther buccally than the distal root, a condition that also occurs in eosimiids and many other basal anthropoid taxa.
M1 is documented in the holotype only by its roots and alveoli. Knowledge of the crown of M1 is based on two referred specimens that are described in the following section.
M2 (length 4.70 mm; width 4.50 mm) is a relatively quadrate, low-crowned tooth characterized by poorly defined cusps and weakly developed crests (figure 3p–r). The M2 crown in the holotype is moderately worn, obscuring some details of its morphology (NMMP 72 is a relatively unworn M2 that is described in the following section). The mesiodistally compressed trigonid shows no evidence of having a paraconid. The protoconid appears to have been slightly larger than the metaconid (at least in terms of its basal circumference), and the two cusps were partly separated by a longitudinal valley. A tiny mesial cingulid occurs in front of the metaconid. The talonid is broad, surrounded by low crests, and simple in construction. It bears a low entoconid and a much higher hypoconid. The cristid obliqua meets the postvallid very buccally, so that the hypoflexid is extremely shallow. Several minor folds of enamel run from the hypoconid and the cristid obliqua toward the central part of the talonid basin. A small interstitial wear facet on the distal side of the talonid demonstrates that M3 was present.
Description of the referred specimens: NMMP 71 (figure 3e–i) is an isolated left lower incisor (apical mesiodistal length, 1.90 mm; basal labiolingual breadth, 1.85 mm) that we tentatively identify as I2 on the basis of the mesiodistal dimension of its root (1.10 mm), which matches the size of the partial I2 alveolus in the holotype lower jaw (see earlier mentioned). The crown is broken apically, but that which remains can be described as being spatulate in shape. In mesial or distal view, the crown is roughly wedge-shaped, being broader at its base than near its apex. The enamel–dentine junction is not uniformly distributed about the base of the crown. Rather, it extends farther toward the root on the labial and lingual surfaces of the crown. Distally and (especially) mesially, the enamel–dentine junction arcs toward the apex of the tooth, thereby covering less of the root. The labial surface of the crown is gently convex, while the lingual surface is more irregular, but generally concave. The lingual surface of the crown is dominated by a central, pillar-like structure that is bounded mesially and distally by well-developed cingulids. The latter structures are connected by a more modest cingulid near the lingual base of the crown.
NMMP 74 (figure 3a–c) is a fragmentary dentary preserving the crowns of P3–4, both of which are double-rooted. P3 (length, 2.80 mm; width, 2.90 mm) is distinctive in showing strong basal distensions of enamel both buccally and lingually. The lingual base of the P3 crown is notably more asymmetrical than its buccal counterpart, because the degree of enamel distension is far more pronounced distally than mesially on the lingual side of the tooth. Similar P3 morphology occurs in Myanmarpithecus and Pondaungia, but Siamopithecus lacks the pronounced basal distension of P3 enamel that characterizes all three Burmese amphipithecids. The trigonid of P3 bears an elevated paraconid that is situated directly mesial to the protoconid. The latter two cusps are connected by the preprotocristid, yielding a relatively trenchant structure that approximates a blade-like morphology. The lingual side of the trigonid bears a relatively complete cingulid, but there is no development of a metaconid. The lingual cingulid is stronger distally than mesially, and it traces the general contour of the base of the crown. As such, the lingual cingulid reaches its inferiormost point near the junction of the trigonid and talonid. From there, it continues superiorly to define the lingual side of the abbreviated talonid. The talonid itself lacks a distinct basin. Rather, it forms a heel-like structure that consists of the hypoconid, the distalmost part of the lingual cingulid, and a vertically oriented crease that separates the trigonid and the talonid. A short, almost vertically oriented crest unites the hypoconid with the protoconid.
P4 (length, 3.05 mm; width, 3.10 mm) shows greater enamel distension buccally than lingually, thereby displaying the muted exodaenodont condition that is typical of most basal anthropoids. The trigonid is more nearly molariform than that of P3, because the metaconid is relatively well developed. The latter cusp is situated inferiorly and distally with respect to the protoconid. A modestly developed crest runs from the apex of the protoconid to the base of the metaconid. As is the case for P3, the preprotocristid is somewhat elevated and it extends almost directly mesially to unite with a small paraconid. The lingual cingulid is restricted to the trigonid, where it forms a thick shelf extending distally and inferiorly from the paraconid. Because the lingual cingulid fails to unite the paraconid with the metaconid, the trigonid is open lingually. The talonid of P4 is abbreviated, but better developed than that of P3. It is dominated by the hypoconid, which occurs at roughly the same height on the crown as the metaconid. Two moderately developed crests emanate from the hypoconid to line the buccal and distal margins of the talonid. The short cristid obliqua climbs the trigonid to become cofluent with the base of the protoconid. The postcristid runs lingually and inferiorly from the hypoconid, connecting that cusp with a tiny entoconid. The talonid itself consists of a short, arcuate valley between the trigonid and the postcristid.
NMMP 69 (figure 3d) (length, 4.30 mm; width, 3.90 mm) and NMMP 73 (figure 3j–l) (length, 4.20 mm; width, 3.80 mm) are isolated left M1s referred to Ganlea megacanina on the basis of their appropriate size and morphology. NMMP 69 shows slightly less wear than NMMP 73, but otherwise the two specimens are remarkably similar in morphology. The trigonid is less compressed mesiodistally than is the case for M2 in the holotype (see earlier mentioned), being roughly triangular in occlusal outline. The protoconid and metaconid are low, rounded cusps separated by either a sinuous valley (NMMP 69) or a tiny depression (NMMP 73). In terms of basal circumference, the protoconid is appreciably larger than the metaconid, but there is no apparent difference in the height of these cusps. Weakly developed crests, including an arcuate crest defining the mesial margin of the trigonid and a transverse crest marking the distal side of the trigonid, connect the protoconid with the metaconid. There is no development of a distinct paraconid. The talonid is remarkably broad and encircled by weak crests. The hypoconid and the cristid obliqua are much taller than the entoconid and its associated crests. The cristid obliqua is relatively straight, and it joins the postvallid very buccally, resulting in an extremely shallow hypoflexid. The hypoconulid is not developed as a distinct cusp.
NMMP 72 (figure 3m–o) (length, 4.45 mm) is an isolated right M2 from Nyaungpinle that appears to be an unerupted tooth germ. We tentatively refer this specimen to Ganlea megacanina here. Although the tooth is broken buccally, it appears to have been relatively narrower than M2 in the holotype. Its unworn condition is reflected by the presence of numerous enamel crenulations.
NMMP 76 (figure 3s) (length, 2.85 mm; width, 4.65 mm) is an isolated left P4 from Paukkaung kyitchaung 2. The crenulated crown is nearly rectangular in occlusal outline, although its lingual margin is rounded and slightly narrower than its buccal counterpart. The buccal margin of the crown is dominated by the paracone, the base of which is buccolingually compressed. Pre- and post-paracristae run mesially and distally from the apex of the paracone toward the margins of the tooth. The mesial cingulum is thick and continuous, running lingually from the terminus of the preparacrista to a point directly mesial to the protocone. The distal cingulum is similar in thickness but less distinct because it is interrupted by multiple enamel crenulations. The protocone is a low, rounded cusp situated near the mesiolingual margin of the crown. The preprotocrista is low but extensive, running buccally more or less parallel to the mesial cingulum until it reaches the base of the paracone. A short postprotocrista is confluent with the distal cingulum. The central part of the crown is covered by an irregular pattern of enamel crenulations that defies any systematic attempt at description.
The upper molar morphology of Ganlea is documented by NMMP 75 (figure 3t), an isolated left M1 or M2 (length, 4.15 mm; width, 5.25 mm). The crown is bunodont, bears crenulated enamel, and is basically tritubercular. The paracone is slightly larger than the metacone, and both buccal cusps are situated near the buccal margin of the crown, rather than being located more internally as is often the case in Pondaungia. Conules are not apparent. The protocone is situated mesial of the midline, such that it is closer to the paracone than the metacone. The pre- and post-protocristae are evident, but neither of these crests are well defined. The postprotocrista runs distally before turning buccally to become confluent with a lingual crest from the metacone (hypometacrista). There is no evidence of a pseudohypocone. The mesial cingulum is relatively strong and continuous, while the distal cingulum is narrower and interrupted by numerous enamel crenulations. A tiny cuspule that could be regarded as an incipient hypocone occurs near the lingual termination of the distal cingulum. Buccal and lingual cingula are either extremely weak or entirely absent.
Discussion: The adult body mass of Ganlea megacanina can be estimated on the basis of regressions of body mass as a function of M1 area in living primates (Conroy 1987). Using the anthropoid regression equation provided by Conroy (1987), we derive a mean adult body mass estimate of roughly 2.4 kg. The all-primate regression equation provided by Conroy (1987) yields a slightly lower estimate of mean adult body mass of roughly 1.9 kg. Hence, Ganlea would have been about the same size as the New World monkey Pithecia.
3. Phylogenetic analysis
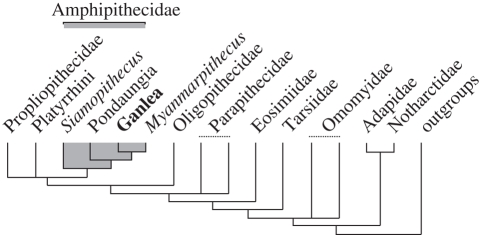
In order to assess the impact of Ganlea on the evolutionary relationships of Amphipithecidae, we performed a phylogenetic analysis based on a data matrix including 39 taxa and 326 characters (see the electronic supplementary material). Twelve maximally parsimonious trees were recovered. These trees have a tree length of 1584, a consistency index of 0.3226 and a retention index of 0.5467. A strict consensus tree that summarizes the phylogenetic resolution obtained by our analysis is depicted in figure 4.
Figure 4.
Strict consensus of 12 equally most parsimonious trees of 1584 steps each (CI = 0.32; RI = 0.54). This cladogram is a simplified tree highlighting the principal dichotomies among higher taxonomic primate groups. Further information is available as electronic supplementary material.
Notable results from this analysis include the novel finding that all three Burmese amphipithecids (Pondaungia, Myanmarpithecus and Ganlea) are monophyletic with respect to the Thai genus Siamopithecus. To reflect their seemingly close phylogenetic relationships, we refer all three Burmese amphipithecids to the subfamily Amphipithecinae, while Siamopithecus is classified here in a separate amphipithecid subfamily, Siamopithecinae. Many previous workers have failed to recognize that Myanmarpithecus is an amphipithecid (Takai et al. 2001; Ciochon & Gunnell 2002; Gunnell et al. 2002, 2008). Those who have accepted the amphipithecid affinities of Myanmarpithecus have typically regarded it as lying outside a putative clade of large-bodied forms including Pondaungia and Siamopithecus (Kay et al. 2004b; Marivaux et al. 2005). All Burmese amphipithecids (or Amphipithecinae) share a distinctive, derived suite of features in the lower premolar dentition that readily distinguishes them from Siamopithecus and other early anthropoids. In general, the crowns of P2–4 show a greater degree of mesiodistal compaction in Amphipithecinae (and particularly in Pondaungia) than is the case in Siamopithecus (table 1). This mesiodistal compaction is manifest in several morphological characters. For example, the root or alveolus for P2 in Amphipithecinae is mesiodistally compressed, such that its buccolingual breadth exceeds its length. Additionally, the bases of the crowns of P2-3 in Amphipithecinae are distended distolingually, often incorporating a strong lingual cingulid into this distolingual lobe. Finally, the talonids of P3–4 are extremely abbreviated in Amphipithecinae, such that they consist of little more than transverse furrows running lingually and inferiorly from the hypoconid. The talonid of P4 in Siamopithecus is less abbreviated, so that it retains a small but distinct talonid basin.
Table 1.
Quantification of the degree of lower premolar compaction among Amphipithecidae, based on the ratio of lower premolar length versus M2 length.
| Species | specimen | length, P2–4 (mm) | length, M2 (mm) | ratio, P2–4 : M2 |
|---|---|---|---|---|
| Ganlea megacanina | NMMP 70 | 9.25 | 4.70 | 1.97 |
| Siamopithecus eocaenus | TF 7624 | 13.67 | 6.75 | 2.02 |
| Pondaungia cotteri (large) | NMMP 17 | 9.26 | 8.20 | 1.13 |
| Pondaungia cotteri (large) | NMMP 24 | 8.42 | 7.50 | 1.12 |
| Pondaungia cotteri (small) | NMMP 30 | 8.64 | 6.70 | 1.29 |
Recognizing the amphipithecid affinities of Myanmarpithecus diminishes the possibility that amphipithecids are adapiforms rather than anthropoids. Previous workers who have argued for the adapiform affinities of amphipithecids have always maintained that Myanmarpithecus pertains to some other higher-level primate taxon, such as Omomyidae (Ciochon & Gunnell 2002; Gunnell et al. 2008). The dentition of Myanmarpithecus diverges radically from that of adapiform primates, rendering detailed comparisons virtually meaningless (Takai et al. 2001). Our phylogenetic analysis conflicts with the hypothesis that amphipithecids are related to adapiforms and places them in or near crown anthropoids instead. Indeed, all 12 of the most parsimonious trees used to generate the strict consensus tree shown in figure 4 recognize amphipithecids as being nested within crown clade anthropoids. These trees differ only in recognizing a closer relationship between amphipithecids and propliopithecids (as originally suggested by Jaeger et al. 1998) or a closer relationship between amphipithecids and platyrrhines. Accordingly, current evidence indicates that amphipithecids are much more advanced anthropoids than eosimiids. Additional evidence, particularly from the postcranium and skull of amphipithecids, is needed to test this hypothesis.
4. Feeding adaptations
The unique morphology of the teeth and jaws of amphipithecids has led many workers to regard them as hard object feeders that specialized on seeds and fruits with tough, resistant exteriors (Gunnell et al. 2002; Ciochon & Gunnell 2004; Kay et al. 2004a). The most detailed analysis of feeding adaptations in amphipithecids published to date is that of Kay et al. (2004a), who cite upper incisor morphology, molar shearing development, molar enamel thickness and mandibular morphology as evidence favoring hard object feeding for this group. Here, we explore the implications of the highly specialized lower canine morphology and lower canine wear pattern found in Ganlea and other amphipithecids for reconstructing their dietary habits.
The holotype of Ganlea megacanina is unique among Amphipithecidae in having a greatly enlarged lower canine relative to M1, which can be quantified using Gingerich's (1981) canine molar ratio (or CMR) (table 2). We interpret this as evidence that the holotype represents a male individual. However, some of the other amphipithecid specimens in our comparative sample, notably including the holotype of Myanmarpithecus yarshensis and a referred lower jaw of Siamopithecus eocaenus, probably also represent male individuals, yet their CMR values are much lower than that of the holotype of Ganlea megacanina (table 2). This suggests that the lower canine of Ganlea megacanina was hypertrophied beyond the level associated merely with canine dimorphism. Among living anthropoid primates, taxa that habitually use their canines to open hard objects such as highly resistant fruits have stouter canines that confer resistance to bending or breaking in both the mesiodistal and buccolingual planes (Plavcan & Ruff 2008). The hypertrophied lower canine of Ganlea could therefore reflect a similar functional adaptation in this amphipithecid.
Table 2.
Quantification of Gingerich's (1981) canine molar ratio (CMR) among Amphipithecidae and Pitheciini.
| species | specimen(s) | length × width, C1 (mm2) | length × width, M1 (mm2) | CMR |
|---|---|---|---|---|
| Ganlea megacanina | NMMP 70, 69, 73 | 25.4 | 16.8 | 1.51 |
| Myanmarpithecus yarshensis | NMMP 9, 37 | 8.1 | 10.5 | 0.77 |
| Siamopithecus eocaenus | TF 7624 | 32.0 | 39.0 | 0.82 |
| Pondaungia cotteri (large) | NMMP 24 | 22.4 | 34.4 | 0.65 |
| Pondaungia cotteri (small) | NMMP 61, 63 | 13.1 | 26.5 | 0.49 |
| Nuciruptor rubricae | IGM 251074 | 14.6 | 15.5 | 0.94 |
| Cebupithecia sarmientoi | UCMP 38762 | 20.8 | 13.3 | 1.56 |
| Chiropotes satanus | CM 51862 | 33.4 | 15.8 | 2.11 |
| Chiropotes satanus | CM 76820 | 33.4 | 15.7 | 2.13 |
The unusual apical wear pattern that occurs on the lower canine of Ganlea megacanina is consistent with this view. Apical wear such as that on the lower canine of Ganlea megacanina can only result from repetitive contact with hard food items that abrade the tooth crown. Normal tooth-on-tooth wear facets (attrition facets) are oriented more obliquely with respect to the tooth crown (Kay & Hiiemae 1974). Modern primate seed predators such as New World monkeys of the tribe Pitheciini habitually use their upper and lower canines to open hard, tough fruits to obtain the seeds contained inside (Kinzey 1992). We interpret both the hypertrophy of the lower canine in Ganlea megacanina and its heavy apical wear as evidence that this taxon engaged in pitheciin-like seed predation in which the canines were used to husk the hard exteriors of resistant fruits.
Although other amphipithecids lack the extreme canine robusticity seen in Ganlea (table 2), many of these taxa also show remarkably heavy apical wear on their canines, suggesting that they too engaged in pitheciin-like husking of hard fruits. For example, the holotype of Myanmarpithecus yarshensis (NMMP 9) shows extremely heavy apical wear on its lower canine (Takai et al. 2001), as does one of the few specimens of Pondaungia (NMMP 24) that preserves the lower canine crown intact (Gunnell et al. 2002; Jaeger et al. 2004). The heavy apical wear patterns on the lower canines of all three genera of Burmese amphipithecids suggest that the entire group was an endemic radiation of hard object feeders. Variation in canine robusticity among Burmese amphipithecids resembles that found among living pitheciins, in which Chiropotes and Cacajao show extreme canine hypertrophy while Pithecia has only modestly enlarged canines (Kinzey 1992). The degree of canine hypertrophy shown by Ganlea closely matches that which occurs in the Miocene pitheciin Cebupithecia sarmientoi, while less specialized amphipithecids resemble the Miocene pitheciin Nuciruptor rubricae in this regard (table 2). The extant pitheciin Chiropotes satanas shows an even greater degree of canine hypertrophy than does Ganlea (table 2). Although our reconstruction of dietary adaptations in amphipithecids is generally consistent with that of Kay et al. (2004a), consideration of the heavy apical wear on the lower canine of Myanmarpithecus yarshensis suggests that this taxon was also a specialized seed predator, rather than a generalized frugivore as Kay et al. (2004a) infer.
5. Discussion
The discovery of Ganlea provides some welcome resolution to the ongoing debate regarding the phylogenetic position of amphipithecids with respect to other primates, while at the same time it clarifies the evolutionary relationships within this extinct group of primates. Ganlea can be referred with confidence to the Amphipithecidae on the basis of its dental morphology, which shares numerous features in common with Pondaungia and Myanmarpithecus. Ganlea has the same lower dental formula that typifies all amphipithecids (and many other basal anthropoid taxa), and its single P2 root is strongly compressed mesiodistally (rather than being circular in cross section), as is also the case in other amphipithecids. However, its lower premolar series shows a lower degree of mesiodistal compaction (both in terms of the crowns and the roots) than is the case in Pondaungia, which has severely compressed its lower premolars relative to M2 length (table 1). In this respect, Ganlea resembles Myanmarpithecus and Siamopithecus, but this resemblance is probably only due to symplesiomorphy. Canine morphology suggests that Ganlea is the sister group of Myanmarpithecus, because both taxa share a distinctive distobuccal distension of the canine crown that yields a sinuous apical wear facet in older individuals. The loss of hypoconulids on M1–2, the greater degree of mesiodistal compaction of the lower premolars, and the strong distolingual distension of enamel on P2-3 that are shared by all of the amphipithecids from Myanmar appear to be derived characters linking these taxa to the exclusion of Siamopithecus.
The inclusion of Ganlea and Myanmarpithecus in Amphipithecidae makes it less probable that this extinct group of primates is closely related to adapiforms, as some recent workers have suggested (Ciochon et al. 2001; Ciochon & Gunnell 2002, 2004; Gunnell et al. 2002, 2008). Even those scholars who have emphasized similarities between certain adapiforms and large amphipithecids such as Pondaungia admit that Myanmarpithecus is unlikely to be related to the former group (Ciochon & Gunnell 2002; Gunnell et al. 2008). In many respects Ganlea is metrically and morphologically intermediate between Pondaungia and Myanmarpithecus, and our phylogenetic analysis suggests that all three of these Burmese taxa are nested within Amphipithecidae. Fossils of all three genera of Burmese amphipithecids show heavy apical wear on their lower canines, suggesting that all Burmese amphipithecids used their canines to husk the hard exteriors of fruits to extract the nutritious seeds contained inside. This apparent shared dietary specialization for hard object feeding corroborates our phylogenetic analysis, suggesting that the Burmese amphipithecid radiation was ecologically analogous to the radiation of modern New World pitheciin primates. Seed predation is an uncommon dietary strategy among primates, and there is no evidence that adapiforms ever invaded this dietary niche.
In terms of both abundance and alpha-level diversity, amphipithecids are the dominant primate group in the Pondaung Formation, although both eosimiids and sivaladapids occur there in much lower numbers (Jaeger et al. 1999; Gebo et al. 2002; Takai et al. 2005; Beard et al. 2007). At first glance, the diversity and abundance of amphipithecids appear to be at odds with the specialized dietary adaptation posited for them here. However, modern pitheciins of the Amazon Basin have radiated into three genera of seed predators that utilize different parts of the floodplain and different foraging strategies to minimize interspecific competition (Ayres 1989). The amphipithecids of the Pondaung Formation inhabited a paleo-Irrawaddy fluvial system that may have resembled the modern Amazon Basin in terms of seasonal flooding and general environmental conditions (Aung Naing Soe et al. 2002). The degree to which sedimentary and taphonomic processes may have biased the fossil record of the Pondaung Formation can only be determined by further field investigations.
Acknowledgements
We thank the many colleagues who helped us in the field, including Stéphane and Agnes Dovert, Mana Rugbumrung, Cornelis Schipper and Xavier Valentin. L. Meslin produced the original art included in figure 3. Richard Cifelli and two anonymous reviewers provided helpful comments on an earlier draft of the manuscript. Special thanks are extended to the villagers of Bahin, Paukkaung, Nyaungpinle and Magyigan, whose kindness and enthusiasm made our work a pleasure to undertake. This research was supported by funding from the US National Science Foundation (BCS 0820602, BCS 0309800), the French CNRS-Eclipse II Programme and the Thai-French TRF-CNRS Biodiversity Project (PICS Thaïlande).
References
- Soe Aung Naing, Myitta, Tun Soe Thura, Aung Aye Ko, Thein Tin, Marandat B., Ducrocq S., Jaeger J.-J.2002Sedimentary facies of the late middle Eocene Pondaung Formation (central Myanmar) and the palaeoenvironments of its anthropoid primates. C. R. Palevol 1, 153–160 [Google Scholar]
- Ayres J. M.1989Comparative feeding ecology of the uakari and bearded saki, Cacajao and. Chiropotes. J. Hum. Evol. 18, 697–716 (doi:10.1016/0047-2484(89)90101-2) [Google Scholar]
- Bajpai S., Kay R. F., Williams B. A., Das D. P., Kapur V. V., Tiwari B. N.2008The oldest Asian record of Anthropoidea. Proc. Natl Acad. Sci. USA 105, 11 093–11 098 (doi:10.1073/pnas.0804159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maw Ba, Ciochon R. L., Savage D. E.1979Late Eocene of Burma yields earliest anthropoid primate Pondaungia cotteri. Nature 282, 65–67 (doi:10.1038/282065a0) [DOI] [PubMed] [Google Scholar]
- Beard K. C.2002Basal anthropoids. In The primate fossil record (ed. Hartwig W. C.), pp. 133–149 Cambridge, UK: Cambridge University Press [Google Scholar]
- Beard K. C.2004. In The hunt for the dawn monkey: unearthing the origins of monkeys, apes, and humans Berkeley, CA: University of California Press [Google Scholar]
- Beard K. C., Jaeger J.-J., Chaimanee Y., Rossie J. B., Soe Aung Naing, Tun Soe Thura, Marivaux L., Marandat B.2005Taxonomic status of purported primate frontal bones from the Eocene Pondaung Formation of Myanmar. J. Hum. Evol. 49, 468–481 (doi:10.1016/j.jhevol.2005.05.008) [DOI] [PubMed] [Google Scholar]
- Beard K. C., Marivaux L., Tun Soe Thura, Soe Aung Naing, Chaimanee Y., Htoon Wanna, Marandat B., Aung Htun Htun, Jaeger J.2007New sivaladapid primates from the Eocene Pondaung Formation of Myanmar and the anthropoid status of Amphipithecidae. Bull. Carnegie Mus. Nat. Hist 39, 67–76 (doi:10.2992/0145-9058(2007)39[67:NSPFTE]2.0.CO;2) [Google Scholar]
- Chaimanee Y., Suteethorn V., Jaeger J.-J., Ducrocq S.1997A new late Eocene anthropoid primate from Thailand. Nature 385, 429–431 (doi:10.1038/385429a0) [DOI] [PubMed] [Google Scholar]
- Chaimanee Y., Thein Tin, Ducrocq S., Soe Aung Naing, Benammi M., Tun Than, Lwin Thit, Wai San, Jaeger J. T.2000aA lower jaw of Pondaungia cotteri from the late middle Eocene Pondaung Formation (Myanmar) confirms its anthropoid status. Proc. Natl Acad. Sci. USA 97, 4102–4105 (doi:10.1073/pnas.97.8.4102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaimanee Y., Khansubha S., Jaeger J.2000bA new lower jaw of Siamopithecus eocaenus from the late Eocene of Thailand. C. R. Acad. Sci. Paris (Sciences de la vie) 323, 235–241 [DOI] [PubMed] [Google Scholar]
- Ciochon R. L., Gunnell G. F.2002Chronology of primate discoveries in Myanmar: influences on the anthropoid origins debate. Yearb. Phys. Anthropol. 45, 2–35 (doi:10.1002/ajpa.10175) [DOI] [PubMed] [Google Scholar]
- Ciochon R. L., Gunnell G. F.2004Eocene large-bodied primates of Myanmar and Thailand: morphological considerations and phylogenetic affinities. In Anthropoid origins: new visions (eds Ross C. F., Kay R. F.), pp. 249–282 New York, NY: Kluwer [Google Scholar]
- Ciochon R. L., Holroyd P. A.1994The Asian origin of Anthropoidea revisited. In Anthropoid origins (eds Fleagle J. G., Kay R. F.), pp. 143–162 New York, NY: Plenum Press [Google Scholar]
- Ciochon R. L., Savage D. E., Tint T., Maw Ba.1985Anthropoid origins in Asia? New discovery of Amphipithecus from the Eocene of Burma. Science 229, 756–759 (doi:10.1126/science.229.4715.756) [DOI] [PubMed] [Google Scholar]
- Ciochon R. L., Gingerich P. D., Gunnell G. F., Simons E. L.2001Primate postcrania from the late middle Eocene of Myanmar. Proc. Natl Acad. Sci. USA 98, 7672–7677 (doi:10.1073/pnas.051003298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert E. H.1937A new primate from the upper Eocene Pondaung Formation of Burma. Am. Mus. Novit. 951, 1–18 [Google Scholar]
- Conroy G. C.1987Problems of body-weight estimation of fossil primates. Int. J. Primatol. 8, 115–137 (doi:10.1007/BF02735160) [Google Scholar]
- Gebo D. L., Gunnell G. F., Ciochon R. L., Takai M., Tsubamoto T., Egi N.2002New eosimiid primate from Myanmar. J. Hum. Evol. 43, 549–553 [Google Scholar]
- Gingerich P. D.1981Cranial morphology and adaptations in Eocene Adapidae. I. Sexual dimorphism in Adapis magnus and Adapis parisiensis. Am. J. Phys. Anthropol. 56, 217–234 (doi:10.1002/ajpa.1330560303) [Google Scholar]
- Gunnell G. F., Ciochon R. L., Gingerich P. D., Holroyd P. A.2002New assessment of Pondaungia and Amphipithecus (Primates) from the late middle Eocene of Myanmar, with a comment on ‘Amphipithecidae’. Contrib. Mus. Paleont. Univ. Mich. 30, 337–372 [Google Scholar]
- Gunnell G. F., Gingerich P. D., Ul-Haq M., Bloch J. I., Khan I. H., Clyde W. C.2008New primates (Mammalia) from the early and middle Eocene of Pakistan and their paleobiogeographical implications. Contrib. Mus. Paleont. Univ. Mich. 32, 1–14 [Google Scholar]
- Jaeger J.-J., Soe Aung Naing, Aung Aye Ko, Benammi M., Chaimanee Y., Ducrocq R.-M., Tun Than, Thein Tin, Ducrocq S.1998New Myanmar middle Eocene anthropoids. An Asian origin for catarrhines? C. R. Acad. Sci. Paris (Sciences de la vie) 321, 953–959 [Google Scholar]
- Jaeger J.-J., Thein Tin, Benammi M., Chaimanee Y., Soe Aung Naing, Lwin Thit, Tun Than, Wai San, Ducrocq S.1999A new primate from the middle Eocene of Myanmar and the Asian early origin of anthropoids. Science 286, 528–530 (doi:10.1126/science.286.5439.528) [DOI] [PubMed] [Google Scholar]
- Jaeger J.-J., et al. 2004Systematics and paleobiology of the anthropoid primate Pondaungia from the late middle Eocene of Myanmar. C. R. Palevol. 3, 243–255 (doi:10.1016/j.crpv.2004.05.003) [Google Scholar]
- Kay R. F., Hiiemae K. M.1974Jaw movement and tooth use in Recent and fossil primates. Am. J. Phys. Anthropol. 40, 227–256 (doi:10.1002/ajpa.1330400210) [DOI] [PubMed] [Google Scholar]
- Kay R. F., Schmitt D., Vinyard C. J., Perry J. M. G., Shigehara N., Takai M., Egi N.2004aThe paleobiology of Amphipithecidae, South Asian late Eocene primates. J. Hum. Evol. 46, 3–25 (doi:10.1016/j.jhevol.2003.09.009) [DOI] [PubMed] [Google Scholar]
- Kay R. F., Williams B. A., Ross C. F., Takai M., Shigehara N.2004bAnthropoid origins: a phylogenetic analysis. In Anthropoid origins: new visions (eds Ross C. F., Kay R. F.), pp. 91–135 New York, NY: Kluwer [Google Scholar]
- Kinzey W. G.1992Dietary and dental adaptations in the Pitheciinae. Am. J. Phys. Anthropol. 88, 499–514 (doi:10.1002/ajpa.1330880406) [DOI] [PubMed] [Google Scholar]
- Marivaux L., Chaimanee Y., Ducrocq S., Marandat B., Sudre J., Soe Aung Naing, Tun Soe Thura, Htoon Wanna, Jaeger J.-J.2003The anthropoid status of a primate from the late middle Eocene Pondaung Formation (central Myanmar): tarsal evidence. Proc. Natl Acad. Sci. USA 100, 13 173–13 178 (doi:10.1073/pnas.2332542100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marivaux L., et al. 2005Anthropoid primates from the Oligocene of Pakistan (Bugti Hills): data on early anthropoid evolution and biogeography. Proc. Natl Acad. Sci. USA 102, 8436–8441 (doi:10.1073/pnas.0503469102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marivaux L., et al. 2008Anatomy of the bony pelvis of a relatively large-bodied strepsirrhine primate from the late middle Eocene Pondaung Formation (central Myanmar). J. Hum. Evol. 54, 391–404 (doi:10.1016/j.jhevol.2007.09.007) [DOI] [PubMed] [Google Scholar]
- Pilgrim G. E.1927A Sivapithecus palate and other primate fossils from India. Mem. Geol. Surv. India, Palaeontol. Indica, n.s 14, 1–26 [Google Scholar]
- Plavcan J. M., Ruff C. B.2008Canine size, shape, and bending strength in primates and carnivores. Am. J. Phys. Anthropol. 136, 65–84 (doi:10.1002/ajpa.20779) [DOI] [PubMed] [Google Scholar]
- Rose K. D., Rana R. S., Sahni A., Kumar K., Missiaen P., Singh L., Smith T.2009Early Eocene primates from Gujarat, India. J. Hum. Evol. 56, 366–404 (doi:10.1016/j.jhevol.2009.01.008) [DOI] [PubMed] [Google Scholar]
- Shigehara N., Takai M., Kay R. F., Aung Aye Ko, Soe Aung Naing, Tun Soe Thura, Tsubamoto T., Thein Tin.2002The upper dentition and face of Pondaungia cotteri from central Myanmar. J. Hum. Evol. 43, 143–166 (doi:10.1006/jhev.2002.0567) [DOI] [PubMed] [Google Scholar]
- Szalay F. S.1970Late Eocene Amphipithecus and the origins of catarrhine primates. Nature 227, 355–357 (doi:10.1038/227355a0) [DOI] [PubMed] [Google Scholar]
- Szalay F. S.1972Amphipithecus revisited. Nature 236, 179–180 (doi:10.1038/236179a0) [Google Scholar]
- Takai M., Shigehara N.2004The Pondaung primates, enigmatic ‘possible anthropoids’ from the latest middle Eocene, central Myanmar. In Anthropoid origins: new visions (eds Ross C. F., Kay R. F.), pp. 283–321 New York, NY: Kluwer [Google Scholar]
- Takai M., Shigehara N., Aung Aye Ko, Soe Thura Tun, Soe Aung Naing, Tsubamoto T., Thein Tin.2001A new anthropoid from the latest middle Eocene of Pondaung, central Myanmar. J. Hum. Evol. 40, 393–409 (doi:10.1006/jhev.2001.0463) [DOI] [PubMed] [Google Scholar]
- Takai M., Shigehara N., Egi N., Tsubamoto T.2003Endocranial cast and morphology of the olfactory bulb of Amphipithecus mogaungensis (latest middle Eocene of Myanmar). Primates 44, 137–144 [DOI] [PubMed] [Google Scholar]
- Takai M., Sein Chit, Tsubamoto T., Egi N., Maung Maung, Shigehara N.2005A new eosimiid from the latest middle Eocene in Pondaung, central Myanmar. Anthropol. Sci. 113, 17–25 (doi:10.1537/ase.04S003) [Google Scholar]
- Simons E. L.1971Relationships of Amphipithecus and Oligopithecus. Nature 232, 489–491 (doi:10.1038/232489a0) [DOI] [PubMed] [Google Scholar]