Abstract
Behavioural syndromes, defined as correlated behaviours in different contexts, have been studied across species and taxa including humans as part of a personality concept. While most studies have focused on solitary individuals, less is known on how shoaling fish compromise between own personality and group behaviour. Risk-taking behaviour in 1-year-old perch (Perca fluviatilis) was observed to compare individual behaviour when in a group and when alone. An experimental design gave the fish the choice between foraging in an open area in the presence of a piscivore and hiding in the vegetation. We quantified the variation accountable by the effect of individuals being in a group, individuals alone and repeated measurements, using hierarchical mixed effects models. Within-group variances were low, but when individuals were later tested alone, individual differences explained most of the variation. Still, the individual best linear unbiased predictors (BLUPs) of time spent in the open area, extracted from the random effects of the mixed effects model, were positively correlated with the corresponding BLUPs when alone. The results indicate that individual behavioural traits are to some degree expressed also within groups. Most fish showed a shyer behaviour when alone, but bolder individuals changed less between treatments than did shyer ones, suggesting a more influential role of bold fish in the group.
Keywords: behavioural syndromes, BLUP, boldness, group influence, Perca fluviatilis, personality
1. Introduction
Recently, there has been an increasing interest in intraspecific variation in behaviour, and studies on personality, temperament, and behavioural syndromes have been performed on a variety of animals, such as birds (Dingemanse et al. 2002; Quinn & Cresswell 2005), mammals (Réale et al. 2000; Fairbanks et al. 2004), fish (Huntingford 1976), cephalopods (Sinn et al. 2008) and spiders (Riechert & Hedrick 1993), both in the wild and in the laboratory. Personality and temperament are terms that cover several components in an individual's behaviour, as boldness, sociability and aggressiveness. In accordance with the observed behaviour, individuals have been divided into coping style categories (Koolhaas et al. 1999; Øverli et al. 2004; Brelin et al. 2008) or been arranged along a behaviour gradient, such as the bold–shy continuum (Wilson et al. 1993; Brown et al. 2005). Behavioural syndromes, defined as correlations between different types of behaviour or between behaviour in different contexts (Sih et al. 2004), have been found in some cases (Ward et al. 2004; Johnson & Sih 2007), but not in others (Sinn et al. 2008). The occurrence of correlations between certain behaviours can also differ between populations within the same species (Bell 2005; Dingemanse et al. 2007).
Most studies on personality have looked at solitary individuals. However, when studying the individual behaviour of animals that naturally live in groups the question arises—how much of the observed behaviour in a natural situation is caused by own personality traits and how much is due to the influence of the group? Individual differences in behaviour have been suggested both to increase (by self-organization, Hemelrijk & Wantia 2005) and to decrease (by consensus decisions, Sumpter et al. 2008), as group size increases. An increased differentiation is mainly an effect of competitive interactions and dominance hierarchies (Hemelrijk & Wantia 2005). However, there are many benefits of consensus decision-making within groups using public information on, for example, food resources and predation risk (Krause & Ruxton 2002), and examples can be found in a variety of taxa (Sumpter & Pratt 2009). Group movements can be decided in a ‘democratic’ manner or led by individuals according to need or social indifference (Conradt et al. 2009). Consensus decision-making without active signalling or individual recognition has also been found in human crowds (Dyer et al. 2008b).
When studying personality in group-living animals, there may be a conflict between the desire to create a natural social environment for the animals, to avoid stress and atypical behaviour, and the problem with interdependency when observing individuals in groups. To avoid pseudoreplication while studying animals in groups, focal individuals have been used (e.g. Ward et al. 2008), but this does not take into account that the individual may be affected by group composition. It is also possible to adjust measurements of behaviour to the group, using relative values for comparisons, as deviation from the group mean (Leblond & Reebs 2006; Magnhagen 2007). However, behaviour within a group may be affected by social influences (Hemelrijk & Wantia 2005; Sumpter et al. 2008; Conradt et al. 2009), and not reflect individual variations in the specific traits under study. The difference between individual behaviour in solitude and in a social context has been studied in only a few cases (Reebs 2000; Magnhagen & Staffan 2005; van Oers et al. 2005; Webster & Hart 2006; Webster et al. 2007).
We have shown earlier that the behaviour of individual young-of-the-year perch (Perca fluviatilis) is influenced by its company (Magnhagen & Staffan 2005). The aim of the current study was to investigate whether behaviour measured in a group of perch reflects individual variation in boldness, or if behaviour patterns in a social environment only show the result of interactions within the group. Here, we have looked at behavioural patterns of perch faced with a trade-off between foraging and predator avoidance. We have compared the behaviour of the same individuals when they were first kept together with other perch and then alone, in order to test the consistency of individual behaviour in different social contexts. We explored perch risk-taking tactics using hierarchical mixed effects models to be able to decompose the total variation in the behavioural data into the effect of individuals being in a group, individuals alone and repeated measurements (Pinheiro & Bates 2000). Using this method, we quantify the variation that is accounted for by individual traits and the influence of the group on these traits.
2. Material and methods
(a). The study fish
One-year-old perch (body length, 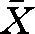 ± s.d.; 93.6 ± 10.0 mm, weight 7.5 ± 2.5 g, n = 64) were collected in August 2006 with a beach seine in the lakes Fisksjön and Ängersjön, close to the city of Umeå (63°47′ N; 20°17′ E) in northern Sweden. The two lakes differ in predator regime and we have earlier found behaviour differences in young perch from these two lakes (Magnhagen 2006; Magnhagen & Borcherding 2008). The fish were transported to Umeå Marine Research Center, 45 km south of Umeå, where the experiments were performed. Prior to the experiments, the perch were kept in tanks (1 × 1 × 1 m) with continuously running water (18–19°C). They were fed daily with pre-frozen red chironomid larvae ad libitum. The predators used were older perch, with a body length of 18–24 cm TL (
± s.d.; 93.6 ± 10.0 mm, weight 7.5 ± 2.5 g, n = 64) were collected in August 2006 with a beach seine in the lakes Fisksjön and Ängersjön, close to the city of Umeå (63°47′ N; 20°17′ E) in northern Sweden. The two lakes differ in predator regime and we have earlier found behaviour differences in young perch from these two lakes (Magnhagen 2006; Magnhagen & Borcherding 2008). The fish were transported to Umeå Marine Research Center, 45 km south of Umeå, where the experiments were performed. Prior to the experiments, the perch were kept in tanks (1 × 1 × 1 m) with continuously running water (18–19°C). They were fed daily with pre-frozen red chironomid larvae ad libitum. The predators used were older perch, with a body length of 18–24 cm TL (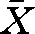 ± s.d.; 20.4 ± 2.1 cm, n = 16), caught in traps in the vicinity of the laboratory. The predators were fed with pieces of fish daily during experiments, after the observations were finished for the day. Although the size of the study fish relative to the potential predator bordered on the maximum ratio of ingestability (literature data: 0.45, Claessen et al. 2000; our data:
± s.d.; 20.4 ± 2.1 cm, n = 16), caught in traps in the vicinity of the laboratory. The predators were fed with pieces of fish daily during experiments, after the observations were finished for the day. Although the size of the study fish relative to the potential predator bordered on the maximum ratio of ingestability (literature data: 0.45, Claessen et al. 2000; our data: 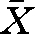 ± s.d.; 0.47 ± 0.06 mm, range 0.33–0.60), the perch reacted to quick movements of the predator by freezing or darting into cover and we thus consider the larger perch as being seen as a threat.
± s.d.; 0.47 ± 0.06 mm, range 0.33–0.60), the perch reacted to quick movements of the predator by freezing or darting into cover and we thus consider the larger perch as being seen as a threat.
(b). Experimental set-up
Experiments were started in mid-September. The experimental aquaria were 170 l (95 × 41 × 44 cm) and had continuously running water (18–19°C). The light regime was 13L∶11D, similar to natural conditions. One-third of each aquarium was used for the predator and the rest for the studied fish. A plastic net with a mesh size of 5 mm was placed between the predator's space and the small perch. During acclimatization and between observations, an opaque plastic screen was placed next to the net to prevent the fish habituating to the predator. The water flowed into the section with the perch group and out through the predator section to minimize olfactory cues. The aquaria had gravel on the bottom and artificial vegetation in the predator space and in the third of the space for the perch group that was furthest away from the predator. Before observations started, the perch were acclimatized to the aquarium for 3 days and were fed daily with red chironomid larvae in the open area. Eight groups of four perch were studied, four from each of the two lakes. Groups were combined randomly, and all the fish were familiar with each other from being held in the same holding tank for a month. To be able to identify individuals, the fish were marked with Alcian blue on their caudal fin.
(c). Behaviour observations
The perch were first observed in groups of four, for three consecutive rounds, twice the first day, and once the second day. After that they were divided up with only one small perch in each aquarium, identified by earlier group number and colour marking. All perch were tested in a different aquarium from the original one. The observations on single fish were made twice on the same day, once in the morning and once in the afternoon, 1 or 2 days after the group study. The order of the treatments could affect the individual behaviour in two ways; the results from the solitude study could depend on (i) habituation to the experimental set-up and (ii) an effect of the previous group composition. We analysed the effect of the group on habituation by looking at individual differences within each test sequence (in a group and alone) and by studying the changes in behaviour from the group to the single study. We also tested for the effect of the previous company. Details on how these tests were carried out can be found in the methods part addressing the statistical set-up.
Before each observation, the small perch were enclosed by the opaque screen in the half of their section that also contained the vegetation. Chironomid larvae (approx. 60–65 larvae) were poured into the aquarium between the predator section and the screen that enclosed the group of perch, and allowed to sink to the bottom. The opaque screen was then removed, making the large perch visible to the smaller perch through the net, and the observations started. The observations lasted for 10 min per aquarium. We used a computer program that recorded every second, for each individual fish, one of three different activities: occurrence in the vegetation, occurrence in the open, and feeding. After each observation the opaque screen was put back next to the net.
(d). Data analyses
As relevant measures of boldness, we used time spent in the open area and the latency to start feeding. These measures were included as response variables in a linear mixed effects model approach (library nlme v.3.1-90) using the free software pack R for statistical computing (R Development Core Team 2009). Time in the open was log + 1 transformed (it contained zeros) and latency was log-transformed to meet normality requirements. The behaviour measurements for boldness were repeated three times when fish were in a group and twice when alone. To avoid pseudoreplication in the analysis, a nested design was created. The repeated measurements within individual were added as random effect at the innermost level. Between individuals within aquarium was added as the next level, and between groups in different aquaria was added as the outer level. Variance components analysis of the random effects were carried out to decompose the variation explained by the different nesting factors within individual, between individual and between aquaria (Pinheiro & Bates 2000; Börger et al. 2006; Bunnefeld et al. 2009). Lake was included as a two-factor fixed effect, and the test sequence (repeated measurement within individual) as a two- or three-factor fixed effect for the model when alone and when in a group, respectively. Note that it is possible to fit an explanatory variable both as random and fixed effects, as these account for different components of the response (Pinheiro & Bates 2000; Crawley 2002). The size ratio (body length) between the focal individual and the predator was added as a continuous fixed effect (asin-transformed). The most parsimonious model was derived by testing the fixed effects using Wald statistics (Pinheiro & Bates 2000).
Best linear unbiased predictors (BLUPs) of the random effect of ‘between individuals’ were extracted from the final model for each individual, one estimate when they were tested within the group and the other when tested alone (Pinheiro & Bates 2000). A mixed effects model was then used to test if the group BLUPs were correlated with the BLUPs when alone. The individual and the group were added as nested random effects from outer to inner most level (library nlme v.3.1-90). Values that exert extreme influence on the regression fit were identified using ‘Cook's distance’ (Fox 2002). One outlier was removed for this analysis (Cook's distance = 4.1).
(e). Behaviour changes across treatments
To analyse the plasticity of the behavioural traits, the difference between the mean measurements when alone and when in group, respectively, was calculated for time in the open and latency to start feeding. The difference between when alone and when in a group was added as a response variable to a mixed effect model with the measurement when alone as explanatory variable. The individual and the group were added as nested random effects from outer to inner most level (library nlme v.3.1-90).
3. Results
(a). The effect of fixed factors
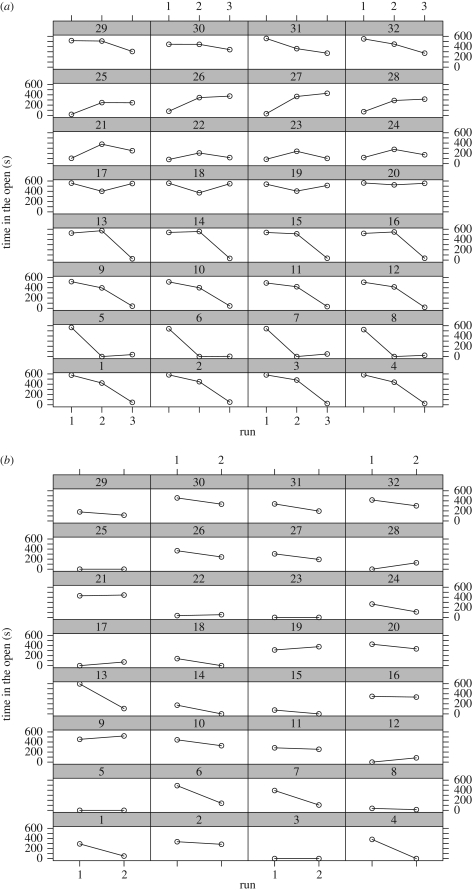
None of the fixed effects (lake, repetition, and predator–prey size ratio) had a significant effect on either of the behaviour measurements when perch were tested alone (table 1). When tested in the group, repetitive run was the only significant effect for both measurements. Time in the open decreased significantly with the sequence of the run (table 1). When the fish were tested for the first time, they spent on average of 319 s in the open, while this decreased to 188 s at the second run and to 97 s for the third (figure 1, table S1 in the electronic supplementary material for model estimates). The latency to start feeding when in a group decreased with the first test showing the longest latency followed by the second and third (90, 52, 24 s, respectively; table S2 and figure S3 in the electronic supplementary material).
Table 1.
Wald statistics for fixed effects for time in the open and latency to start feeding in perch studied alone and in groups, tested in a mixed effects model. (Significant tests in italics.)
| lake |
repetition |
prey/predator size ratio |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| F | d.f. | p | F | d.f. | p | F | d.f. | p | |
| when alone | |||||||||
| time in open | 0.10 | 1,6 | 0.8 | 1.64 | 1,31 | 0.2 | 0.18 | 1,23 | 0.7 |
| latency | 2.06 | 1,6 | 0.2 | 0.22 | 1,31 | 0.6 | 1.32 | 1,23 | 0.3 |
| in group | |||||||||
| time in open | 2.20 | 1,6 | 0.2 | 7.10 | 2,62 | <0.002 | 0.07 | 1,23 | 0.8 |
| latency | 0.35 | 1,6 | 0.6 | 24.26 | 2,62 | <0.001 | 0.29 | 1,23 | 0.6 |
Figure 1.
The time spent in the open (out of 600 s) shown for (a) three repetitions (runs) when individuals were in groups and (b) for two runs when individuals were alone. Individuals in the same row of the graph are from the same group. Each group contains four individuals.
(b). Variance component analysis
For fish that were alone, the highest variance explained in the models, for both time in the open and latency to start feeding, was between individuals, followed by the variance explained by within-individual differences (table 2). This indicates that individuals differed greatly in their behaviour, and also that the behaviour within individual is more similar than between individuals. In contrast, when fish were tested in groups, the highest variation was explained by within-individual differences and by between-group differences (table 2), which shows that individuals changed their behaviour between different runs of the experiment, and also adjusted to the group. Figure 1 illustrates the change over time in time spent in the open for each individual in the two treatments.
Table 2.
The percentage of total variance explained by the nesting factors within individual, between individuals, between groups and the residual variance of the random effects of the mixed effect model, for the experiments with groups and single perch, respectively.
| within individual (%) | between individuals (%) | between groups (%) | residual (%) | |
|---|---|---|---|---|
| alone | ||||
| time in open | 49 | 51 | <0.1 | <0.1 |
| latency | 23 | 77 | <0.1 | <0.1 |
| in group | ||||
| time in open | 71 | <0.1 | 29 | <0.1 |
| latency | 67 | <0.1 | 33 | <0.1 |
(c). Correlation of behaviour when alone and in group
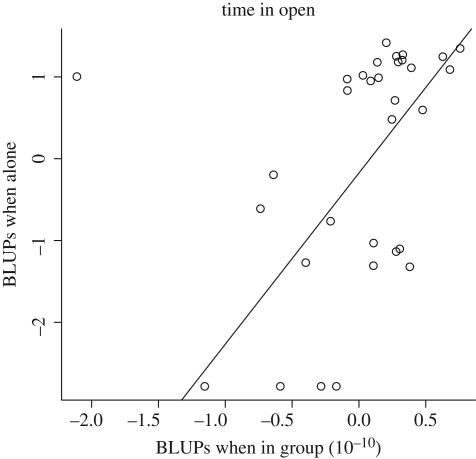
BLUPs for time in the open for individual perch when kept in groups were significantly correlated with the BLUPs when kept alone (t = 4.4, d.f. = 22, p < 0.001; figure 2). For the latency to start feeding, no significant correlation was found (t = 0.4, d.f. = 23, p = 0.7).
Figure 2.
Correlation between the best linear unbiased predictors (BLUPs) as extracted from a mixed effects model for individuals when alone and individuals tested in groups, respectively. Please note that one outlier, the most extreme point in the upper-left corner, was removed from the analysis according to Cooks' distance.
(d). Behaviour changes across treatments
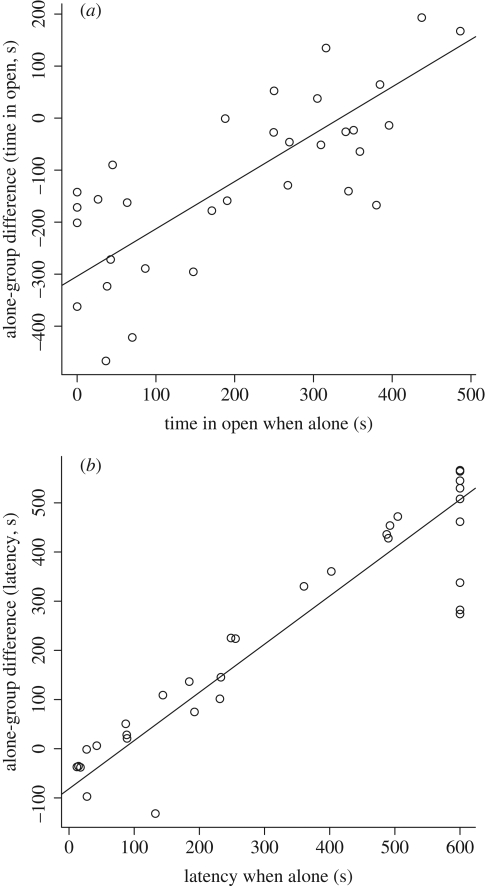
The difference in behaviour from being alone to being in a group was correlated to the individual's behaviour when alone, both regarding time in the open (t = 29.1, d.f. = 23, p < 0.001) and latency to start feeding (t = 65.5, d.f. = 23, p < 0.001). Only a few fish (5–6) spent less time in the open, or waited longer to start feeding when they were in a group compared with when they were alone (figure 3). Thus, most of the fish show an overall shyer behaviour when alone compared with when in a group. Bolder individuals when alone changed their behaviour less when tested in the group than did shy individuals.
Figure 3.
The difference between the behaviour when alone and when in a group plotted against the behaviour when alone. The difference was calculated for (a) time in the open and (b) latency to start feeding. The line was estimated by a mixed effects model taking into account the group and individual as random effects.
4. Discussion
In this study individual perch expressed behavioural tactics according to their personality on a bold–shy continuum both when in groups and when alone. Even though the variation explained by the between-individual random effect was low when in a group, the boldness predictors (BLUPs, time in the open) were still correlated with the boldness predictors when alone. These results show that the group shaped the behaviour of its members, but each member followed a certain tactic or personality, where bold and shy individuals when alone were also the bold and shy ones compared with the other group members when observed in the group. To consider a behaviour pattern to be caused by an individual personality trait, it should be possible to find a consistency within context (Réale et al. 2007). This requirement was fulfilled when the individuals were tested alone, since the between-individual differences explained the total variation in behaviour to a great extent. In the group study, on the other hand, there was a higher variance within individuals, while almost no variation in behaviour was found among group members. There was an effect of the order of the repeated runs for the group part of the study, but not for the individual trials. Differences in behaviour between runs may depend on predator activity during the observations, or on more efficient food depletion with time, making the perch go back to the vegetation when food was finished. However, the most interesting aspect of this result is that the changes over time were very similar within groups (figure 1). Also, even though the behaviour when in a group was highly influenced by the group, it is clear that this influence did not affect the individual behaviour when later tested alone.
The effect of the group is in line with the study by Magnhagen (2007), where risk-taking and explorative behaviours in young-of-the-year perch were correlated, but only when data were adjusted for the behaviour of the other group members. Similarly, in great tits (Parus major), the latency to feed depended on the behavioural type of the company (van Oers et al. 2005). Furthermore, Dyer et al. (2008a) showed that composition of personalities in a shoal of guppies (Poecilia reticulata) influenced the foraging success of all its individual members.
Young perch live in shoals in their natural habitat, and conformity of behaviour is probably adaptive, because of the benefit of using public information during foraging and predator avoidance (Krause & Ruxton 2002). Three-spined sticklebacks (Gasterosteus aculeatus) were found to adopt a quorum decision-making about movements (Ward et al. 2008). Public information was also used by sticklebacks during foraging, and individuals sometimes prioritized social conformity over the use of private information (Coolen et al. 2003; Webster & Hart 2006). Sticklebacks seem to be able to weigh private and public information appropriately depending on circumstances (van Bergen et al. 2004).
We found that most individuals became shyer when alone, thus refuting the possibility that the order of the treatments would confound the effects of solitude and habituation. Webster et al. (2007) also found that three-spined sticklebacks showed higher activity levels when conspecifics were present compared with when tested alone, although the same individuals were not tested in both situations. They interpreted their result with social facilitation effects on behaviour, and stated that the lower per capita risk within a larger group could be assessed by the fish. A change in perceived risk may similarly explain the decreased boldness of solitary fish in our study. Interestingly, we found that bolder individuals changed less in behaviour between treatments than did shyer ones. The behaviour within a group can be determined by leaders (Reebs 2000; Conradt et al. 2009), or by key-stone individuals affecting group dynamics (Sih & Watters 2005). Conradt et al. (2009) suggested that group movements may be led by individuals more socially indifferent than others. In sticklebacks, bold individuals showed a reduced shoaling tendency and a willingness to occupy front positions in a shoal (Ward et al. 2004). Our results are in line with these studies and suggest that the bolder individuals may have an influential role in the group, thus explaining the higher changes of behaviour in shy individuals when later tested alone. An alternative explanation is that bold individuals are less affected by a perceived increase of risk in solitude, compared with shyer ones, therefore maintaining their behaviour in the changed social situation.
In conclusion, the behaviour patterns observed in perch tested together in groups seemed mainly influenced by the behaviour of the other shoal mates, leading to a consensus. However, it is also clear that the small differences found within groups reflect differences in individual traits, for example, in boldness as tested here. Using the same individuals in different contexts, we quantified the individual variation and how the group affected behavioural decisions. Mixed effects models allowed us to study the effects of the different factors that form an animal's behaviour, and to tease apart individual personality traits and the influence of the group. In future studies, the combination of individual personalities and group dynamics should provide a fruitful line of research to explain behaviour patterns within animal groups.
Acknowledgements
The experiments in this study comply with the guidelines of the Association for the Study of Animal Behaviour, and were approved by the Local Ethics Committee of the Swedish National Board for Laboratory Animals (CFN).
We are grateful to Jost Borcherding who participated in a preceding experiment, thus being involved in the experimental set-up, and Markus Volpers who programmed the computer software to record behaviour. We are also grateful to Lynsey McInnes, Tom Ezard and two anonymous referees for their invaluable comments on the manuscript. The study was financially supported by CF Lundström's Foundation (no 1298). N.B. was funded by the SLU thematic programme Wildlife and Forestry.
References
- Bell A. M.2005Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473 (doi:10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- Brelin D., Petersson E., Dannewitz J., Dahl J., Winberg S.2008Frequency distribution of coping strategies in four populations of brown trout (Salmo trutta). Horm. Behav. 53, 546–556 (doi:10.1016/j.yhbeh.2007.12.011) [DOI] [PubMed] [Google Scholar]
- Brown C., Jones F., Braithwaite V.2005In situ examination of boldness-shyness traits in the tropical poeciliid Brachyraphis episcopi. Anim. Behav. 70, 1003–1009 (doi:10.1016/j.anbehav.2004.12.022) [Google Scholar]
- Börger L., Franconi N., Ferretti F., Meschi F., De Michele G., Gantz A., Coulson T.2006An integrated approach to identify spatiotemporal and individual-level determinants of animal home range size. Am. Nat. 168, 471–485 (doi:10.1086/507883) [DOI] [PubMed] [Google Scholar]
- Bunnefeld N., Baines D., Newborn D., Milner-Gulland E. J.2009Factors affecting unintentional harvesting selectivity in a monomorphic species. J. Anim. Ecol. 78, 485–492 (doi:10.1111/j.1365-2656.2008.01500.x) [DOI] [PubMed] [Google Scholar]
- Claessen D., de Roos A. M., Persson L.2000Dwarfs and giants: cannibalism and competition in size-structured populations. Am. Nat. 155, 219–237 (doi:10.1086/303315) [DOI] [PubMed] [Google Scholar]
- Conradt L., Krause J., Couzin I. D., Roper T. J.2009‘Leading according to need’ in self-organizing groups. Am. Nat. 173, 304–312 (doi:10.1086/596532) [DOI] [PubMed] [Google Scholar]
- Coolen I., van Bergen Y., Day R. L., Laland K. N.2003Species difference in adaptive use of public information in sticklebacks. Proc. R. Soc. Lond. B 270, 2413–2419 (doi:10.1098/rspb.2003.2525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley M. J.2002Statistical computing London, UK: John Wiley & Sons [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Van Oers K., Van Noordwijk A. J.2002Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938 (doi:10.1006/anbe.2002.2006) [Google Scholar]
- Dingemanse N. J., Wright J., Kazem A. J. N., Thomas D. K., Hickling R., Dawnay N.2007Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138 (doi:10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- Dyer J. R. G., Croft D. P., Morrell L. J., Krause J.2008aShoal composition determines foraging success in the guppy. Behav. Ecol. 20, 165–171 (doi:10.1093/beheco/arn129) [Google Scholar]
- Dyer J. R. G., Ioannou C. C., Morrell L. J., Croft D. P., Couzin I. D., Waters D. A., Krause J.2008bConsensus decision making in human crowds. Anim. Behav. 75, 461–470 (doi:10.1016/j.anbehav.2007.05.010) [Google Scholar]
- Fairbanks L. A., Newman T. K., Bailey J. N., Jorgensen M. J., Breidenthal S. E., Ophoff R. A., Comuzzie A. G., Martin L. J., Rogers J.2004Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol. Psychol. 55, 642–647 (doi:10.1016/j.biopsych.2003.12.005) [DOI] [PubMed] [Google Scholar]
- Fox J.2002An R and S-Plus companion to applied regression Thousand Oaks, CA: Sage Publications [Google Scholar]
- Hemelrijk C. K., Wantia J.2005Individual variation by self-organisation. Neurosci. Biobehav. R 29, 125–136 (doi:10.1016/j.neubiorev.2004.07.003) [DOI] [PubMed] [Google Scholar]
- Huntingford F. A.1976The relation between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 24, 245–260 (doi:10.1016/S0003-3472(76)80034-6) [Google Scholar]
- Johnson J. C., Sih A.2007Fear, food, sex and parental care: a syndrome of boldness in the fishing spider Dolomedes triton. Anim. Behav. 74, 1131–1138 (doi:10.1016/j.anbehav.2007.02.006) [Google Scholar]
- Koolhaas J. M., Korte S. M., De Boer S. F., Van Der Vegt B. J., Van Reenen C. G., Hopster H., De Jong I. C., Ruis M. A. W., Blokhuis H. J.1999Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. R 23, 925–935 (doi:10.1016/S0149-7634(99)00026-3) [DOI] [PubMed] [Google Scholar]
- Krause J., Ruxton G. D.2002Living in groups Oxford Series in Ecology and Evolution Oxford, UK: Oxford University Press [Google Scholar]
- Leblond C., Reebs S. G.2006Individual leadership and boldness in shoals of golden shiners (Notemigonus crysoleucas). Behaviour 143, 1263–1280 (doi:10.1163/156853906778691603) [Google Scholar]
- Magnhagen C.2007Social influence on the correlation between behaviours in young-of-the-year perch. Behav. Ecol. Sociobiol. 61, 525–531 (doi:10.1007/s00265-006-0280-3) [Google Scholar]
- Magnhagen C., Borcherding J.2008Risk-taking behaviour in foraging perch: does predation pressure influence age-specific boldness? Anim. Behav. 75, 509–517 (doi:10.1016/j.anbehav.2007.06.007) [Google Scholar]
- Magnhagen C., Staffan F.2005Is boldness affected by group composition in young-of-the-year perch (Perca fluviatilis)? Behav. Ecol. Sociobiol. 57, 295–303 (doi:10.1007/s00265-004-0834-1) [Google Scholar]
- Øverli Ø, et al. 2004Stress coping style predicts aggression and social dominance in rainbow trout. Horm. Behav. 45, 235–241 (doi:10.1016/j.yhbeh.2003.12.002) [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D.2000Mixed-effects models in S and S-Plus New York, NY: Springer-Verlag [Google Scholar]
- Quinn J. L., Cresswell W.2005Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behaviour 142, 1377–1402 (doi:10.1163/156853905774539391) [Google Scholar]
- R Development Core Team 2009R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; (ISBN 3-900051-07-0). http://www.R-project.org [Google Scholar]
- Réale D., Gallant B. Y., Leblanc M., Festa-Bianchet M.2000Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim. Behav. 60, 589–597 (doi:10.1006/anbe.2000.1530) [DOI] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J.2007Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318 (doi:10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- Reebs S. G.2000Can a minority of informed leaders determine the foraging movements of a fish shoal? Anim. Behav. 59, 403–409 (doi:10.1006/anbe.1999.1314) [DOI] [PubMed] [Google Scholar]
- Riechert S. E., Hedrick A. V.1993A test for correlations among fitness-linked behavioral traits in the spider Agelenopsis aperta (Araneae, Agelenidae). Anim. Behav. 46, 669–675 (doi:10.1006/anbe.1993.1243) [Google Scholar]
- Sih A., Watters J. V.2005The mix matters: behavioural types and group dynamics in water striders. Behaviour 142, 1417–1431 (doi:10.1163/156853905774539454) [Google Scholar]
- Sih A., Bell A., Johnson J. C.2004Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- Sinn D. L., Gosling S. D., Moltschaniwskyj N. A.2008Development of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim. Behav. 75, 433–442 (doi:10.1016/j.anbehav.2007.05.008) [Google Scholar]
- Sumpter D. J. T., Pratt S. C.2009Quorum responses and consensus decision making. Phil. Trans. R. Soc. B. 364, 743–753 (doi:10.1098/rstb.2008.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter D. J. T., Krause J., James R., Couzin I. D., Ward A. J. W.2008Consensus decision making by fish. Curr. Biol. 18, 1773–1777 (doi:10.1016/j.cub.2008.09.064) [DOI] [PubMed] [Google Scholar]
- van Bergen Y., Coolen I., Laland K. N.2004Nine-spined sticklebacks exploit the most reliable source when public and private information conflict. Proc. R. Soc. Lond. B 271, 957–962 (doi:10.1098/rspb.2004.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers K., Klunder M., Drent P. J.2005Context dependence of personalities: risk-taking behavior in a social and a nonsocial situation. Behav. Ecol. 16, 716–723 (doi:10.1093/beheco/ari045) [Google Scholar]
- Ward A. J. W., Thomas P., Hart P. J. B., Krause J.2004Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 55, 561–568 (doi:10.1007/s00265-003-0751-8) [Google Scholar]
- Ward A. J. W., Sumpter D. J. T., Couzin L. D., Hart P. J. B., Krause J.2008Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953 (doi:10.1073/pnas.0710344105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M. M., Hart P. J. B.2006Subhabitat selection by foraging threespine stickleback (Gasterosteus aculeatus): previous experience and social conformity. Behav. Ecol. Sociobiol. 60, 77–86 (doi:10.1007/s00265-005-0143-3) [Google Scholar]
- Webster M. M., Ward A. J. W., Hart P. J. B.2007Boldness is influenced by social context in threespine sticklebacks (Gasterosteus aculeatus). Behaviour 144, 351–371 (doi:10.1163/156853907780425721) [Google Scholar]
- Wilson D. S., Coleman K., Clark A. B., Biederman L.1993Shy-bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J. Comp. Psychol. 107, 250–260 (doi:10.1037/0735-7036.107.3.250) [Google Scholar]