Abstract
Fishes farmed in sea pens may become infested by parasites from wild fishes and in turn become point sources for parasites. Sea lice, copepods of the family Caligidae, are the best-studied example of this risk. Sea lice are the most significant parasitic pathogen in salmon farming in Europe and the Americas, are estimated to cost the world industry €300 million a year and may also be pathogenic to wild fishes under natural conditions.
Epizootics, characteristically dominated by juvenile (copepodite and chalimus) stages, have repeatedly occurred on juvenile wild salmonids in areas where farms have sea lice infestations, but have not been recorded elsewhere. This paper synthesizes the literature, including modelling studies, to provide an understanding of how one species, the salmon louse, Lepeophtheirus salmonis, can infest wild salmonids from farm sources. Three-dimensional hydrographic models predicted the distribution of the planktonic salmon lice larvae best when they accounted for wind-driven surface currents and larval behaviour. Caligus species can also cause problems on farms and transfer from farms to wild fishes, and this genus is cosmopolitan. Sea lice thus threaten finfish farming worldwide, but with the possible exception of L. salmonis, their host relationships and transmission adaptations are unknown. The increasing evidence that lice from farms can be a significant cause of mortality on nearby wild fish populations provides an additional challenge to controlling lice on the farms and also raises conservation, economic and political issues about how to balance aquaculture and fisheries resource management.
Keywords: Caligus, Lepeophtheirus, trout, epizootics, aquaculture, ectoparasites
1. Introduction
Floating sea cages (or net pens) allow free movement of pathogens between farmed and wild finfish. Modern mollusc culture similarly exposes farm stock to natural pathogens, but is at similar densities as found in nature, for example, in mussel and oyster beds. However, even at low farm stocking densities, sea-cage culture holds fishes for months in the same location at high host densities; a situation that does not occur in nature for such long time periods (Bergh 2007). These conditions facilitate disease and parasite transmission within the farm (Murray & Peeler 2005). Thus, should pathogens from wild hosts infest a farm, their population may grow exponentially and release the pathogens back into the same environment (Murray 2008). Depending on how the pathogen finds new hosts, the behaviour and ecology of the wild hosts and local hydrographic conditions, this may or may not have a significant impact on the wild fish populations. The sea lice interaction between aquaculture and wild fish stocks has important implications for the management and viability of both resources (Rosenberg 2008).
The best-studied example of this interaction has been the epidemiology of sea lice, namely, the ectoparasitic copepod crustaceans Lepeophtheirus salmonis (Krøyer) and several species of the genus Caligus; recently reviewed by Costello (2006). Their life cycle consists of non-feeding planktonic larvae (nauplii), an infective planktonic copepodite,1 immature ‘chalimus’ embedded on the host skin and mobile pre-adults and adults that move freely over the host skin. Sea lice are the most pathogenic parasite in salmon farming and may cost the industry €300 million (US$480 million) a year and 6 per cent of product value (Costello 2009). The planktonic larvae and mobile adults infest farmed fishes from natural populations and adjacent farms, but their progeny are then released from the net pens into the surrounding environment where they may infect wild hosts.
(a). Epizootics
Sea lice epizootics (exceptionally heavy and fatal infestations) appear to be rare in wild fish populations. However, it is possible that heavily infested fishes die and are not observed, and evidence from wild fish species, including salmonids, suggests that pathogenicity may occur naturally (Costello 1993, 2006; Hvidsten et al. 2007). In 1989, sea lice epizootics were recorded on wild sea trout, Salmo trutta L., in Ireland for the first time, and it was proposed that salmon farms were the primary source (Tully & Whelan 1993). Similar epizootics were found on wild salmonid species in Scotland, Norway and British Columbia, including sea trout, char Salvelinus alpinus (L.) and pink salmon Oncorhynchus gorbuscha (Walbaum). In all cases, they only occurred in regions where Atlantic salmon was farmed in net pens (e.g. Tully et al. 1999; Butler 2002; Gargan et al. 2003; Krkošek et al. 2005, 2006b; Morton et al. 2004, 2005) and were characterized by heavy infestations of chalimii. In Europe, epizootics were also characterized by the premature return of the juvenile sea trout to freshwater (Birkeland 1996; Gargan et al. 2003; Hatton-Ellis et al. 2006). In British Columbia, juvenile pink salmon heavily infested with sea lice have been observed to congregate where freshwater streams mix with sea water, such as under waterfalls, but whether this is a response to sea lice or not requires further research (A. Morton in Minutes of the Sea Lice Meeting of Scientists, Simon Fraser University, 26 March 2002 & 2008, personal communication). Thus, whether lice-infested salmonids exhibit freshwater return in Atlantic or Pacific Canada remains uncertain. A return to freshwater is beneficial to the host, because it results in reduced osmoregulatory stress and loss of sea lice (Wagner et al. 2004; Wells et al. 2006, 2007; Webster et al. 2007). However, even if epizootics and altered wild fish behaviour occur in areas with salmon farms, such correlation does not indicate cause and effect unless the observations become sufficiently repeated in space and time that other factors can be ruled out and are supported by experimental data.
The occurrence of epizootics on both wild and farmed fishes may have been caused by their sharing environments that favoured the parasite, such as the location of most salmon farms in wave-sheltered coastal inlets. Other reasons proposed as to why sea lice may not be contributing to significant wild fish mortality have included that: Atlantic salmon and sea trout populations had been declining for decades before farming began; infested fishes would have died from other causes; lice may have other wild hosts than farm fishes and whether the lice burdens observed were pathogenic (McVicar 2004). The rarity of sea lice larvae in plankton samples (except close to salmon pens), and limited knowledge of their behaviour, made it difficult to understand how lice from farms could cause mass infestations on wild fishes many kilometres distant (Pike & Wadsworth 2000). When the epizootics of sea lice on juvenile pink salmon later arose in British Columbia, the same issues were debated in this context with two additions. First, that infestations of wild fishes may have been limited if the lice were washed out of inlets in low salinity currents that would also have compromised lice development and survival. Second, there were the study-specific arguments about how appropriate the different mathematical models and choice of wild fish data were, and these have been addressed in responses in the literature (Brooks 2005; Brooks & Stucchi 2006; Krkošek et al. 2006a, 2008; Brooks & Jones 2008).
In the absence of good information on sea lice ecology, the controversy about whether lice from farms were significantly impacting wild fishes grew, and scientists were unable to provide the same interpretations of available data (McVicar 2004); most notably whether the correlations between lice abundance on farms and wild fishes were due to farm lice progeny infesting wild fishes. A similar debate arose in British Columbia when epizootics were observed on juvenile pink and chum salmon (e.g. Brooks & Stucchi 2006; Krkošek et al. 2008; Diana 2009). The anti-fish farming lobby added sea lice to their criticisms of the industry, and government agencies prevaricated owing to insufficient knowledge. Such debate is part of the scientific process. However, at some point, a synthesis is required to advise policy makers on the risk posed by sea lice from farms to wild fisheries. Recently, a series of papers by independent groups of researchers from different countries have provided models and data of how lice infestations can occur for the salmon louse, L. salmonis. This marks a milestone in understanding the ecology of sea lice, but perhaps more importantly, in how aquaculture may impact on wild fish populations. Here, these findings are brought together to clarify what is now known, what further research is required (see the appendix in the electronic supplementary material) and what are the policy implications for aquaculture, and fisheries conservation.
2. Research progress
(a). Larval dispersal and transport
The most significant progress in the understanding of sea lice in recent years has been the new data on larval dispersal, associated with oceanographic, mathematical and conceptual models. Significant correlations between lice abundance on farms and wild sea trout repeated over several years in Ireland suggested that lice may disperse for up to 30 km (Tully et al. 1999; Gargan et al. 2003). Field data from British Columbia and Scotland, supported by dispersal models, indicated that lice larvae could be transported 30 km in one area (Krkošek et al. 2005), concentrated in patches (Murray 2002) and their dispersal was influenced by hydrographic conditions and wind-driven circulation (Murray & Gillibrand 2006; Amundrud & Murray 2009). A review of dispersal distances of larvae of other marine species in relation to the range of typical coastal ocean current conditions further suggested that lice larvae may disperse an average of 27 km (11–45 km range) over 5–15 days, depending on current velocity (Costello 2006).
Earlier studies had found that L. salmonis had a greater tolerance to low salinity than Caligus species and survived longer when attached to the host than free-swimming, suggesting some adaptation to survival in estuarine conditions (reviewed in Costello 1993, 2006). Thus, the reduced salinities to which migrating pink and chum (Oncorhynchus keta (Walbaum)) salmon are normally exposed to in British Columbia are unlikely to significantly affect survival of L. salmonis (Connors et al. 2008a). Salmon lice abundance has been found to be reduced on salmon in farms in areas of low salinity in Scotland (Revie et al. 2003), Norway (Heuch et al. 2009), British Columbia (Jones & Hargreaves 2007), and for Caligus rogercresseyi in Chile (Bravo et al. 2008). However, in contrast to lice on fishes in sea cages, lice on wild and feral salmon may not be exposed to low salinities for long enough to affect their abundance until the fishes migrate into freshwater. Experiments in the laboratory and sea pens have shown that L. salmonis copepodites swim upwards towards light and do not cross into low salinity waters (Heuch 1995; Heuch et al. 1995; Bricknell et al. 2006). Plankton sampling discovered higher densities of L. salmonis copepodites in very shallow water along the seashore and in estuarine areas of Ireland and Scotland (Costelloe et al. 1995, 1998; McKibben & Hay 2004). While the same copepodite distribution may be expected in British Columbia, it requires confirmation because the Pacific and Atlantic populations of L. salmonis are genetically distinct (Tjensvoll et al. 2006; Todd et al. 2006) and may have different evolutionary adaptations. Thus, at least in Europe, empirical data indicated that the infective copepodites of L. salmonis concentrated in the path of migrating salmonids in estuaries. However, whether these copepodites arose from salmon entering rivers to spawn, escaped farm fishes, farms or wild fishes further offshore, was uncertain. Since then, extensive plankton surveys in a Scottish sea loch supporting a wild salmon population have indicated that gravid L. salmonis on farmed salmon were the major contributor to sea lice larvae recovered from the plankton (Penston et al. 2008a,b; Penston & Davies 2009).
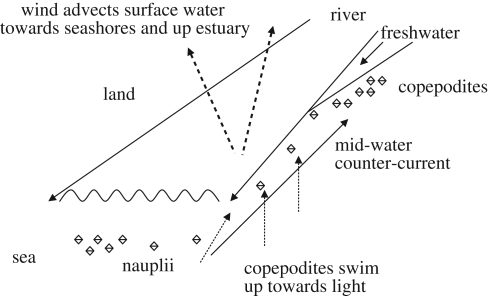
A conceptual model of how larvae of L. salmonis may be transported to intercept migrating salmonid hosts proposed that copepodites swim to the surface during daylight where the onshore wind moves the surface water towards the shore and into estuaries (Costello 2006) (figure 1). Gillibrand & Willis (2007) mathematically demonstrated such a model for the dispersal of sea lice larvae under typical coastal environmental conditions (including tidal, riverine and wind-driven currents) and showed that inclusion of larval behaviour in the model best explained field observations of copepodite distribution. In addition, their hydrographic model showed how below the seaward freshwater current is an upstream mid-depth current that can transport larvae towards land and into estuaries (figure 1). While some model studies (e.g. Brooks & Stucchi 2006; Gillibrand & Willis 2007) suggested that lice larvae could be washed out of inlets during high freshwater flows, other models found that gyres within sea lochs could retain larvae (Gillibrand & Amundrud 2007). Importantly, such models may be misleading if larvae avoid entrainment in freshwater as laboratory experiments indicate (discussed earlier). Larvae may thereby avoid salinity-delayed development or mortality, and seaward transport, and be retained in the inner estuaries as plankton sampling suggests (Amundrud & Murray 2009). Naturally, variation in wind force and direction owing to weather conditions and landscape, and in current speed and direction owing to seabed topography, will further affect water movement and larval dispersal. Whether a freshwater current is present or absent, wind-driven surface currents are critical in larval dispersal (Amundrud & Murray 2009). Penston et al. (2008b) sampled plankton at 0 and 5 m depth (in the absence of a freshwater surface layer) and found sea lice nauplii most abundant at 5 m, but copepodites at the surface. Thus copepodites would be subjected to dispersal by wind-driven surface currents, and nauplii most abundant near their sources (e.g. farms), as Costelloe et al. (1995) found in Ireland and Penston et al. (2008a,b) in Scotland. Comparable plankton sampling has not been reported in Canada or Norway, nor for any Caligus species.
Figure 1.
Diagram of how larvae of L. salmonis move into an estuary. Nauplii may be entrained and transported in a mid-water current that runs counter to the seaward freshwater current. Copepodites swim to the light and may be dispersed towards the seashore and up the estuary (inland) in wind-driven surface water. Solid lines indicate water flow direction. Heavy dashed lines indicate wind-driven water movement. Dotted lines indicate movement of copepodites.
(b). Identifying lice origin
While the presence of L. salmonis chalimii on Atlantic and Pacific salmon sampled in offshore seas indicates that infestation may occur there (reviewed by Costello 2006), the most significant infestations will occur in coastal waters owing to the congregation of hosts, larval behaviour and hydrography. The timing of salmonid migrations is important in L. salmonis infestations and similar in both the Atlantic and Pacific Oceans. The juvenile salmon migrate from rivers to the sea in spring (mainly April to May), whereas adults return to the coast during the summer (mainly June to October) before entering the rivers to spawn; where their lice will die in freshwater (Dill et al. 2009). Thus juvenile fishes will suffer less exposure to lice transferred from returning adults the sooner they migrate away from the coast, a situation called migratory allopatry between host and parasite (Krkošek et al. 2007a). The source of the sea lice causing the epizootics on juvenile salmonids in spring must be either wild salmonids that did not migrate offshore, escaped or feral farm fishes, salmon farms or non-salmonid wild hosts. Escaped farm fishes are a significant reservoir of lice in Norway in comparison with wild salmonids (Heuch & Mo 2001), but whether they may be a reservoir elsewhere is not clear.
In British Columbia, mobile L. salmonis have been found to increase on inshore juvenile salmonids coincident with the return of adult salmon from the open ocean to spawn in rivers, indicating that adult sea lice transferred from returning wild salmon to farm, and to wild juvenile, salmonids (Krkošek et al. 2007a; Saksida et al. 2007a; Gottesfeld et al. 2009). A similar phenomenon may be expected in other regions with migratory salmonids. Furthermore, large adult Pacific and Atlantic salmon returning to spawn typically have several ovigerous (egg bearing) lice, which will release their larvae inshore. If these lice find new hosts before the host enters freshwater, they may produce more strings of eggs as lice on farm fishes do. Beamish et al. (2007) found that although most fishes migrate away from the coast, some individuals of adult and juvenile Pacific salmon were present in coastal waters of British Columbia throughout the year and provided a continuous reservoir of sea lice. Similarly, in European seas, some wild salmonids, such as sea trout S. trutta, and farm escapes, may be present in coastal waters most of the year. However, over 90 per cent of Atlantic salmon in Irish, Scottish, Norwegian and Gulf of Maine (New Brunswick, Atlantic Canada, and Maine, USA) coastal waters are in farms (e.g. Tully & Whelan 1993; Heuch & Mo 2001; Butler 2002; McKenzie et al. 2004). Despite the greater abundance of wild Pacific compared with wild Atlantic salmon, most Pacific salmon are offshore until the summer (reviewed by Krkošek in press), and Orr (2007) predicted significant lice production (billions of larvae) from farms in British Columbia from 2003 to 2004. Atlantic salmon are typically grown in sea pens for about 18 months and so can provide a significant reservoir of sea lice all year around.
While Caligus species seem to have wide host preferences, L. salmonis is largely restricted to, and only matures on, salmonids. Even wild non-salmonid fishes congregated around salmon pens in Scotland (Bruno & Stone 1990) and Ireland (M. J. Costello 1994, unpublished data), and wrasse held within salmon pens (Costello et al. 1996), do not host this species. In British Columbia, field surveys found that not only were juvenile pink and chum salmon infested, but 84 per cent of sticklebacks Gasterosteus aculeatus L. also were, of which 97 per cent were copepodite and chalimus stages (Jones et al. 2006a). However, laboratory studies found a loss of lice on all three hosts over time, and no mature L. salmonis occurred on sticklebacks (Jones et al. 2006b). Neither the source nor fate of these lice was known, but some may re-infest other fishes. Thus sticklebacks may act as a temporary reservoir for L. salmonis, albeit not as significant a reservoir as hosts of ovigerous lice. Because the epizootics on juvenile salmonids consist of the same early life-stages as found on sticklebacks, it is likely that both are receiving lice from the same sources rather than contributing to each other's infestations.
Efforts to find indicators of whether juvenile lice on wild fishes have come from farmed fishes have met little success. Although it is possible to distinguish lice from farmed and wild fishes using stable isotopes, elemental signatures and colourants derived from feeding on farmed fishes, these indicators do not identify farm lice progeny that may infect wild fishes (Todd 2006). Genetic analyses indicate that L. salmonis is one well-mixed population across the North Atlantic, but that there has been some genetic drift from the North Pacific population; thus genetics are unlikely to distinguish farm and wild host populations (Costello 2006; Todd 2006). While Caligus elongatus von Nordmann has two genotypes in the North Atlantic, whether they have diverged based on seasonal reproductive cycles, host selection or other environmental factors remains to be determined (Øines & Heuch 2007). However, indirect support from spatial transmission models indicates that farms can be significant sources of lice on wild fishes (Krkošek et al. 2005, 2006b; Frazer 2008, 2009).
(c). Host capture
In the vicinity of a host, the L. salmonis copepodite increases swimming speed and attaches faster to slower moving hosts (Genna et al. 2005; Mordue & Birkett 2009). It recognizes a host's movement by mechanosensors from approximately 26 mm distance (Heuch et al. 2006), but may also be attracted by water-borne host odours, as well as contact chemosensors once attached to the host (Mordue Luntz et al. 2006; Fields et al. 2007; Pino-Marambio et al. 2007; Mordue & Birkett 2009). Both copepodite and adult lice can discriminate their preferred host species using both odour and taste, and pre-adult female lice attract males using pheromones (Mordue & Birkett 2009).
Mobile (pre-adult and adult) sea lice can transfer between hosts, and Caligus mobiles are found in the plankton (Hewitt 1971; Costello 2006; A. Morton 2008, unpublished data). The rapid arrival and loss of C. elongatus from fish farms suggest that transmission by mobiles is significant. Mobile L. salmonis can transfer from returning Pacific salmon to migrating juvenile pink salmon (discussed earlier). Another method of transmission may be for lice to transfer to a predator of their host. Connors et al. (2008b) found 70 per cent of L. salmonis (mostly males) transferred from their host when it was predated. In this instance, the host was also a salmonid. Were it a less suitable host, the lice may transfer again when an opportunity arose. Thus, transmission of mobile stages may be important for lice but is largely unstudied. It would enable redistribution of lice among hosts so as to prevent pathology, leading to host mortality and loss of lice habitat. By increasing the number of hosts infested, it would further spread the lice population. Hosts may be attracted to what appears to be a crustacean prey item in the plankton, but upon approach, the lice may dodge predation and attach to the host, as aquarium observations indicate may occur (Connors et al. 2008b; M. J. Costello 1993, personal observation). This transmission of adult life stages may be more important for Caligus than for Lepeophtheirus species, as only the former are commonly found in the plankton. The success of mobile louse transmission may vary with light conditions as found for ectoparasitic isopod praniza larvae (Smit & Davies 2004). Experiments on the behavioural interactions between mobile lice and their hosts are required to understand the significance of this route of infestation.
(d). Host impacts
It had been suggested that lice may preferentially infest and cause mortality to weaker fishes that would have died from other causes (McVicar 2004). However, Todd et al. (2006) found that wild Atlantic salmon in poor condition were not more likely to have high lice burdens. At low density (less than three lice per fish or less than 0.65 lice g−1 fish), lice had no effect on the condition of juvenile pink salmon (Butterworth et al. 2008). Even high densities of chalimus on sticklebacks in British Columbia did not correlate with fish condition (Jones et al. 2006a). Comparable studies have not been conducted for farm fishes. Similarly, helminth parasites do not appear to normally affect host condition and mortality (reviewed by Thomas 2002). In an evolutionary context, this benefits the survival of both the parasite and its (host) habitat. Higher parasite burdens on fishes in better condition may reflect the occurrence of more parasites in locations where the fishes find more food.
Lice can cause reduced swimming and cardiac performance in Atlantic salmon (Wagner et al. 2004). Although lice-infested fishes show increased leaping behaviour, presumably to dislodge lice (Stone et al. 2002), which may attract predators, it would seem disadvantageous for the host to be predated because the lice may not easily find an alternative host (but see above). At present, whether fishes suffer more or less predation owing to lice infestation is unknown. However, a comparison of lice abundance between wild fishes and epizootics suggests that lice may sometimes be sufficiently abundant to cause host mortality under natural conditions (Costello 2006).
Recent reviews have stated that greater than 0.5 to 0.75 mobile L. salmonis per gram host weight, or more than 5 to 10 per fish (greater than 0.1 lice g−1 fish) can be pathogenic to Atlantic salmon, Salmo salar (Costello 2006; Wagner et al. 2007). Todd et al. (2006) found that 0.2 to 0.7 L. salmonis per gram were pathogenic to the sea trout S. trutta. Although chalimii are not known to be pathogenic, they can become so when they mature into mobile stages if sufficiently abundant. Survival models for juvenile pink salmon indicated significantly greater population mortality if L. salmonis abundance was greater than or equal to 0.75 or 2.0 mobile lice per fish, depending on whether predators selected fish that were lice infested or not, respectively (Krkošek in press). These pink salmon were less than 1 g in weight so these levels are similar to levels pathogenic to the Salmo species. However, pathogenicity varies with host size, species and other health conditions (Costello 2006; Jones et al. 2007; Wagner et al. 2007; Bravo et al. 2008), and how fish sensitivity changes with size and age needs to be better quantified (Krkošek in press).
Chalimus abundance during epizootics is typically greater than 10 per fish (sometimes over 60) on sea trout and Arctic charr (Bjorn et al. 2001; Gargan et al. 2003; Heuch et al. 2005) and over three per pink and chum salmon, which are a much smaller fish (Morton et al. 2004). Such levels will at least irritate and stress the host and, when they moult to mobile stages, will be fatal (Bjorn & Finstad 1997; Tully & Nolan 2002). However, experimental studies find lice tend to be lost from hosts over time. Almost all pre-adult lice were lost from wild-captured Pacific salmon hosts in laboratory and sea-cage experiments (Krkošek et al. in press). Whether these lice die and fall off, are dislodged and move onto a different host, are eaten by the fish or are lost due to the experimental conditions is not clear. Data on the mortality of sea lice on wild and farmed fishes are required for predictions about future host impacts. Such estimates may be possible by monitoring lice population dynamics on farms.
(e). Caligus species
How Caligus species maximize their chance of finding a suitable host is uncertain, but appears to involve adults dispersing in the plankton, and moving between a wider range of host species than Lepeophtheirus species (Ho 2004; Costello 2006). Where L. salmonis is absent, Caligus species can be a problem on farms and on wild fishes and can be a more difficult parasite to control.
Species of Caligus have been pathogenic to salmonids on farms in Europe and Chile, and observations suggest that C. elongatus can be pathogenic to at least wild herring Clupea harengus L. and haddock Melanogrammus aeglinfinus (L.) (reviewed by Costello 1993), and subpopulations may have host preferences (Øines et al. 2006). Infestation of bluefin tuna, Thunnus maccoyii Castelnau, in farms in South Australia by mobile Caligus chiastos Lin & Ho (2003) and Caligus amblygenitalis Tripathi (1961) resulted in host blindness and reduced weight (Hayward et al. 2008, 2009). The absence of chalimus stages indicated direct transfer of mobile lice from wild hosts. A candidate aquaculture fish species in Australia, mulloway Argyrosomus japonicas (Temminck & Schegel), can be infested by another Caligus species tentatively identified as C. elongatus (Hayward et al. 2007). While Caligus spinosus Yamaguti infests farmed yellowtail amberjacks Seriola quinqueradiata Temminck & Schlegel in Japan, the larger C. lalandei Barnard has appeared in the region on wild yellowtail and may cause more severe infestations on farms (Ho et al. 2001). A variety of wild and farmed marine finfish species from Australia to southeast Asia have had pathogenic infestations of Caligus epidemicus Hewitt (Ho 2000; Ho et al. 2004). Although only 1–3 mm in size, C. epidemicus may hold the record for lice abundance on a single host; over 5000 were taken from a single host surgeonfish in captivity (Ho et al. 2004).
Young-of-the-year herring, Clupea pallasi Valenciennes have been infected by Caligus clemensi (Parker & Margolis) in areas of British Columbia with high numbers of fish farms (Morton et al. 2008). In Chile, the abundance of C. rogercresseyi (Boxshall & Bravo) on wild hosts decreased after farm fallowing, indicating transfer of lice from farm to wild hosts (C. Levicoy & G. Asencio 2009, personal communication). However, with the possible exception of a heavy infestation of C. rogercresseyi on feral trout S. trutta in southern Argentina (near Chile) (Bravo et al. 2006), sea lice epizootics have not been reported on wild fishes in South America.
While farm data show replacement of Caligus species by L. salmonis on farms (Revie et al. 2002; Saksida et al. 2007b), there can also be positive correlations in both species abundance on farmed salmon (Todd et al. 2006). Such contrasting correlations may be due to density-dependent competition between the species, whereby populations of both species may increase until the larger L. salmonis begins to displace C. elongatus. There are little-to-no benefits of fallowing and parasiticides in controlling C. elongatus on farms, perhaps because adult Caligus transfer between wild and farmed hosts more readily than L. salmonis (Treasurer & Grant 1994; Revie et al. 2002). Thus, some Caligus species are already causing problems to farm and wild fish populations, and more threaten to do so. Studies on the behaviour of Caligus species' larvae and adults are required to understand how these sea lice find their hosts (appendix in the electronic supplementary material) and manage the risks of transmission between farm and wild fish stocks.
(f). Geographical extent of the problem
The L. salmonis epizootics would have been afflicting juvenile salmonids in Ireland, Scotland, Norway and British Columbia for some time before being observed. Similar epizootics may also have occurred in the Gulf of Maine, but not have been noticed. Other pathogenic infestations of L. salmonis on wild salmonids, and Caligus on other fish species, were adult populations (Hewitt 1971; reviewed by Costello 1993). Indeed, were it not for the premature return of heavily infested sea trout to freshwater where they were easily observed, the phenomenon may not have been discovered in Ireland. The countries that produce most farmed salmonids have a problem with sea lice on farms whereas the minor producers do not (see the appendix, electronic supplementary material), suggesting a relationship between the number of farms and/or farmed fishes, and the development of sea lice infestations on farms. Persistent infestations on farms increase the risk of lice transferring to wild fishes. Krkošek et al. (2007b) estimated the mortality of pink salmon owing to L. salmonis in areas with salmon farms to be 80 per cent, levels that could extirpate populations in four generations (eight years). Two of their co-authors conducted a global analysis and found a significant decrease in wild salmon abundance in areas with salmon farming compared with areas with no farms since the late 1980s (Ford & Myers 2008). They compared all regions of the world where both wild and farmed salmonids co-occurred, and thus controlled for environmental conditions in the freshwater and marine phases, and for fishery impacts. While salmon farming may have several impacts on wild stocks, including escaped farmed fishes interfering in wild fish spawning and genetic dilution, the most likely cause of the global decline in wild salmonids in areas with farms was sea lice transmission from farms. Frazer (2009) argues that the ‘wild fish decline in proportion to the ratio of lice abundance on farm … to … wild fish’, largely because each female louse produces thousands of eggs in her lifetime so less than 0.1 per cent need to survive to maintain the lice population. Should this be the situation, sea lice-infested farms would lead to the extirpation of the local wild fish population, unless the total number of lice is less on the farm than on the wild fish population.
3. Conclusions
The evidence that salmon farms are the most significant source of the epizootics of sea lice on juvenile wild salmonids in Europe and North America is now convincing (Heuch et al. 2005; Costello 2006; Krkošek et al. 2006b, 2007a,b, in press; Todd 2006). Farms may contain millions of fishes almost year round in coastal waters and, unless lice control is effective, may provide a continuous source of sea lice, although the amount of infestation pressure will vary over time owing to seasonal and farm management practices (e.g. fallowing). If escaped farm fishes remain in coastal waters, they will be an additional reservoir of lice. Experimental and field data in conjunction with mathematical models provide an explanation of how the larvae of the most common and pathogenic species, L. salmonis, disperse and congregate to infest wild salmonids in coastal waters. The correlation of epizootics on wild fishes in areas with fish farms, compared with (control) areas without farms, has been repeated over years and in different countries (see the appendix, electronic supplementary material). Analyses that controlled for the effects of environmental conditions and fisheries found that salmon population declines were coincident with salmon farming in both North America and Europe (Ford & Myers 2008). However, these are general patterns. Not all salmon farms have sea lice problems, and local hydrographic conditions vary and will influence larval dispersal. Despite improved knowledge about how to control sea lice on farms, including fallowing and a wider range of parasiticides, sea lice epizootics persist. In Ireland, where the impact on wild salmonids was first discovered, government monitoring of farms found that L. salmonis abundance had increased since 2001 after an earlier decline (O'Donohoe et al. 2007), and the problem continues to be raised in the parliament and the press (e.g. Viney 2008). Some salmon farms will not be a source of sea lice for wild fishes, and epizootics may not always occur in areas with salmon farms. However, if one of a group of neighbouring farms is infested, then both the farms who have lice under control and the wild fish populations are at risk of infestation. To coordinate sea lice control, industry, wild fishery and regulatory agencies have increasingly become involved in sea lice control on farms to minimize risks to wild fishes.
4. Outlook
The globalization of the salmon-farming industry may help expedite the development and implementation of measures to improve fish health and reduce sea lice transfer to wild fishes. However, moving farm sites to reduce the risk of sea lice transmission and to improve coordinated fallowing requires cooperation of farm owners and staff, local communities (who may object to new farm locations) and government authorities. In some regions, reductions in farm density would reduce overall farm production and may have financial consequences for the companies involved and local economy. However, this may result in a more sustainable salmon-farming industry and help protect wild populations. Salmon farming is an important agribusiness and employer in many countries and remote coastal regions of Europe and the Americas. Fisheries for wild Atlantic salmon and sea trout were once a significant economic resource, and the health of Pacific salmon stocks is also of concern. Sea lice thus raise important social, economic and political issues, as well as priorities for nature conservation and fish health. In addition, they provide a case study of problems that can arise in aquaculture that could be repeated elsewhere.
Acknowledgements
I thank Sandra Bravo, John Burka, Kai (Ju-Shey) Ho, Martin Krkošek, Cristobel Levicoy, Alexandra Morton, Sandy Murray, Michael Penston, Jim Treasurer and anonymous reviewers for very helpful information and/or suggestions on earlier versions of this paper.
Endnote
The term copepodite or copepodid refers to the infective plankton stage of Caligidae (Copepoda Crustacea). A search on Google Scholar found that the former term is most used in the scientific literature (6260 versus 3710 responses on 15 December 2008), although the latter tends to be more used in the sea lice literature (57 versus 361 results when term was combined with ‘sea lice’). There seems to be no benefit to have two terms for such similar life stages.
References
- Amundrud T. L., Murray A. G.2009Modelling sea lice dispersion under varying environmental forcing in a Scottish sea loch. J. Fish Dis. 32, 27–44 (doi:10.1111/j.1365-2761.2008.00980.x) [DOI] [PubMed] [Google Scholar]
- Beamish R. J., Neville C. M., Sweeting R. M., Jones S. R. M., Ambers N., Gordon E. K., Hunter K. L., McDonald T. E.2007A proposed life history strategy for the salmon louse, Lepeophtheirus salmonis in the subarctic Pacific. Aquaculture 264, 428–440 (doi:10.1016/j.aquaculture.2006.12.039) [Google Scholar]
- Bergh Ø.2007The dual myths of the healthy wild fish and the unhealthy farmed fish. Dis. Aquat. Organ. 75, 159–164 (doi:10.3354/dao075159) [DOI] [PubMed] [Google Scholar]
- Birkeland K.1996Consequences of premature return by sea trout (Salmo trutta L.) infested with the salmon louse (Lepeophtheirus salmonis Krøyer); migration, growth and mortality. Can. J. Fish Aquat. Sci. 53, 2808–2813 (doi:10.1139/cjfas-53-12-2808) [Google Scholar]
- Bjorn P. A., Finstad B.1997The physiological effects of salmon lice infection on sea trout smolts. Nord. J. Freshwater Res. 73, 60–72 [Google Scholar]
- Bjorn P. A., Finstad B., Kristoffersen R.2001Salmon lice infection of wild sea trout and Arctic char in marine and freshwaters: the effects of salmon farms. Aquacult. Res. 73, 947–962 (doi:10.1046/j.1365-2109.2001.00627.x) [Google Scholar]
- Bravo S., Perroni M., Torres E., Silva M. T.2006Report of Caligus rogercresseyi in the anadromous brown trout (Salmo trutta) in the Río Gallegos Estuary, Argentina. Bull. Eur. Ass. Fish Pathol. 26, 186–193 [Google Scholar]
- Bravo S., Pozo V., Silva M.2008The tolerance of Caligus rogercresseyi to salinity reduced in southern Chile. Bull. Eur. Ass. Fish Pathol. 28, 197–204 [Google Scholar]
- Bricknell I. R., Dalesman S. J., O'shea B., Pert C. C., Mordue Luntz A. J.2006Effect of environmental salinity on sea lice Lepeophtheirus salmonis settlement success. Dis. Aquat. Organ. 71, 201–212 (doi:10.3354/dao071201) [DOI] [PubMed] [Google Scholar]
- Brooks K. M.2005The affects of water temperature, salinity and currents on the survival and distribution of the infective copeopodid stage of sea lice (Lepeophtheirus salomins) originating on Atlantic salmon farms in the Broughton Archipelago of British Columbia, Canada. Rev. Fish. Sci. 13, 177–204 (doi:10.1080/10641260500207109) [Google Scholar]
- Brooks K. M., Jones S. R. M.2008Perspectives on pink salmon and sea lice: scientific evidence fails to support the extinction hypothesis. Rev. Fish. Sci. 16, 403–412 (doi:10.1080/10641260801937131) [Google Scholar]
- Brooks K. M., Stucchi D. J.2006The effects of water temperature, salinity and currents on the survival and distribution of the infective copepodid stage of the salmon louse (Lepeophtheirus salmonis) originating on Atlantic salmon farms in the Broughton Archipelago of British Columbia, Canada (Brooks, 2005): a response to the rebuttal of Krkošek et al. (2005a). Rev. Fish. Sci. 14, 13–23 (doi:10.1080/10641260500448893) [Google Scholar]
- Bruno D. W., Stone J.1990The role of saithe, Pollachius virens L., as a host for sea lice Lepeophtheirus salmonis Krøyer and Caligus elongatus Nordmann. Aquaculture 89, 201–207 (doi:10.1016/0044-8486(90)90125-7) [Google Scholar]
- Butler J. R.2002Wild salmonids and sea louse infestations on the west coast of Scotland: sources of infection and implications for the management of marine salmon farms. Pest Manag. Sci. 58, 595–608 (doi:10.1002/ps.490) [DOI] [PubMed] [Google Scholar]
- Butterworth K. G., Cubitt K. F., McKinley R. S.2008The prevalence, density and impact of Lepeophtheirus salmonis (Krøyer) infestation on juvenile pink salmon (Oncorhynchus gorbuscha) from the central coast of British Columbia, Canada. Fish. Res. 91, 35–41 (doi:10.1016/j.fishres.2007.11.018) [Google Scholar]
- Connors B. M., Juarez-Colunga E., Dill L. M.2008aEffects of varying salinities on Lepeophtheirus salmonis survival on juvenile pink and chum salmon. J. Fish Biol. 72, 1825–1830 (doi:10.1111/j.1095-8649.2008.01839.x) [Google Scholar]
- Connors B. M., Krkošek M., Dill L. M.2008bSea lice escape predation on their host. Biol. Lett. 4, 455–457 (doi:10.1098/rsbl.2008.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello M. J.1993Review of methods to control sea-lice (Caligidae, Crustacea) infestations on salmon farms. In Pathogens of wild and farmed fish: sea lice (eds Boxshall G. A., Defaye D.), pp. 219–252 London, UK: Ellis Horwood [Google Scholar]
- Costello M. J.2006Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol. 22, 475–483 (doi:10.1016/j.pt.2006.08.006) [DOI] [PubMed] [Google Scholar]
- Costello M. J.2009The global economic cost of sea lice to the salmonid farming industry. J. Fish Dis. 32, 115–118 (doi:10.1111/j.1365-2761.2008.01011.x) [DOI] [PubMed] [Google Scholar]
- Costello M. J., Deady S., Pike A., Fives J. M.1996Parasites and diseases of wrasse being used as cleaner-fish on salmon farms in Ireland and Scotland. In Wrasse biology and use in aquaculture (eds Sayer M. D. J., Treasurer J. W., Costello M. J.), pp. 211–227 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- Costelloe J., Costelloe M., Roche N.1995Variation in sea lice infestation on Atlantic salmon smolts in Killary Harbour, west coast of Ireland. Aquacult. Int. 3, 379–393 (doi:10.1007/BF00121626) [Google Scholar]
- Costelloe M., Costelloe J., O'Donohoe G., Coghlan N. J., Oonk M., Van der Heijden Y.1998Planktonic distribution of sea lice larvae, Lepeophtheirus salmonis, in Killary Harbour, west coast of Ireland. J. Mar. Biol. Ass. UK 78, 853–874 (doi:10.1017/S0025315400044830) [Google Scholar]
- Diana J. S.2009Aquaculture production and biodiversity conservation. BioScience 59, 27–38 (doi:10.1525/bio.2009.59.1.7) [Google Scholar]
- Dill L. M., Losos C. J. C., Connors B. M., Mages P.2009Comment on Beamish et al. (2007) ‘A proposed life history strategy for the salmon louse, Lepeophtheirus salmonis in the subarctic Pacific’. Aquaculture 286, 154–155 (doi:10.1016/j.aquaculture.2008.09.024) [Google Scholar]
- Fields D. M., Weissburg M. J., Browman H. I.2007Chemoreception in the salmon louse Lepeophtheirus salmonis: an electrophysiological approach. Dis. Aquat. Organ. 78, 161–168 (doi:10.3354/dao01870) [DOI] [PubMed] [Google Scholar]
- Ford J. S., Myers R. A.2008A global assessment of salmon aquaculture impacts on wild salmonids. PLoS Biol. 6, e33 (doi:10.1371/journal.pbio.0060033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer L. N.2008Sea-lice infection models for fishes. J. Math. Biol. 57, 595–611 (doi:10.1007/s00285-008-0181-3) [DOI] [PubMed] [Google Scholar]
- Frazer L. N.2009Sea-cage aquaculture, sea lice and declines of wild fish. Conserv. Biol. 23, 599–607 (doi:10.1111/j.1523-1739.2008.01128.x) [DOI] [PubMed] [Google Scholar]
- Gargan P. G., Tully O., Poole W. R.2003Relationship between sea lice infestation, sea lice production, and sea trout survival in Ireland, 1992–2001. In Salmon at the edge (ed. Mills D.), pp. 119–135 Oxford, UK: Blackwell Science [Google Scholar]
- Genna R. L., Mordue W., Pike A. W., Mordue A. J.2005Light intensity, salinity, and host velocity influence presettlement intensity and distribution on hosts by copepodites of sea lice, Lepeophtheirus salmonis. Can. J. Fish. Aquat. Sci. 62, 2675–2682 (doi:10.1139/f05-163) [Google Scholar]
- Gillibrand P. A., Amundrud T. L.2007A numerical study of the tidal circulation and buoyancy effects in a Scottish fjord: Loch Torridon. J. Geophys. Res. 112, CO5030 (doi:10.1029/2006JC003806) [Google Scholar]
- Gillibrand P. A., Willis K. J.2007Dispersal of sea louse larvae from salmon farms: modelling the influence of environmental conditions and larval behaviour. Aquat. Biol. 1, 63–75 [Google Scholar]
- Gottesfeld A. S., Proctor B., Rolston L. D., Carr-Harris C.2009Sea lice, Lepeophtheirus salmonis, transfer between wild sympatric adult and juvenile salmon on the north coast of British Columbia, Canada. J. Fish Dis. 32, 45–57 (doi:10.1111/j.1365-2761.2008.01003.x) [DOI] [PubMed] [Google Scholar]
- Hatton-Ellis M., Hay D., Walker A. F., Northcott S. J.2006Sea lice Lepeophtheirus salmonis infestations of post-smolt sea trout in Loch Shieldaig, Wester Ross, 1999–2003. In Sea trout: biology, conservation and management (eds Harris G. S., Milner N. J.), pp. 372–376 Oxford, UK: Blackwell Publishing [Google Scholar]
- Hayward C. J., Bott N. J., Itoh N., Iwashita M., Okihiro M., Nowak B. F.2007Three species of parasites emerging on the gills of mulloway, Argyrosomus japonicus (Temminck & Schegel, 1843), cultured in Australia. Aquaculture 265, 27–40 (doi:10.1016/j.aquaculture.2007.02.004) [Google Scholar]
- Hayward C. J., Aiken H. M., Nowak B. F.2008An epizootic of Caligus chiastos on farmed southern bluefin tuna Thunnus maccoyii off South Australia. Dis. Aquat. Organ. 79, 57–63 (doi:10.3354/dao01890) [DOI] [PubMed] [Google Scholar]
- Hayward C. J., Bott N. J., Nowak B. F.2009Seasonal epizootics of sea lice, Caligus spp., on southern bluefin tuna, Thunnus maccoyii (Castelnau), in a long-term farming trial. J. Fish Dis. 32, 101–106 (doi:10.1111/j.1365-2761.2008.01010.x) [DOI] [PubMed] [Google Scholar]
- Heuch P. A.1995Experimental evidence for aggregation of salmon louse copepodids (Lepeophtheirus salmonis) in steep salinity gradients. J. Mar. Biol. Ass. UK 75, 927–939 (doi:10.1017/S002531540003825X) [Google Scholar]
- Heuch P. A., Mo T. A.2001A model of salmon louse production in Norway: effects of increasing salmon production and public management measures. Dis. Aquat. Organ. 45, 145–152 (doi:10.3354/dao045145) [DOI] [PubMed] [Google Scholar]
- Heuch P. A., Parsons A., Boxaspen K.1995Diel vertical migration: a possible host-finding mechanism in salmon louse (Lepeophtheirus salmonis) copepodids? Can. J. Fish. Aquat. Sci. 52, 681–689 [Google Scholar]
- Heuch P. A., Bjørn P. A., Finstad B., Holst J. C., Asplin L., Nilsen F.2005A review of the Norwegian ‘National Action Plan Against Salmon Lice on Salmonids’: the effect on wild smolts. Aquaculture 246, 79–92 (doi:10.1016/j.aquaculture.2004.12.027) [Google Scholar]
- Heuch P. A., Doall M. H., Yen J.2006Water flow around a fish mimic attracts a parasitic and deters a planktonic copepod. J. Plankt. Res. 29, 13–116 [Google Scholar]
- Heuch P. A., Olsen R. S., Malkenes R., Revie C. W., Gettinby G., Baillie M., Lees F., Finstad B.2009Temporal and spatial variations in lice numbers on salmon farms in the Hardanger fjord 2004–06. J. Fish Dis. 32, 89–100 (doi:10.1111/j.1365-2761.2008.01002.x) [DOI] [PubMed] [Google Scholar]
- Hewitt G. C.1971Two species of Caligus (Copepoda, Caligidae) from Australia waters, with a description of some developmental stages. Pac. Sci. 25, 145–164 [Google Scholar]
- Ho J. S.2000The major problem of cage aquaculture in Asia relating to sea lice. In Proc. First Int. Symp. on Cage Aquaculture in Asia (eds Liao I. C., Lin C. K.), pp. 13–19 Manila: Asian Fisheries Society, Bangkok: World Aquaculture Society Southeast Asian Chapter [Google Scholar]
- Ho J. S.2004Invasiveness of sea lice (Copepoda, Caligidae) in marine aquaculture. J. Fish. Soc. Taiwan 31, 85–99 [Google Scholar]
- Ho J. S., Nagasawa K., Kim I. H., Ogawa K.2001Occurrence of Caligus lalandei Barnard, 1948 (Copepoda: Siphonostomatoida) on amberjacks (Seriola spp.) in the Western North Pacific. Zool. Sci. 18, 423–431 (doi:10.2108/zsj.18.423) [Google Scholar]
- Ho J. S., Kim I. H., Cruz-Lacierda E. R., Nagasawa K.2004Sea lice (Copepoda: Caligidae) parasitic on marine cultured and wild fishes of the Philippines. J. Fish. Soc. Taiwan 31, 235–249 [Google Scholar]
- Hvidsten N. A., Finstad B., Kroglund F., Johnsen B. O., Strand R., Arnekleiv J. V., Bjørn P. A.2007Does increased abundance of sea lice influence survival of wild Atlantic salmon post-smolt? J. Fish Biol. 71, 1639–1648 [Google Scholar]
- Jones S. R. M., Hargreaves N. B.2007The abundance and distribution of Lepeophtheirus salmonis (Copepoda: Caligidae) on pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon in coastal British Columbia. J. Parasitol. 93, 1324–1331 (doi:10.1645/GE-1252.1) [DOI] [PubMed] [Google Scholar]
- Jones S. R. M., Prosperi-Porta G., Kim E., Callow P., Hargreaves N. B.2006aThe occurrence of Lepeophtheirus salmonis and Caligus clemensi (Copepoda: Caligidae) on three-spine stickleback Gasterosteus aculeatus in coastal British Columbia. J. Parasitol. 92, 473–480 (doi:10.1645/GE-685R1.1) [DOI] [PubMed] [Google Scholar]
- Jones S. R. M., Kim E., Dawe S.2006bExperimental infections with Lepeophtheirus salmonis (Krøyer) on threespine sticklebacks, Gasterosteus aculeatus L. and juvenile Pacific salmon, Oncorhynchus spp. J. Fish Dis. 29, 489–495 (doi:10.1111/j.1365-2761.2006.00742.x) [DOI] [PubMed] [Google Scholar]
- Jones S. R. M., Fast M. D., Johnson S. C., Groman D. B.2007Differential rejection of salmon lice by pink and chum salmon: disease consequences and expression of proinflammatory genes. Dis. Aquat. Organ. 75, 229–238 (doi:10.3354/dao075229) [DOI] [PubMed] [Google Scholar]
- Krkošek M.In press Sea lice and salmon in Pacific Canada: ecology and policy. Front. Ecol. Environ (doi:10.1890/080097) [Google Scholar]
- Krkošek M., Lewis M. A., Volpe J. P.2005Transmission dynamics of parasitic sea lice from farm to wild salmon. Proc. R. Soc. B 272, 689–696 (doi:10.1098/rspb.2004.3027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkošek M., Lewis M. A., Volpe J. P., Morton A.2006aFish farms and sea lice infestations of wild juvenile salmon in the Broughton Archipelago: a rebuttal to Brooks (2005). Rev. Fish. Sci. 14, 1–11 (doi:10.1080/10641260500433531) [Google Scholar]
- Krkošek M., Lewis M. A., Morton A., Frazer L. N., Volpe J. P.2006bEpizootics of wild fish induced by farm fish. Proc. Natl Acad. Sci. USA 103, 15 506–15 510 (doi:10.1073/pnas.0603525103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkošek M., Gottesfeld A., Proctor B., Rolston D., Carr-Harris C., Lewis M. A.2007aEffects of host migration, diversity and aquaculture on sea lice threats to Pacific salmon populations. Proc. R. Soc. B 274, 3141–3149 (doi:10.1098/rspb.2007.1122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkošek M., Ford J. S., Morton A., Lele S., Myers R. A., Lewis M. A.2007bDeclining wild salmon populations in relation to parasites from farm salmon. Science 318, 1772–1775 (doi:10.1126/science.1148744) [DOI] [PubMed] [Google Scholar]
- Krkošek M., Ford J. S., Morton A., Lele S., Lewis M. A.2008Sea lice and pink salmon declines: a response to Brooks and Jones. Rev. Fish. Sci. 16, 413–420 (doi:10.1080/10641260802013692) [Google Scholar]
- Krkošek M., Morton A., Volpe J., Lewis M.In press Sea lice and salmon population dynamics: effects of exposure time for migratory fish. Proc. R. Soc. B. (doi:10.1098/rspb.2009.0317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E., Gettinby G., McCart K., Revie C. W.2004Time-series models of sea lice Caligus elongatus (Nordmann) abundance on Atlantic salmon Salmo salar L. in Loch Sunart, Scotland. Aquacult. Res. 35, 764–772 (doi:10.1111/j.1365-2109.2004.01099.x) [Google Scholar]
- McKibben M. A., Hay D. W.2004Distributions of planktonic sea lice larvae Lepeophtheirus salmonis in the inter-tidal zone in Loch Torridon, Western Scotland in relation to salmon farm production cycles. Aquacult. Res. 35, 742–750 (doi:10.1111/j.1365-2109.2004.01096.x) [Google Scholar]
- McVicar A. H.2004Management actions in relation to the controversy about salmon lice infections in fish farms as a hazard to wild salmonid populations. Aquacult. Res. 35, 751–758 (doi:10.1111/j.1365-2109.2004.01097.x) [Google Scholar]
- Mordue A. J., Birkett M. A.2009A review of host finding behaviour in the parasitic sea louse, Lepeophtheirus salmonis (Caligidae: Copepoda). J. Fish Dis. 32, 3–13 (doi:10.1111/j.1365-2761.2008.01004.x) [DOI] [PubMed] [Google Scholar]
- Mordue Luntz A. J., Bailey R. J. E., Birkett M. A., Ingvarsdóttir A., Mordue W., O'Shea B., Pickett J. A., Wadhams L. J.2006The role of semiochemicals in host location and non-host avoidance by salmon louse (Lepeophtheirus salmonis) copepodids. Can. J. Fish. Aquat. Sci. 63, 448–456 (doi:10.1139/f05-231) [Google Scholar]
- Morton A., Routledge R., Peet C., Ladwig A.2004Sea lice (Lepeophtheirus salmonis) infection rates on juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon in the nearshore marine environment of British Columbia, Canada. Can. J. Fish. Aquat. Sci. 61, 147–157 (doi:10.1139/f04-016) [Google Scholar]
- Morton A. B., Routledge R., Williams R.2005Temporal patterns of sea lice infestation on wild Pacific salmon in relation to the fallowing of Atlantic salmon farms. Am. J. Fish. Manag. 25, 811–821 (doi:10.1577/M04-149.1) [Google Scholar]
- Morton A., Routledge R., Krkošek M.2008Sea louse infestation in wild juvenile salmon and Pacific herring associated with fish farms off the east-central coast of Vancouver Island, British Columbia. N. Am. J. Fish. Manag. 28, 523–532 (doi:10.1577/M07-042.1) [Google Scholar]
- Murray A. G.2002Using observed load distributions with a simple model to analyse the epidemiology of sea lice (Lepeophtheirus salmonis) on sea trout (Salmo trutta). Pest Manag. Sci. 58, 585–594 (doi:10.1002/ps.470) [DOI] [PubMed] [Google Scholar]
- Murray A. G.2008Using simple models to review the application and implications of different approaches used to simulate transmission of pathogens among aquatic animals. Prevent. Vet. Med. 88, 167–177(doi:10.1016/j.prevetmed.2008.09.006) [DOI] [PubMed] [Google Scholar]
- Murray A. G., Gillibrand P. A.2006Modelling salmon lice dispersal in Loch Torridon, Scotland. Mar. Poll. Bull. 53, 128–135 (doi:10.1016/j.marpolbul.2005.09.013) [DOI] [PubMed] [Google Scholar]
- Murray A. G., Peeler E. J.2005A framework for understanding the potential for emerging diseases in aquaculture. Prevent. Vet. Med. 67, 223–235 (doi:10.1016/j.prevetmed.2004.10.012) [DOI] [PubMed] [Google Scholar]
- O'Donohoe P., Kane F., Kennedy S., Nixon P., Power A., Naughton O., Jackson D.2007National survey of sea lice (Lepeophtheirus salmonis Krøyer and Caligus elongatus Nordmann) on fish farms in Ireland: 2006. Ir. Fish. Bull. 28, 35 [Google Scholar]
- Øines Ø., Heuch P. A.2007Caligus elongatus Nordmann genotypes on wild and farmed fish. J. Fish Dis. 30, 81–91 (doi:10.1111/j.1365-2761.2007.00783.x) [DOI] [PubMed] [Google Scholar]
- Øines Ø., Simonsen J. H., Knutsen J. A., Heuch P. A.2006Host preference of adult Caligus elongatus Nordmann in the laboratory and its implications for Atlantic cod aquaculture. J. Fish Dis. 29, 167–174 (doi:10.1111/j.1365-2761.2006.00702.x) [DOI] [PubMed] [Google Scholar]
- Orr C.2007Estimated sea louse egg production from Marine Harvest Canada farmed Atlantic salmon in the Broughton Archipelago, British Columbia, 2003–2004. North American. J. Fish. Manag. 27, 187–197 (doi:10.1577/M06-043.1) [Google Scholar]
- Penston M. J., Davies I. M.2009An assessment of salmon farms and wild salmonids as sources of Lepeophtheirus salmonis (Krøyer) copepodids in the water column in Loch Torridon, Scotland. J. Fish Dis. 32, 75–88 (doi:10.1111/j.1365-2761.2008.00986.x) [DOI] [PubMed] [Google Scholar]
- Penston M. J., Millar C. P., Davies I. M.2008aReduced Lepeophtheirus salmonis larval abundance in a sea loch on the west coast of Scotland between 2002 and 2006. Dis. Aquat. Organ. 81, 109–117 (doi:10.3354/dao01946) [DOI] [PubMed] [Google Scholar]
- Penston M. J., Millar C. P., Zuur A., Davies I. M.2008bSpatial and temporal distribution of Lepeophtheirus salmonis (Krøyer) larvae in a sea loch containing salmon, Salmo salar L. farms on the north-west coast of Scotland. J. Fish Dis. 31, 361–371 (doi:10.1111/j.1365-2761.2008.00915.x) [DOI] [PubMed] [Google Scholar]
- Pike A. W., Wadsworth S. L.2000Sea lice on salmonids: their biology and control. Adv. Parasitol. 44, 233–337 (doi:10.1016/S0065-308X(08)60233-X) [DOI] [PubMed] [Google Scholar]
- Pino-Marambio J., Mordue (Luntz) A. J., Birkett M., Carvajal J., Asencio G., Mellado A., Quiroz A.2007Behavioural studies of host, non-host and mate location by the sea louse, Caligus rogercresseyi Boxshall & Bravo, 2000 (Copepoda: Caligidae). Aquaculture 271, 70–76 [Google Scholar]
- Revie C. W., Gettinby G., Treasurer J. W., Rae G. H.2002The epidemiology of sea lice, Caligus elongatus Nordmann, in marine aquaculture of Atlantic salmon, Salmo salar L., in Scotland. J. Fish Dis. 25, 391–399 (doi:10.1046/j.1365-2761.2002.00388.x) [Google Scholar]
- Revie C. W., Gettinby G., Treasurer J. W., Wallace C.2003Identifying epidemiological factors affecting sea lice (Lepeophtheirus salmonis) abundance on Scottish salmon farms using general linear models. Dis. Aquat. Organ. 57, 85–95 (doi:10.3354/dao057085) [DOI] [PubMed] [Google Scholar]
- Rosenberg A. A.2008The price of lice. Nature 451, 23–24 (doi:10.1038/451023a) [DOI] [PubMed] [Google Scholar]
- Saksida S., Karreman G. A., Constantine J., Donald A.2007aDifferences in Lepeophtheirus salmonis abundance levels on Atlantic salmon farms in the Broughton Archipelago, British Columbia, Canada. J. Fish Dis. 30, 357–366 (doi:10.1111/j.1365-2761.2007.00814.x) [DOI] [PubMed] [Google Scholar]
- Saksida S., Constantine J., Karreman G. A., Donald A.2007bEvaluation of sea lice abundance levels on farmed Atlantic salmon (Salmo salar L.) located in the Broughton Archipelago of British Columbia from 2003 to 2005. Aquacult. Res. 38, 219–231 (doi:10.1111/j.1365-2109.2007.01651.x) [Google Scholar]
- Smit N. J., Davies A. J.2004The curious life-style of the parasitic stages of gnathiid isopods. Adv. Parasit. 58, 289–391 (doi:10.1016/S0065-308X(04)58005-3) [DOI] [PubMed] [Google Scholar]
- Stone J., Roy W. J., Sutherland I. H., Ferguson H. W., Sommerville C., Endris R.2002Safety and efficacy of emamectin benzoate administered in-feed to Atlantic salmon, Salmo salar L., smolts in freshwater, as a preventative treatment against infestations of sea lice, Lepeophtheirus salmonis (Kroyer). Aquaculture 210, 21–34 (doi:10.1016/S0044-8486(01)00822-5) [Google Scholar]
- Thomas J. D.2002The ecology of fish parasites with particular reference to helminth parasites and their salmonid fish hosts in Welsh rivers: a review of some of the central questions. Adv. Parasitol. 52, 1–154 (doi:10.1016/S0065-308X(02)52011-X) [DOI] [PubMed] [Google Scholar]
- Tjensvoll K., Glover K. A., Nylund A.2006Sequence variation in four mitochondrial genes of the salmon louse Lepeophtheirus salmonis. Dis. Aquat. Organ. 68, 251–259 (doi:10.3354/dao068251) [DOI] [PubMed] [Google Scholar]
- Todd C. D.2006The copepod parasite (Lepeophtheirus salmonis (Krøyer), Caligus elongatus Nordman) interactions between wild and farmed Atlantic salmon (Salmo salar L.) and wild sea trout (Salmo trutta L.): a mini review. J. Plankt. Res. 29(Suppl. 1), 161–171 [Google Scholar]
- Todd C. D., Whyte B. D. M., MacLean J. C., Walker A. M.2006Ectoparasitic sea lice (Lepeophtheirus salmonis and Caligus elongatus) infestations of wild, adult, and one sea-winter Atlantic salmon Salmo salar returning to Scotland. Mar. Ecol. Prog. Ser. 328, 183–193 (doi:10.3354/meps328183) [Google Scholar]
- Treasurer J., Grant A.1994‘Second’ louse species must not be ignored. Fish Farmer 17, 46–47 [Google Scholar]
- Tully O., Nolan D. T.2002A review of population biology and host–parasite interactions of the sea louse Lepeophtheirus salmonis (Copepoda: Caligidae). Parasitology 124, 165–182 (doi:10.1017/S0031182002001889) [DOI] [PubMed] [Google Scholar]
- Tully O., Whelan K. F.1993Production of nauplii of Lepeophtheirus salmonis (Krøyer) (Copepoda: Caligidae) from farmed and wild salmon and its relation to the infestation of wild sea trout (Salmo trutta L.) off the west coast of Ireland. Fish. Res. 17, 187–200 (doi:10.1016/0165-7836(93)90018-3) [Google Scholar]
- Tully O., Gargan P., Poole W. R., Whelan K. F.1999Spatial and temporal variation in the infestation of sea trout (Salmo trutta L.) by the caligid copepod Lepeophtheirus salmonis (Krøyer) in relation to sources of infection in Ireland. Parasitology 119, 41–51 (doi:10.1017/S003118209900445X) [DOI] [PubMed] [Google Scholar]
- Viney M.2008From small sea-lice do great problems grow. The Irish Times, Saturday 24 January 2008 [Google Scholar]
- Wagner G. N., McKinley R. S., Bjørn P. A., Finstad B.2004Short-term freshwater exposure benefits sea lice-infected Atlantic salmon. J. Fish Biol. 64, 1593–1604 (doi:10.1111/j.0022-1112.2004.00414.x) [Google Scholar]
- Wagner G. N., Fast M. D., Johnson S. C.2007Physiology and immunology of Lepeophtheirus salmonis infections of salmonids. Trends Parasitol. 24, 176–183 (doi:10.1016/j.pt.2007.12.010) [DOI] [PubMed] [Google Scholar]
- Webster S. J., Dill L. M., Butterworth K.2007The effect of sea lice infestation on the salinity preference and energetic expenditure of juvenile pink salmon (Oncorhynchus gorbuscha). Can. J. Fish. Aquat. Sci. 64, 672–680 (doi:10.1139/F07-043) [Google Scholar]
- Wells A., et al. 2006Physiological effects of simultaneous, abrupt seawater entry and sea lice (Lepeophtheirus salmonis) infestation of wild, sea-run brown trout (Salmo trutta) smolts. Can. J. Fish. Aquat. Sci. 63, 2809–2821 (doi:10.1139/F06-160) [Google Scholar]
- Wells A., et al. 2007Physiological consequences of ‘premature freshwater return’ for wild sea-run brown trout (Salmo trutta) postsmolts infested with sea lice (Lepeophtheirus salmonis). Can. J. Fish. Aquat. Sci. 64, 1360–1369 (doi:10.1139/F07-107) [Google Scholar]