Abstract
In vertebrates, the adrenocortical stress response activates an emergency life-history stage, which is thought to promote survival by helping individuals escape life-threatening situations. Although the adrenocortical stress response promotes many behavioural and physiological changes, it remains unclear whether this stress response actually translates into higher survival in wild vertebrates. We measured the adrenocortical stress response of non-breeding American redstarts (Setophaga ruticilla), a migratory bird that wintered in habitats of either high (mangroves) or low suitability (scrubs), and subsequently monitored their return rate during the following non-breeding seasons. The intensity of the adrenocortical stress response was consistent within individuals across the non-breeding season and was positively correlated with return rates in redstarts that wintered in scrubs, but not in redstarts that wintered in mangroves. Thus, in a context-dependent manner, the ability of an individual to physiologically react to stress determines its ability of returning to its non-breeding territory the following winters. For an individual, the ability to mount an important adrenocortical stress response probably benefits to survival. However, this beneficial effect probably depends on an individual's environment and phenotypic characteristics because these two variables are likely to affect its probability of being confronted with life-threatening stressors during its annual life cycle.
Keywords: corticosterone, survival, stress, environmental context, habitat
1. Introduction
In confronting habitat destruction and fragmentation, there is an urgent need to understand how organisms cope with an increasing intensity and frequency of stressful events associated with habitat degradation, reduced food abundance, increased competition or predation risk and anthropogenic disturbance (Walther et al. 2002; Wikelski & Cooke 2006). Studies of the physiological and endocrine mechanisms can provide unique insights into how individuals functionally respond to habitat degradation because of the role the endocrine system plays in mediating interactions among environment, physiology, behaviour and, ultimately, individual fitness (Ricklefs & Wikelski 2002; Wingfield et al. 2008). In vertebrates, focusing on glucocorticosteroid hormones (cortisol and corticosterone) is especially judicious when examining the relationship between stressors and survival because these hormones have clearly been demonstrated to be important mediators of allostasis and responses to potential stressors throughout the life of an individual (Wingfield & Romero 2001; McEwen & Wingfield 2003; Wingfield & Sapolsky 2003). Plasma levels of glucocorticosteroid hormones rapidly increase in response to potential stressors (the ‘adrenocortical stress response’ or ‘corticosterone stress response’ in birds), and this rise triggers behavioural and physiological changes that are thought to promote survival by helping individuals escape life-threatening situations (‘the emergency life-history stage’, Wingfield et al. 1998; Wingfield & Romero 2001; Breuner et al. 2008).
Although the adrenocortical stress response appears to be promising to the study of how wild vertebrates cope with environmental changes, it is not currently well understood if it actually translates into higher survival for animals living in the wild (Breuner et al. 2008). Short-term increases in glucocorticoid levels are known to promote behaviours and physiological mechanisms thought to ultimately increase survival (reviewed in Sapolsky et al. 2000; Breuner et al. 2008). However, only three studies have explicitly examined the potential benefits of elevated stress-induced glucocorticoid levels (a measure of the ability of an individual to mount an acute adrenocortical stress response) on survival. Surprisingly, all three reported that elevated stress-induced glucocorticoid levels were not positively correlated with survival or return probability (Romero & Wikelski 2001; Brown et al. 2005; Blas et al. 2007). Therefore, despite strong theoretical evidence (Wingfield et al. 1998; Wingfield & Sapolsky 2003), there is currently no direct empirical evidence supporting the idea that the strength of an acute adrenocortical response is a reliable indicator of an individual's intrinsic probability of long-term survival (Breuner et al. 2008).
The underlying endocrine control mechanisms responsible for the adjustment of physiology and behaviour to the environment are complex and can be regulated by multiple extrinsic (environmental) and intrinsic factors (e.g. energy reserves, reproductive state, life-history stage; Sapolsky et al. 2000; Romero 2004). Understanding these complex interactions is crucial because the multiple levels of regulation can complicate the relationships linking environmental factors, endocrine stress mechanisms and fitness components (e.g. survival). Recent research has demonstrated that the physiological and behavioural effects of circulating glucocorticoid levels can be context dependent (Orchinik 1998; Angelier et al. 2007; Breuner et al. 2008). For example, Almasi et al. (2008) have shown that the effect of an experimental increase in corticosterone levels on parental effort clearly depends on phenotype and parental strategy in barn owls (Tyto alba). Therefore, it is likely that the effect of an acute adrenocortical stress response on survival depends on the interaction between environmental situations and an individual's intrinsic state, and this could explain the unexpected findings reported thus far (reviewed in Breuner et al. 2008). To better evaluate the influence of an acute adrenocortical stress response on survival, it may be crucial to investigate the context-dependent effect of glucocorticoids on survival and return probability.
In this study, our objective was to determine whether the corticosterone stress response can predict return probability in wild birds. We focused on a species of Neotropical–Nearctic long-distance migrant, the American redstart (Setophaga ruticilla). We selected this species because of an existing dataset on corticosterone stress responses (Marra & Holberton 1998), in which many individuals were sampled for baseline corticosterone levels (within 3 min of capture) and stress-induced corticosterone levels (after 30 min of restraint) both early and late in their non-breeding period (autumn and spring). All birds were individually colour-marked and have been looked for in every year for 10 subsequent years (Marra & Holmes 2001; Johnson et al. 2006). According to the adaptive benefit hypothesis of the effect of the adrenocortical stress response on survival (Wingfield et al. 1998; Wingfield & Sapolsky 2003; Lendvai et al. 2007; Breuner et al. 2008; Bokony et al. 2009), we predict that a high corticosterone stress response will be associated with a high return rate. Interestingly, American redstarts are known to occupy sites along a habitat quality gradient where food availability limits condition and survival (Marra & Holmes 2001; Studds & Marra 2007). Marra & Holberton (1998) sampled redstarts wintering in two contrasting winter habitats: a mangrove habitat known to have high food availability and a scrub habitat known to have low food availability (Marra & Holberton 1998; Marra & Holmes 2001; Studds & Marra 2007). This natural situation represents a unique opportunity to investigate a potential habitat-dependent relationship between the corticosterone stress response and the return probability.
2. Material and methods
(a). Study site and species
Our study was conducted at the Font Hill Nature Preserve, 13 km west of Black River, St Elizabeth Parish, Jamaica, West Indies. American redstarts begin arriving in Jamaica and establishing territories in mid-September and remain on those territories for six to seven months until they depart on spring migration in April through mid-May. During this wintering period, redstarts are territorial and occur in a variety of habitat types that range in suitability (Marra & Holberton 1998; Marra & Holmes 2001; Studds & Marra 2007).
(b). Capture, blood sample and body condition
Captured redstarts from Marra & Holberton (1998) were resighted annually to quantify survival and return rate (Marra & Holmes 2001; Johnson et al. 2006). To summarize these methods, 73 adult redstarts were captured during the autumn, i.e. early in the wintering period, between 27 September and 3 November 1995. Moreover, 31 of these redstarts and nine additional redstarts were captured during the spring, i.e. late in the wintering period, between 19 March and 14 April 1996. Thus, 31 birds were captured in both autumn and spring, allowing for the monitoring of consistency in the corticosterone stress response. In this study, we considered adults only—birds that had completed at least one wintering season prior to the season of our sampling—because they are known to show a high fidelity to their wintering territory between seasons (Marra & Holmes 2001), which allowed for greater accuracy in our estimates of survival and return probability.
During the initial study by Marra & Holberton (1998), all birds were bled according to a standardized capture–restraint stress protocol. As described in this initial paper, an initial blood sample was collected immediately after capture from the brachial vein into heparinized microcapillary tubes. After collection of the initial blood sample, birds were placed into cloth bags, and a subsequent sample was collected 30 min later. For some redstarts, blood sampling was difficult or too much time elapsed between capture and the initial sampling to reliably measure baseline corticosterone levels, and so we were not able to determine either baseline or stress-induced corticosterone levels. Thus, baseline and stress-induced corticosterone levels were, respectively, available for 50 and 67 redstarts in autumn and for 29 and 35 redstarts in spring. Among the 31 redstarts sampled in both autumn and spring, baseline and stress-induced corticosterone levels were, respectively, available for 15 and 24 redstarts. All birds were weighed to the nearest 0.1 g and measured (unflattened wing chord, tail length and tarsus length) to the nearest 0.1 mm. We calculated a measure of body size for each from a factor analysis. Factors were extracted by a principal component analysis performed on the three measurement variables, and the resulting factor for each individual (PC1) was used as a measure of overall body size (Studds & Marra 2007). We then calculated our residual indices of body condition by using the residuals from a regression of body mass against our body size factor (r = 0.537, p < 0.001). All birds were banded with a USFWS aluminium ring and three coloured plastic bands for the individual identification from a distance.
(c). Hormone assay
Corticosterone values were determined for the initial study by Marra & Holberton (1998). Briefly, blood samples were stored at 4°C for up to 8 h until centrifuged at 13 000g for 10 min. Plasma was then recovered from each sample with a 50 µl Hamilton syringe and kept frozen in microcentrifuge tubes until assayed for corticosterone by radioimmunoassay. The sensitivity of the assay was measured as the lowest concentration used in the standard curve and was 7.8 pg per tube. For this assay, interassay variation was 14.6 per cent and within-assay variation was 1.1 per cent.
(d). Survival and statistical analyses
Extensive and exhaustive field surveys of colour-banded birds have been conducted in each of the 10 years following our sampling season in order to monitor the return rate of redstarts wintering in Font Hill (Marra & Holmes 2001; Johnson et al. 2006). Specifically, colour-banded birds of the study site were intensively followed and mapped during several weeks each year to define the boundaries of their territories (Marra & Holmes 2001). During this 10-year period, no bird of our study site was found to dramatically change the location of its territory between years. However, some birds that failed to return to the study site in the following wintering season could have survived, but upgraded their territory and, thus, wintered in another location that we did not survey. We could not totally disentangle dispersal processes from survival ones. For this reason, we measured apparent survival (i.e. return rate) and we refer to return rate in our analyses. A preliminary capture–mark–recapture analysis of our dataset showed that the recapture probability is estimated to be 1 for the birds that were sampled in this study. It means that every bird that was sampled for corticosterone and which was resighted at any point during the following 10 years was resighted during the wintering season directly following our sampling. Therefore, we used classic return rate analyses (logistic models) in our study to reduce the number of parameters and, thus, to analyse the probability of return with a higher statistical power.
In this study, the magnitude of the corticosterone stress response was expressed as stress-induced corticosterone levels (measured after 30 min of restraint). We also calculated the rate of increase from baseline to stress-induced corticosterone levels per minute (ng ml−1 min−1). This measure was highly correlated with stress-induced corticosterone levels in both autumn and spring (r > 0.850, p < 0.0001), and our sample size for stress-induced corticosterone levels was larger than our sample size for the rate of increase from baseline to stress-induced corticosterone levels. Therefore, we report only the results for stress-induced corticosterone levels.
We used generalized mixed linear models (GLMMs, binomial errors, logit link function) to test whether baseline or stress-induced corticosterone levels predicted the return of redstarts in the following winter. The dependent variable was a binary datum (bird that returned the following winter = 1 and birds that did not return the following winter = 0), and the independent variables or factors were habitat (scrub versus mangrove), period of sampling (autumn versus spring), baseline corticosterone levels, stress-induced corticosterone levels and their interactions (table 1). Bird identity was included as a random factor. Because previous studies and preliminary analyses have reported that corticosterone levels and return rates do not vary between sexes (Marra & Holberton 1998; Marra & Holmes 2001; Studds & Marra 2007), we pooled data from males and females in our analyses to test our hypotheses with enough statistical power. Moreover, we were not able to include baseline and stress-induced corticosterone levels in the same models because those values were not available for all redstarts. Thus, we built two distinct models to test our two biological hypotheses (table 1). In addition, we tested whether baseline and stress-induced corticosterone levels were consistent across the wintering period according to the procedure detailed in Romero & Reed (2008). To test this hypothesis, we used birds that were sampled in both autumn and spring of a single wintering period. For the autumn corticosterone values (baseline and, then, stress-induced levels), we ranked individuals from lowest to highest values. This ranking procedure was repeated during the spring. We then used an ANOVA in order to look for consistency of this ranking across the wintering season (dependent variable: ranking and independent variable: individual identity). A significant effect of individual identity on our dependent variable would mean that autumn ranks are consistent with spring ranks, whereas a non-significant effect would mean that autumn ranks are not consistent with spring ranks (Romero & Reed 2008). For each analysis of ranked corticosterone value, we also calculated the repeatability statistic r (Lessels & Boag 1987). We also tested whether corticosterone levels (baseline and stress-induced) were correlated with body condition and whether body condition was affected by habitat by using GLMM with individual identity as a random factor.
Table 1.
Model selection and GLMM (binomial error distribution and logit link function) to test the influence of baseline corticosterone (cort) levels (model A) and stress-induced corticosterone levels (model B) on annual return rate of American redstarts that were sampled twice during one wintering period (in autumn and spring). Significant variables are in italic.
| model | n | dependent variable | independent variables | d.f. | F-value | p-value |
|---|---|---|---|---|---|---|
| A | 79 | survival | habitat | 1,42 | 0.45 | 0.505 |
| period | 1,42 | 0.71 | 0.404 | |||
| baseline cort level | 1,42 | 1.13 | 0.295 | |||
| habitat×period | 1,42 | 0.07 | 0.794 | |||
| baseline cort level×habitat | 1,42 | 0.22 | 0.642 | |||
| baseline cort level×period | 1,42 | 0.80 | 0.377 | |||
| baseline cort level×habitat×period | 1,42 | 1.54 | 0.221 | |||
| B | 102 | survival | habitat | 1,58 | 0.37 | 0.544 |
| period | 1,58 | 0.09 | 0.765 | |||
| stress-induced cort level | 1,58 | 0.04 | 0.994 | |||
| habitat×period | 1,58 | <0.01 | 0.845 | |||
| stress-induced cort level×habitat | 1,58 | 8.59 | 0.004 | |||
| stress-induced cort level×period | 1,58 | 0.06 | 0.802 | |||
| stress-induced cort level×habitat×period | 1,58 | 1.46 | 0.231 |
3. Results
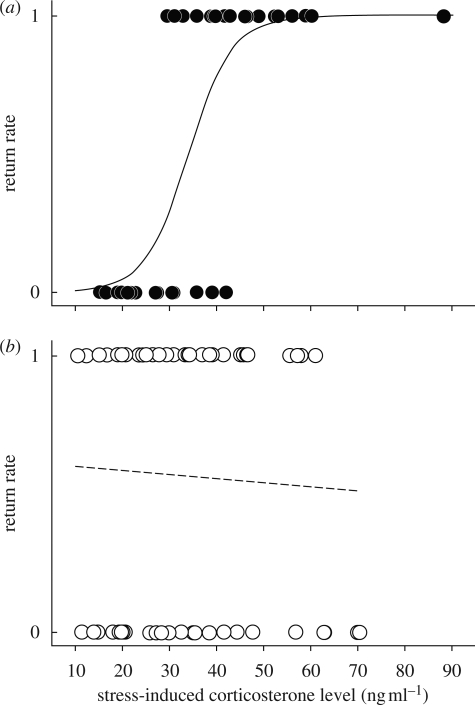
The return probability of individual American redstarts from one non-breeding season to another was independent of habitat (scrub or mangrove), period of sampling (autumn or spring) and baseline corticosterone levels (table 1, model A). In contrast, there was a highly significant effect of the interaction ‘habitat’בstress-induced corticosterone levels’ on the return probability of redstarts (table 1, model B). Specifically, the return probability of redstarts wintering in low-quality scrub habitat was positively correlated with their stress-induced corticosterone levels (a proxy of the intensity of the adrenocortical stress response). Birds with elevated stress-induced corticosterone levels were more likely to return than those with low stress-induced corticosterone levels (parameter estimates, t = 3.49, p < 0.001; figure 1a). However, this relationship was not found for redstarts that wintered in high-quality mangrove habitat (parameter estimates, t = −0.35, p = 0.727; figure 1b). For individual redstarts, stress-induced corticosterone levels were consistent across the wintering season (n = 24 individuals, F = 4.46, p < 0.001, r = 0.634). A bird with relatively low stress-induced corticosterone level in autumn had a similarly low stress-induced corticosterone level in spring. Baseline corticosterone levels, on the other hand, were not significantly consistent across the wintering season (n = 15 individuals, F = 1.418, p = 0.255, r = 0.173). A bird with a relatively low baseline corticosterone level in autumn could have either a low, an intermediate or an elevated baseline corticosterone level in spring. Baseline corticosterone levels and stress-induced corticosterone levels were not correlated with body condition (p > 0.25). Moreover, body condition did not differ between habitats in autumn and spring (p > 0.15).
Figure 1.
Return rate from one wintering season to the other as a function of stress-induced corticosterone levels of redstarts wintering in (a) a scrub habitat of low suitability or (b) in a mangrove habitat of high suitability. The probability of returning from one wintering season to another was significantly positively correlated with stress-induced corticosterone levels in birds that wintered in scrub habitats (filled symbols and solid line), but not in birds that wintered in mangrove habitats (open symbols and dashed line). The relationship is still highly significant (p < 0.01) if the bird with elevated stress-induced corticosterone levels is considered as an outlier and removed.
4. Discussion
In the present study, we report that the intensity of the acute corticosterone stress response (measured by stress-induced corticosterone levels) in redstarts on the non-breeding ground is a predictor of individual return from one year to the next. For those birds that wintered in low-quality scrub habitats, elevated stress-induced corticosterone levels were associated with a high return probability. Interestingly, this relationship appears to be context dependent since stress-induced corticosterone levels were not correlated with return probability in birds that wintered in high-quality mangrove habitats. To our knowledge, this is the first study to show that stress-induced corticosterone levels can serve as a predictor of return rate in a habitat-dependent manner in a wild vertebrate species.
Although our results support the hypothesis that the ability to respond rapidly to stressors is beneficial to survival, we cannot exclude the possibility that long-distance and permanent dispersal could also play a role in the patterns that we described. Moreover, confounding environmental and energetic factors may also explain this positive relationship between stress-induced corticosterone levels and return rate. However, this last interpretation is very unlikely because neither baseline nor stress-induced corticosterone levels were correlated with body condition (i.e. energetic factor) in our study. Moreover, we did not find any difference in return probability or body condition between habitats (i.e. environmental factor). These results are somewhat surprising because mangrove habitats have been shown to be of higher suitability than scrub habitats (Marra & Holberton 1998; Marra & Holmes 2001; Studds & Marra 2007). However, rainfall and food availability were unusually high in both habitats during the year of sampling, which probably resulted in attenuated differences in environmental and energetic constraints between mangrove and scrub birds (Marra & Holmes 2001; Studds & Marra 2007). Importantly, we measured the capacity of an individual to respond to a standardized stress protocol in our study (Wingfield & Sapolsky 2003), not to an uncontrolled environmental stressor. We also showed that this response is consistent in an individual throughout the wintering period, even though environmental conditions are known to vary dramatically across this period (Marra & Holberton 1998; Marra & Holmes 2001; Studds & Marra 2007). In addition, the corticosterone stress response is, at least partially, heritable and genetically determined in birds (Satterlee & Johnson 1988; Evans et al. 2006). Therefore, because the expression of the adrenocortical response was consistent within individuals, and less influenced, at least during our sampling periods, by environmental factors, we believe that this endocrine measurement represents the intrinsic ability or potential of an individual to react to environmental stressors (see also Blas et al. 2007). Supporting this interpretation, a recent study has demonstrated that the corticosterone stress response can be correlated with individual quality in male zebra finches, Taeniopygia guttata, living in a controlled environment (Wada et al. 2008). Earlier studies in a variety of species have similarly shown that while expression of the adrenocortical response can change across different stages of the annual cycle or season (reviewed in Romero 2002) that are associated with different levels of environmental predictability (Holberton & Able 2000), corticosterone stress response can be repeatable and can, therefore, represent a consistent individual characteristic (Cockrem & Silverin 2002; Wada et al. 2008; Williams 2008; Cockrem et al. in press). In other words, some birds probably always have a higher corticosterone stress response than others, despite seasonal variability in the corticosterone stress response.
How could the corticosterone stress response mechanistically promote return and survival of redstarts wintering in poor habitats? Mounting a stress response probably provides survival benefits by influencing aspects of an individual animal's behaviour and physiology (Sapolsky et al. 2000; Romero 2004; Breuner et al. 2008). From a behavioural perspective, the corticosterone stress response may govern personality and risk-taking strategies, which can affect survival probability in specific environmental situations (Wingfield 2003; Korte et al. 2005; Blas et al. 2007; Cockrem 2007). Moreover, some transient increases in corticosterone levels can be beneficial by promoting locomotor and feeding activity (Wingfield et al. 1998; Angelier et al. 2007; Breuner et al. 2008) and by improving cognitive abilities such as memory or those used in food retrieval (Saldanha et al. 2000; Roozendaal 2003). From a physiological perspective, the corticosterone stress response promotes a rapid mobilization of energy storage (Sapolsky et al. 2000), a reduced allocation of energy to non-essential physiological components (Lendvai et al. 2007; Wada & Breuner 2008; Müller et al. 2009) and has some transient immunoprotective effects (Dhabhar 2002). Such behavioural and physiological responses to stressors may be beneficial to redstarts during the non-breeding period, as well as the rest of their annual life cycle, and could, therefore, explain why a strong adrenocortical stress response is associated with a high return rate for the birds wintering in poor habitats.
How then can we explain that the relationship between return rate and the corticosterone stress response depends on the quality of the non-breeding habitat? Indeed, the ability to mount a strong stress response may only be beneficial when birds are repeatedly faced with unpredictable perturbations (Holberton & Able 2000). Depending on their environment and their phenotypic characteristics, individuals of a population may differ in the probability of being confronted with frequent stressors during their annual life cycle. In redstarts, wintering habitat is a reliable representation of individual performances. Birds wintering in poor habitats are individuals with a low dominance rank, and have been excluded from good territories by more dominant redstarts (Marra 2000). Moreover, birds wintering in poor habitats decline in condition over winter, depart later from the wintering grounds (Studds & Marra 2007), arrive later on the breeding grounds (Marra et al. 1998) and have lower breeding success (Reudink et al. 2009) than those wintering in good habitats. Indeed, birds wintering in good habitats are less likely to face major or prolonged stressors during their life cycle than those wintering in poor habitats because of better physical (more aggressive and dominant, larger size) and physiological abilities (immunity, metabolism, etc.) and because of better environmental conditions (high-quality breeding and non-breeding territories). Therefore, their survival may not predominantly depend on their ability to react to stressors, but rather on the accumulated effects of life-history decisions made throughout the life cycle such as breeding investment (Reid et al. 2003), migration and molt strategies (Barta et al. 2008; Gillis et al. 2008). On the contrary, the survival of birds wintering in poor habitats may very well primarily depend on their ability to react to the numerous unpredictable perturbations that they encounter during their annual life cycle. This may explain why we did not find any correlation between the return rate and the corticosterone stress response in birds wintering in high-quality mangrove habitats, but that return rate did depend on the corticosterone stress response in birds that wintered in low-quality scrub habitat. Thus, our results illustrate the need to consider phenotypic characteristics, environmental context and coping strategies when examining the relationship between survival, return rate and the corticosterone stress response (Breuner et al. 2008).
In our study, we found that baseline corticosterone levels were not a good predictor of return rate in redstarts. Interestingly, we also found that baseline corticosterone levels were not consistent across the wintering season in redstarts. Actually, baseline corticosterone levels probably vary quickly and frequently depending on environmental variables during the non-breeding season, such as food availability, predator or parasite density, status of body reserves or climatic events (Love et al. 2005; Raouf et al. 2006; Jenni-Eiermann et al. 2008). Because of this variability, it appears to be difficult to reliably link return rate with only one measurement of baseline corticosterone levels in vertebrates. This interpretation is supported by other empirical studies that did not find any correlation between endogenous corticosterone levels and survival in wild vertebrates (Romero & Wikelski 2001; Blas et al. 2007). Indeed, baseline corticosterone levels can probably predict reduced survival or return probability only when they are maintained at extremely elevated levels during a prolonged period and thus mirror a state of chronic stress (Brown et al. 2005; Kitaysky et al. 2007).
Most studies have assumed that the primary adaptive function of the corticosterone stress response is to promote survival, but, until now, this had not been demonstrated. Here, we show that the corticosterone stress response is repeatable within individuals as previously reported in other species (Cockrem & Silverin 2002; Wada et al. 2008; Cockrem et al. in press) and it can be linked to return probability in a wild migratory bird. Habitat quality, a natural stressor to wild birds, played an important role in the reliability of the stress response to predict return rate. Thus, it is essential to consider the phenotype of individuals and their environmental context when focusing on the fitness consequences of the adrenocortical response to stress (Orchinik 1998; Angelier et al. 2007; Kitaysky et al. 2007; Almasi et al. 2008; Breuner et al. 2008).
Acknowledgements
This study was supported by an NSF grant (0649679) to P.P.M. and R.L.H. and an NSF grant (0717338) to P.P.M. We thank M. Dawkins, O. Chastel, C. M. Vleck, D. Vleck, M. Palacios, M. Shultz and two anonymous reviewers, who gave very helpful comments on a previous version of the manuscript. We thank the Petroleum Corporation of Jamaica for permission to conduct this research at the Font Hill Nature Preserve, and Yvette Strong and Andrea Donaldson of the Jamaica National Environmental Planning Agency for their cooperation.
References
- Almasi B., Roulin A., Jenni-Eiermann S., Jenni L.2008Parental investment and its sensitivity to corticosterone is linked to melanin-based coloration in barn owls. Horm. Behav. 54, 217–223 (doi:10.1016/j.yhbeh.2008.02.021) [DOI] [PubMed] [Google Scholar]
- Angelier F., Clément-Chastel C., Gabrielsen G. W., Chastel O.2007Corticosterone and time-activity budget: an experiment with black-legged kittiwakes. Horm. Behav. 52, 482–491 (doi:10.1016/j.yhbeh.2007.07.003) [DOI] [PubMed] [Google Scholar]
- Barta Z., McNamara J. M., Houston A. I., Weber T. P., Hedenstrom A., Fero O.2008Optimal moult strategies in migratory birds. Phil. Trans. R. Soc. B 363, 221–229 (doi:10.1098/rstb.2007.2136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blas J., Bortolotti G. R., Tella J. L., Marchant T. A.2007Stress response during development predicts fitness in a wild, long-lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884 (doi:10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokony V., Lendvai A. Z., Liker A., Angelier F., Wingfield J. C., Chastel O.2009Stress response and the value of reproduction: are birds prudent parents? Am. Nat. 173, 589–598 (doi:10.1086/597610) [DOI] [PubMed] [Google Scholar]
- Breuner C. W., Patterson S. H., Hahn T. P.2008In search of relationships between the acute stress adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295 (doi:10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- Brown C. R., Brown M. B., Raouf S. A., Smith L. C., Wingfield J. C.2005Effects of endogenous steroid hormone levels on annual survival in cliff swallows. Ecology 86, 1034–1046 (doi:10.1890/04-0740) [Google Scholar]
- Cockrem J. F.2007Stress, corticosterone responses and avian personalities. J. Ornithol. 148, S169–S178 (doi:10.1007/s10336-007-0175-8) [Google Scholar]
- Cockrem J. F., Silverin B.2002Variation within and between birds in corticosterone response of great tits (Parus major). Gen. Comp. Endocrinol. 125, 197–206 (doi:10.1006/gcen.2001.7750) [DOI] [PubMed] [Google Scholar]
- Cockrem J. F., Barrett P., Candy E. J., Potter M. A.In press.Corticosterone responses in birds: individual variation and repeatability in Adélie penguins (Pygoscelis adeliae) and other species, and the use of power analysis to determine sample size. Gen. Comp. Endocrinol. [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S.2002Stress-induced augmentation of immune function. The role of stress hormones, leucocyte trafficking, and cytokines. Brain Behav. Immun. 16, 785–798 (doi:10.1016/S0889-1591(02)00036-3) [DOI] [PubMed] [Google Scholar]
- Evans M. R., Roberts M. L., Buchanan K. L., Goldsmith A. R.2006Heritability of corticosterone response and changes in life-history traits in the zebra finch. J. Evol. Biol. 19, 343–352 (doi:10.1111/j.1420-9101.2005.01034.x) [DOI] [PubMed] [Google Scholar]
- Gillis E. A., Green D. J., Middleton H. A., Morrissey C. A.2008Life history correlates of alternative migration strategies in American dippers. Ecology 89, 1687–1695 (doi:10.1890/07-1122.1) [DOI] [PubMed] [Google Scholar]
- Holberton R. L., Able K. P.2000Differential migration and an endocrine response to stress in wintering dark-eyed juncos (Junco hyemalis). Proc. R. Soc. Lond. B 267, 1889–1896 (doi:10.1098/rspb.2000.1226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni-Eiermann S., Glaus E., Grüebler M., Schwabl H., Jenni L.2008Glucocorticoid response to food availability in breeding barn swallows (Hirunda rustica). Gen. Comp. Endocrinol. 155, 558–565 (doi:10.1016/j.ygcen.2007.08.011) [DOI] [PubMed] [Google Scholar]
- Johnson M. D., Sherry T. W., Holmes R. T., Marra P. P.2006Assessing habitat quality for a migratory songbird wintering in natural and agricultural habitats. Cons. Biol. 20, 1433–1444 (doi:10.1111/j.1523-1739.2006.00490.x) [DOI] [PubMed] [Google Scholar]
- Kitaysky A. S., Piatt J. F., Wingfield J. C.2007Stress hormones link food availability and population processes in seabirds. Mar. Ecol. Prog. Ser. 352, 245–258 (doi:10.3354/meps07074) [Google Scholar]
- Korte S. M., Koolhaas J. M., Wingfield J. C., McEwen B. S.2005The Darwinian concept of stress: benefits of allostatis and costs of allostatic load and the trade-off in health and disease. Neurosci. Biobehav. Rev. 29, 3–38 (doi:10.1016/j.neubiorev.2004.08.009) [DOI] [PubMed] [Google Scholar]
- Lendvai A. Z., Giraudeau M., Chastel O.2007Reproduction and modulation of the stress response: an experimental test in the house sparrow. Proc. R. Soc. B 274, 391–397 (doi:10.1098/rspb.2006.3735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessels C. M., Boag P. T.1987Unrepeatable repeatabilities—a common mistake. Auk 104, 116–121 [Google Scholar]
- Love O. P., Chin E. H., Wynne-Edwards K. E., Williams T. D.2005Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 166, 751–766 (doi:10.1086/497440) [DOI] [PubMed] [Google Scholar]
- Marra P. P.2000The role of behavioral dominance in structuring patterns of habitat occupancy in a migrant bird during the non-breeding season. Behav. Ecol. 11, 299–308 (doi:10.1093/beheco/11.3.299) [Google Scholar]
- Marra P. P., Holberton R. L.1998Corticosterone levels as indicator of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 116, 284–292 (doi:10.1007/s004420050590) [DOI] [PubMed] [Google Scholar]
- Marra P. P., Holmes R. T.2001Consequences of dominance-mediated habitat segregation in American redstarts during the non-breeding season. Auk 118, 92–104 (doi:10.1642/0004-8038(2001)118[0092:CODMHS]2.0.CO;2) [Google Scholar]
- Marra P. P., Hobson K. A., Holmes R. T.1998Linking winter and summer events in a migratory bird using stable carbon isotopes. Science 282, 1884–1886 (doi:10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Wingfield J. C.2003The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15 (doi:10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- Müller C., Jenni-Eiermann S., Jenni L.2009Effects of short period of elevated circulating corticosterone on post-natal growth in free-living Eurasian kestrels Falco tinnunculus. J. Exp. Biol. 212, 1405–1412 (doi:10.1242/jeb.024455) [DOI] [PubMed] [Google Scholar]
- Orchinik M.1998Glucocorticoids, stress, and behavior: shifting the timeframe. Horm. Behav. 34, 320–327 (doi:10.1006/hbeh.1998.1488) [DOI] [PubMed] [Google Scholar]
- Raouf S. A., Smith L. C., Brown M. B., Wingfield J. C., Brown C. R.2006Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim. Behav. 71, 39–48 (doi:10.1016/j.anbehav.2005.03.027) [Google Scholar]
- Reid J. M., Bignal E. M., Bignal S., McCracken D. I., Monaghan P.2003Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J. Anim. Ecol. 72, 765–776 (doi:10.1046/j.1365-2656.2003.00750.x) [Google Scholar]
- Reudink M. W., Marra P. P., Kyser T. K., Boag P. T., Langin K. M., Ratcliffe L. M.2009Non-breeding season events influence sexual selection in a long-distance migratory bird. Proc. R. Soc. B 276, 1619–1626 (doi:10.1098/rspb.2008.1452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R., Wikelski M.2002The physiology/life history nexus. Tr. Ecol. Evol. 17, 462–468 (doi:10.1016/S0169-5347(02)02578-8) [Google Scholar]
- Romero L. M.2002Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24 (doi:10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- Romero L. M.2004Physiological stress in ecology: lessons from biomedical research. Tr. Ecol. Evol. 19, 249–255 (doi:10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- Romero L. M., Reed J. M.2008Repeatability of baseline corticosterone concentrations. Gen. Comp. Endocrinol. 156, 27–33 (doi:10.1016/j.ygcen.2007.10.001) [DOI] [PubMed] [Google Scholar]
- Romero L. M., Wikelski M.2001Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Niño events. Proc. Natl Acad. Sci. USA 98, 7366–7370 (doi:10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B.2003Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 27, 1213–1223 (doi:10.1016/j.pnpbp.2003.09.015) [DOI] [PubMed] [Google Scholar]
- Saldanha C. J., Schlinger B. A., Clayton N. S.2000Rapid effects of corticosterone on cache recovery in mountain chickadees (Parus gambeli). Horm. Behav. 37, 109–115 (doi:10.1006/hbeh.2000.1571) [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U.2000How do glucocorticoids influence stress responses: integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- Satterlee D. G., Johnson W. A.1988Selection of Japanese quail for contrasting blood corticosterone response to immobilization. Poult. Sci. 67, 25–32 [DOI] [PubMed] [Google Scholar]
- Studds C. E., Marra P. P.2007Linking fluctuations in rainfall to non-breeding season performance in a long-distance migratory bird, Setophaga ruticilla. Clim. Res. 35, 115–122 (doi:10.3354/cr00718) [Google Scholar]
- Wada H., Breuner C. W.2008Transient elevation of corticosterone alters begging behavior and growth of white-crowned sparrow nestlings. J. Exp. Biol. 211, 1696–1703 (doi:10.1242/jeb.009191) [DOI] [PubMed] [Google Scholar]
- Wada H., Salvante K. G., Stables C., Wagner E., Williams T. D., Breuner C. W.2008Adrenocortical responses in zebra finches (Taeniopygia guttata): individual variation, repeatability, and relationship to phenotypic quality. Horm. Behav. 53, 472–480 (doi:10.1016/j.yhbeh.2007.11.018) [DOI] [PubMed] [Google Scholar]
- Walther G. R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J. M., Hoegh-Guldberg O., Bairlein F.2002Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- Wikelski M., Cooke S. J.2006Conservation physiology. Tr. Ecol. Evol. 21, 38–46 (doi:10.1016/j.tree.2005.10.018) [DOI] [PubMed] [Google Scholar]
- Williams T. D.2008Individual variation in endocrine systems: moving beyond the ‘tyranny of the golden mean’. Phil. Trans. R. Soc. B 363, 1687–1698 (doi:10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J. C.2003Control of behavioural strategies for capricious environments. Anim. Behav. 66, 807–816 (doi:10.1006/anbe.2003.2298) [Google Scholar]
- Wingfield J. C., Romero L. M.2001Adrenocortical responses to stress and their modulation in free-living vertebrates. In Handbook of physiology (eds McEwen B. S., Goodman H. M.), pp. 211–234 New York, NY: Oxford University Press [Google Scholar]
- Wingfield J. C., Sapolsky R. M.2003Reproduction and resistance to stress: when and how? J. Neuroendocrinol. 15, 711–724 [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Maney D. L., Breuner C. W., Jacobs J. D., Lynn S., Ramenofsky M., Richardson R. D.1998Ecological bases of hormone–behavior interactions: the ‘emergency life-history stage’. Am. Zool. 38, 191–206 [Google Scholar]
- Wingfield J. C., Visser M. E., Williams T. D.2008Introduction. Integration of ecology and endocrinology in avian reproduction: a new synthesis. Phil. Trans. R. Soc. B 363, 1581–1588 (doi:10.1098/rstb.2007.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]