Abstract
Historically, ecomorphological inferences regarding theropod (i.e. ‘predatory’) dinosaurs were guided by an assumption that they were singularly hypercarnivorous. A recent plethora of maniraptoran discoveries has produced evidence challenging this notion. Here, we report on a new species of maniraptoran theropod, Nothronychus graffami sp. nov. Relative completeness of this specimen permits a phylogenetic reassessment of Therizinosauria—the theropod clade exhibiting the most substantial anatomical evidence of herbivory. In the most comprehensive phylogenetic study of the clade conducted to date, we recover Therizinosauria as the basalmost maniraptoran lineage. Using concentrated changes tests, we present evidence for correlated character evolution among herbivorous and hypercarnivorous taxa and propose ecomorphological indicators for future interpretations of diet among maniraptoran clades. Maximum parsimony optimizations of character evolution within our study indicate an ancestral origin for dietary plasticity and facultative herbivory (omnivory) within the clade. These findings suggest that hypercarnivory in paravian dinosaurs is a secondarily derived dietary specialization and provide a potential mechanism for the invasion of novel morpho- and ecospace early in coelurosaurian evolution—the loss of obligate carnivory and origin of dietary opportunism.
Keywords: ecomorphology, phylogeny, diet, Maniraptora, Theropoda, Nothronychus
1. Introduction
Recent work by several authors casts doubt on the notion that the majority of coelurosaurs were obligate carnivores, and raises the possibility that dietary diversification was more commonplace among ‘predatory’ dinosaurs than previously appreciated (Holtz et al. 1998; Kobayashi et al. 1999; Xu et al. 2002a; Zhou & Zhang 2002; Zhou et al. 2004; Barrett 2005; Barrett & Rayfield 2006; Wings & Sander 2007). A fundamental advance in our understanding of the palaeobiology of Coelurosauria was the recent substantiation of therizinosaurs as members of the clade (Russell & Dong 1993; Xu et al. 1999; Kirkland et al. 2005). Therizinosaurs are the most widely regarded candidate for herbivory among theropods (e.g. Paul 1984; Weishampel & Norman 1989; Russell & Russell 1993; Zhang et al. 2001; Kirkland et al. 2005). Members of the clade possess a suite of morphological features consistent with a hypothesis of predominant herbivory, including: (i) diminutive, tightly packed, coarsely serrated, lanceolate teeth; (ii) low tooth-replacement rate; (iii) rostral rhamphotheca; (iv) inset tooth row suggesting fleshy cheeks; (v) elongate neck and diminutive skull; (vi) proportionally enormous gut capacity (as estimated by gastralia/trunk rib circumference and lateral flare of preacetabular process of ilium); and (vii) secondary loss of cursorial hind limb adaptations, including development of a functionally tetradactl pes.
Whether by historical artefact or as a result of our previously limited understanding of the anatomy and relationships of the group, therizinosaurs have not played a major role in most modern systematic studies. Consequently, current hypotheses of coelurosaurian evolution are lacking data fundamental to achieving a broad perspective on the palaeobiological history of the clade. Fortunately, the past decade has witnessed a surge in therizinosaur discoveries, propelling our understanding of their evolution at an unequalled pace. Here, we report on the most complete derived therizinosaur yet discovered—a new species of Nothronychus—from the Late Cretaceous, southern Utah, USA. On the basis of this and other recent discoveries (e.g. the basalmost taxon Falcarius utahensis), we produce, to our knowledge, the first coelurosaurian phylogeny to include a comprehensive character analysis of Therizinosauria. This study provides the most rigorous phylogenetic framework generated to date for evaluating the evolutionary history of diet, specifically the advent of herbivory among maniraptorans, and fills a former gap in our understanding of the radiation and success of Coelurosauria—the most diverse theropod clade in the Cretaceous.
2. Description and comparative anatomy
(a). Systematic palaeontology
Theropoda (Marsh 1881).
Maniraptora (Gauthier 1986).
Therizinosauridae (Maleev 1954).
Nothronychus (Kirkland & Wolfe 2001).
N. graffami new taxon.
(b). Holotype
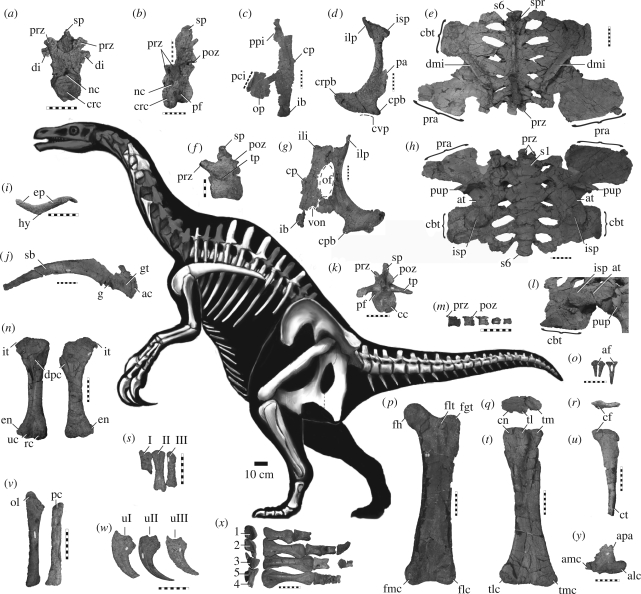
UMNH VP 16420, nearly complete postcranial skeleton (figure 1).
Figure 1.
Skeletal reconstruction (preserved shown in white) and representative elements of N. graffami (UMNH VP 16420): (a) cranial dorsal vertebra, cranial view; (b) mid-dorsal vertebra, craniolateral view; (c) left ischium, lateral view; (d) left pubis, lateral view; (e) dorsoventrally crushed iliosacrum, dorsal view; (f) proximal caudal vertebra, left lateral view; (g) right ischium/pubis, lateral view; (h) iliosacrum, ventral view; (i) furcula, cranial view; (j) right scapulacoracoid, lateral view; (k) proximal caudal vertebra, caudal view; (l) right iliosacrum, oblique ventrolateral view, showing crushed peduncles, acetabulum, brevis fossa and cubic tuberosity of postacetabular process; (m) four distalmost caudal vertebrae, lateral view; (n) left and right humeri, cranial view; (o) chevrons, cranial view; (p) left femur, cranial view; left tibia (q) proximal and (t) caudal views; left fibula (r) proximal and (u) lateral views; (s) left metacarpus, dorsal view; (v) antebrachium; (w) manual unguals, lateral view; (x) proximal (left) and dorsal (right) views of pes (missing PI–II, PIII–II, PIII–IV and PIV–V); and (y) left astragalus, cranial view. For abbreviations, see electronic supplementary material. Scale bars: 10 cm (a–e, g–y); 5 cm (f). Skeletal drawing modified from Victor O. Leshyk, copyright 2007.
(c). Etymology
In honour of M. Graffam who discovered the holotype.
(d). Locality and horizon
Tropic Shale, 65 m above the local top of the Dakota Formation; lower portion of the Mammites nodosoides Ammonoid Biozone (Albright et al. 2007) (early Turonian), Kaiparowits Basin, Kane County, UT, USA.
(e). Diagnosis
A therizinosaurid with the following autapomorphies: pubic boot with diminutive caudal process; ventral margin of pubic boot dorsally convex; subtriangular caudal process of ischium diminutive (less than 5% of the total length of ischium); and caudal process of ischium located proximal to obturator foramen. Nothronychus graffami can be differentiated from Nothronychus mckinleyi by the following five features: strongly amphicoelus cranial caudal centra; caudoventral aspect of caudal centra poorly developed; glenoid with pronounced caudal buttress; ulna straight; and ventral notch between obturator process and iliac shaft craniocaudally wide (electronic supplementary material, figure S1).
(f). Remarks
The discovery of N. graffami necessitated rediagnosis of the type species N. mckinleyi and formation of a genus-level diagnosis (see electronic supplementary material, A for revised diagnoses of these taxa and Therizinosauridae).
(g). Description and comparisons
Owing to its recovery from a marine shale unit, UMNH VP 16420 is moderately to heavily crushed. On several elements, the periosteal bone was removed (possibly bioeroded) before burial. Despite these conditions, UMNH VP 16420 is the most complete derived therizinosaur individual yet discovered and serves as a keystone for interpreting the anatomy of other derived therizinosaurs known from disparate aspects of the skeleton.
Representative portions of two badly damaged, elongate cervical vertebrae, nine amphicoelus dorsal vertebrae, a complete sacrum and 23 complete caudal vertebrae are preserved. As in other derived therizinosauroids (e.g. Suzhousaurus, Nanshiungosaurus brevispinus and Neimongosaurus), pneumatic invasion of lateral dorsal centra is extensive; pneumatopores incise the entire cervical and at least half the dorsal axial column. Cranialmost dorsals are characteristically therizinosaurian, exhibiting hypaxially inflated neural arches, bearing dorsally elevated pre- and postzygapophyseal facets, accessory centrodiapophyseal laminae, pendant transverse processes and reduced neural spines, as well as centra with hypertrophied, deeply concave parapophyses, enlarged lateral pleurocoels and moderately developed hypapophyses. Mid-dorsal vertebrae possess spool-shaped centra and tall neural spines, as in Neimongosaurus. A complete sacrum bearing six coossified vertebrae as in other derived therizinosaurs and many other coelurosaurs is preserved. In contrast to Segnosaurus and Suzhousaurus (Li et al. 2008) the sacral neural spines are indistinguishable, forming a low coossified ridge; due to crushing, sacral pleurocoels cannot be confirmed. The caudal axial series is shortened and numerically reduced as in other derived therizinosaurs and oviraptorosaurs, and cranially pneumatic as in Neimongosaurus, Ingenia and Chirostenotes.
The left scapula and coracoid are unfused, whereas the right are coossified. As in other derived therizinosaurs, paravians and some oviraptorosaurs, the glenoid is laterally extensive. As in N. mckinleyi and Ingenia, the distal scapular blade tapers (in contrast to previous reports, distal tapering cannot be confirmed in Erliansaurus or Therizinosaurus). The dorsal flange on the scapular blade of Suzhousaurus and Therizinosaurus is absent.
Humeri possess a depression between the well-developed internal tuberosity and humeral head, cranially oriented distal condyles and a hypertrophied, crest-like entepicondyle, as in other therizinosaurids. The deltopectoral crest occupies approximately 42–45% of the total humeral length, less than Therizinosaurus, yet greater than Neimongosaurus and Suzhousaurus. As in other derived therizinosaurs (except N. mckinleyi), the ulna is straight. Elements of the manus are difficult to identify due to severe crushing and erosion; however, metacarpals I–III are tentatively identified and appear distally ginglymoid as in other therizinosaurids. Penultimate manual phalanges, if correctly identified, are shortened as in Erliansaurus. Manual unguals are transversely compressed, markedly recurved and enlarged (approximately twice the length of the penultimate phalanx).
The highly derived pelvis of N. graffami is the most complete known for a therizinosaurid. Although the iliosacrum is severely crushed dorsoventrally, numerous features of phylogenetic significance can be ascertained. Proportionally, the preacetabular portion is longer than the postacetabular portion, as in Falcarius, Suzhousaurus, Segnosaurus and some oviraptorosaurs (e.g. Chirostenotes and Nomingia). The preacetabular portion of the ilium is ventrally extensive and laterally deflected, whereas the postacetabular portion is reduced in length and forms a hypertrophied ‘cubic’ tuberosity as in other derived species. The pubic peduncle is dorsoventrally elongate, craniocaudally compressed and caudally recurved, whereas the ischiadic peduncle forms an enlarged spherical boss, confluent with the antitrochanter. The ischium is nearly subequal to the pubis in length and the proximal contact between these elements is sinuous as in other derived therizinosaurs. As in Suzhousaurus, Enigmosaurus and Segnosaurus, the pubic shaft is cranially convex. In contrast to other coelurosaurs, the pubic apron extends from the caudal aspect of the shaft in N. graffami as in other therizinosaurids. The pubic boot is enlarged and cranially focused; however, the caudal aspect of the pubic boot in N. graffami possesses a small process of distinct morphology. The ischium of N. graffami is transversely flattened, with a distinct ventral caudal process and distal ‘boot’. In contrast to the dorsoventrally extensive ventral process of N. mckinleyi, Segnosaurus and Enigmosaurus, this feature is diminutive and located entirely dorsal to the obturator process on N. graffami. A longitudinal ridge divides the lateral aspect of the ischium as in Segnosaurus, Engimosaurus and Beipiaosaurus; a similar ridge is convergently present on dromaeosaurids (e.g. Buitreraptor, Sinornithosaurus and Achillobator). As in other advanced therizinosaurs, the obturator process is hypertrophied, yet unlike other taxa, it is incompletely fused to the pubis (as in N. mckinleyi). A notch incises the contact between the ventral obturator process and the ischiadic shaft in both species of Nothronychus.
As in Segnosaurus, Suzhousaurus, Neimongosaurus and Erliansaurus, the femora possess a dorsally inclined, poorly differentiated head, separated from the greater trochanter by a marked depression. In dorsal view, the greater trochanter has greater craniocaudal depth than the femoral head and the bridging region is constricted. The lesser trochanter is cylindrical as in other therizinosaurs, oviraptorosaurs and paravians. The tibia is only 90 per cent of the length of the femur and exhibits a robust, laterally deflected cnemial crest, with a poorly developed lateral fossa. An elongate, distally positioned fibular crest extends from the tibial shaft as in Neimongosaurus, Erliansaurus and Segnosaurus. Proximally, the cranial aspect of the fibula is dorsally elevated; medially the fibula is flat as in other therizinosaurids. The cranial tubercle is distally displaced as in other therizinosaurs (e.g. Neimongosaurus and Erliansaurus). As is synapomorphic for therizinosaurids, the astragalar body is reduced, exposing the craniolateral aspect of the distal tibia. Although the ascending process is only partially preserved, its morphology suggests that it was laterally deflected as in Segnosaurus and Therizinosaurus (IGM 100/45). A nearly complete right pes and partial left pes are known. The pes is functionally tetradactyl as in other derived therizinosaurs, with a robust first metatarsal and subequal third and fourth metatarsals. The pedal unguals are transversely thick and moderately recurved, unlike Erlikosaurus.
3. Phylogenetic analysis
Here, we present a new phylogenetic study of therizinosaurs and one of the most data-rich coelurosaurian analyses conducted to date (see electronic supplementary material, D1–D4). Results contest the widely regarded hypothesis of a monophyletic relationship between therizinosaurs and oviraptorosaurs (e.g. Xu et al. 1999, 2002a; Rauhut 2003; Barrett 2005; Kirkland et al. 2005; Barrett & Rayfield 2006; Turner et al. 2007a). This study suggests that a large proportion of the noted morphological similarities shared by members of these clades represent convergent evolution (probably from similar ecological and/or dietary constraints) and that therizinosaurs are a more basal lineage than was previously appreciated.
Here, Therizinosauria is recovered as the sister taxon to the five other major maniraptoran clades (i.e. as the basalmost maniraptoran clade) (figure 2; electronic supplementary material, figures S2 and S3)—a position supported by a suite of primitive features retained in Falcarius including: acromion of scapula with dorsocranially extensive blade; supracetabular crest on ilium; well-developed obturator notch on pubis with ventral process; propubic pelvis; pubic apron extends medially from medial aspect of cylindrical pubic shaft; peg-and-socket articulation between ischium and ilium; craniocaudal depth of greater trochanter less than depth of femoral head; lesser trochanter craniocaudally elongate and alariform; lesser trochanter separated from greater trochanter by a deep cleft; fourth trochanter prominent; ascending process of astragalus tall with medial notch; proximal end of MT IV overlaps plantar side of MT III; and metatarsals not appressed.
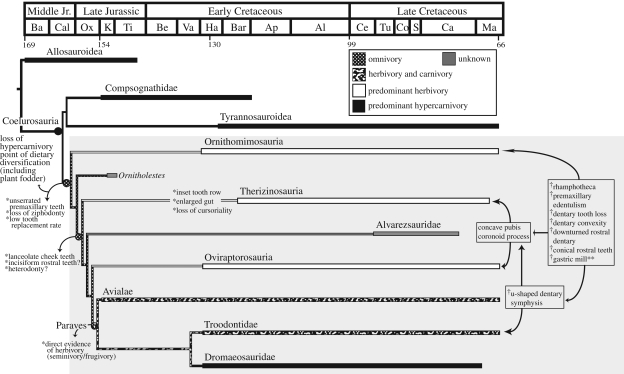
Figure 2.
Time-calibrated phylogeny showing the temporal range of the 76 coelurosaurian dinosaurs included in this study only, with diet interpreted for nine major coelurosaurian subclades based on ecomorphological traits supported by CCTs and MP optimizations. Clades interpreted as predominant herbivory or hypercarnivory possess species-level ecomorphology correlated with direct evidence of diet using CCTs. Interpretation of at least facultative herbivory in basal ornithomosaurs, therizinosaurs and oviraptorosaurs based on the presence of at least two CCT-supported traits per basal species (CCT characters 263 and 349 in Pelecanimimus, 263 and 355 in Falcarius, 349 and 355 in Incisivosaurus). Ecomorphological characters potentially related to herbivory listed at select nodes. Traits supported by CCTs are listed at the right of the tree and denoted by the ‘dagger’ symbol (distribution illustrated in flow chart). Optimization of ecomorphological dental characteristics based on MP is listed at the left of the tree and denoted by an asterisk. MP optimization of direct fossil evidence of herbivory listed at node Paraves. The origin of facultative herbivory (i.e. omnivory) and the point of dietary diversification for the clade is posited at the base of Maniraptorformes (grey box). Coelurus fragilis is excluded from the tree owing to widely conflicting interpretations regarding its taxonomic and phylogenetic relationships (e.g. Senter 2007; Turner et al. 2007a,b; this study).
Among the features previously purported to support the monophyly of therizinosaurs and oviraptorosaurs (Sues 1997; Makovicky & Sues 1998; Xu et al. 2002a; Rauhut 2003), several are contested as synapomorphic by the anatomy of basal members of these subclades, including those associated with the development of a rostral rhamphotheca (e.g. rostral edentulism, downturned dentary) and reduction of the tail (e.g. shortened, homogeneous caudal vertebrae), whereas others are known to have a wider distribution (e.g. pronounced lip on manual unguals). The present study brings to light numerous additional convergences, which are also refuted as possible synapomorphies by the anatomy of basal oviraptorosaurs and/or therizinosaurs (see electronic supplementary material, D2).
The therizinosaur ingroup relationships recovered in this analysis differ from previous hypotheses—most notably in the positions of Neimongosaurus, Erliansaurus, Nothronychus, Suzhousaurus and N. brevispinus, as well as the taxonomic composition of Therizinosauridae (electronic supplementary material, figure S2 and B). This analysis serves as the basis for a redefinition of the clade Therizinosauridae and is, to our knowledge, the first study to provide phylogenetic support for: (i) the intermediate status of Neimongosaurus, Erliansaurus and Enigmosaurus (Zhang et al. 2001; Xu et al. 2002b); and (ii) derived placement of Suzhousaurus and Nothronychus (Kirkland & Wolfe 2001), contra several recent studies (Xu et al. 2002b; Li et al. 2007, 2008).
4. Palaeobiological implications
Although ecomorphological interpretations of coelurosaurian diet have been wide ranging, direct evidence of diet (e.g. gut contents, coprolites) among members of the clade are rare. Direct evidence of herbivory is preserved in the small-bodied, Early Cretaceous birds Jeholornis (Zhou & Zhang 2002) and Yanornis (Zhou & Zhang 2006), and may also be preserved in the diminutive basal-troodontid Jinfengopteryx (Ji et al. 2005; sensu Turner et al. 2007b), indicating that some small-bodied maniraptorans were at least facultative herbivores. However, speculations regarding diet in other coelurosaurs are, by necessity, based on ecomorphological evidence alone. In addition to therizinosaurs, several maniraptoran clades are hypothesized to have been predominantly or facultatively herbivorous (i.e. omnivorous) (see electronic supplementary material for dietary terminology). Specifically, the discovery of diminutive conical teeth, keratinous rhamphotheca (Norell et al. 2001) and gastric mill (Kobayashi et al. 1999) has been proposed as evidence for herbivory in ornithomimosaurs; the ‘glirodont’ dentition of Incisivosaurus (Xu et al. 2002a) and Protarchaeopteryx (Ji & Ji 1997) and the gastric mill of Caudipteryx (Ji et al. 1998) are espoused as evidence for herbivory in oviraptorosaurs; and the widely spaced, coarse dental serrations of some derived troodontids are more congruent with the dentition of extant herbivorous squamates such as iguanids than of obligate carnivores (Holtz et al. 1998). Widespread speculation of herbivory among coelurosaurian dinosaurs notwithstanding, the utility of morphological features alleged to correlate with diet in coelurosaurian theropods has not been tested analytically, nor have reconstructions of dietary evolution been subjected to empirical scrutiny within a character-comprehensive, species-level phylogenetic framework.
Among coelurosaurian dinosaurs, two patterns of character evolution are relevant to our goal of assessing dietary evolution in the clade: (i) the prevalence of convergent ecomorphology among known members of several subclades (e.g. Therizinosauria, Ornithomimosauria and Oviraptorosauria); and (ii) ancestral character optimizations at the base of Maniraptora. To investigate the implication of these patterns, it is necessary to employ dual analytical methodologies—concentrated changes tests (CCTs) (Maddison 1990)—to identify repeated evolution of characters probably carrying an ecomorphological signature and maximum parsimony (MP) analysis (Kluge & Farris 1969; Farris 1970; Fitch 1971) to infer basal character optimizations. As CCTs are only useful in identifying suites of morphological features that evolve under scenarios of repeated evolution (and thus evolve several times within the group in question), they are uninformative in evaluating traits with a single basal origination point—i.e. traits inferred to be ancestral for Maniraptora—within the scope of our study.
(a). Concentrated changes tests
To identify patterns of correlated character evolution within coelurosaurian clades, we performed CCTs on an amended version of our 76-taxon phylogenetic character matrix (see electronic supplementary material, E2–6). We tested the correlation between the evolution (appearance) of select morphological features when herbivory was interpreted for the clades Ornithomimosauria, Therizinosauria, Oviraptorosauria (except Oviraptor philoceratops) and the avialans Jeholornis and Yanornis. We also tested for the non-random evolution of features when hypercarnivory was interpreted for the clades Tyrannosauridae, Compsognathidae, Dromaeosauridae, basal troodontids and O. philoceratops. Our goal was to examine whether traits present in Yanornis and Jeholornis (fossil taxa possessing direct fossil evidence of herbivory (Zhou & Zhang 2002, 2006)) and Sinosauropteryx, Compsognathus, Tyrannosaurus and O. philoceratops (fossil taxa possessing direct fossil evidence of carnivory (Ostrom 1978; Chen et al. 1998; Chin et al. 1998)) are present in taxa inferred to possess, yet lacking direct evidence of, the same diet (e.g. Paul 1984; Weishampel & Norman 1989; Russell & Russell 1993; Holtz et al. 1998; Kobayashi et al. 1999; Xu et al. 2002a; Barrett 2005; Kirkland et al. 2005; Barrett & Rayfield 2006). In our study, the following morphologies evolve with non-random distribution (i.e. are significantly correlated statistically (p < 0.1; see electronic supplementary material, E4)) in taxa inferred to be at least facultatively herbivorous: edentulous premaxilla (premaxillary rhamphotheca); caudally, rostrally or fully edentulous dentary (dentary rhamphotheca); U-shaped dentary; conical rostral teeth; downturned rostral dentary; dorsally convex dentary; and gastric mill (distribution shown in figure 2). Likewise, the evolution of ziphodonty, small denticle size and/or replacement gaps is significantly correlated in taxa inferred to have a hypercarnivorous diet. These findings demonstrate that in the absence of direct fossil evidence, morphological features can be regarded as reliable ecomorphological indicators of diet in coelurosaurs and statistically support prior interpretations of herbivory and hypercarnivory among taxa within our study. The convergent evolution of a suite of morphological features correlated with direct evidence of herbivory within several coelurosaurian subclades by CCTs in our analysis (figure 2) may indicate parallel trends of increased morphological adaptation to herbivory among closely related coelurosaurian lineages. Finally, CCTs also support our interpretation that previous hypotheses of therizinosaur/oviraptorosaur monophyly were based, in part, on convergent ecomorphology, as several features previously corroborating this relationship are herein found to be correlative with herbivory.
(b). Parsimony optimizations
To infer diet at the base of Maniraptora, we mapped character transformations within our phylogeny. MP-based optimization of characters hypothesizes that the following traits are ancestral for Maniraptora: (i) unserrated premaxillary teeth; (ii) loss of ziphodont dentition; (iii) low tooth-replacement rate; (iv) lanceolate cheek teeth; and (v) constriction between crown and root (figure 2). As the scope of our study precludes the statistical correlation of these basally optimized maniraptoran traits with direct evidence of diet using CCTs, we take a conservative approach by not assigning a particular diet to this node. Nonetheless, the basalmost members of the clades Ornithomimosauria, Therizinosauria and Oviraptorosauria each possess at least two characters found to be correlative with herbivory in our study using CCTs. Thus, our data support two significant conclusions: (i) the evolution of dental features basally optimized by MP are inconsistent with hypercarnivory and suggest an ancestral origin for dietary diversification within Maniraptora—specifically, the incorporation of plant fodder into the diet; and (ii) these data combined with the presence of features correlative with direct evidence of herbivory in basal ornithomimosaurs, therizinosaurs and oviraptorosaurs are indicative of, at minimum, an omnivorous common ancestor.
Although missing data among Alvarezsauridae precludes basal optimization of some features shared in basal therizinosaurs and oviraptorosaurs, our model predicts that the presence of heterodontous dentition and elongate, incisiform rostral teeth may also represent ancestral maniraptoran traits and may ultimately be found to characterize basal members of other maniraptoran subclades.
(c). Conclusions
The phylogenetic relationships recovered here are notable in that: (i) they group several coelurosaurian subclades identified by CCTs as possessing ecomorphological characteristics of herbivory (e.g. Ornithomimosauria, Therizinosauria and Oviraptorosauria) in succession at the base of Maniraptora; (ii) basalmost members of these clades each possess at least two features statistically correlated with direct evidence of herbivory; and (iii) MP optimization of character transformations reconstructs a basal origination for several features incongruent with hypercarnivory, including a loss of ziphodont dentition. These conclusions support one of the two evolutionary scenarios: either herbivorous habits evolved independently at least two and upwards of five times in coelurosaurian dinosaurs, or the primitive condition for Maniraptora represents a deviation from obligate carnivory (i.e. at least facultative herbivory) and hypercarnivory within Paraves is a secondarily derived dietary specialization.
Ecomorphological studies have demonstrated a correlation between low morphological disparity and hypercarnivory, both in terms of occupied morphospace and rate of character change (Holliday & Steppan 2004); however, this relationship has not been tested in dinosaurs. An insufficient pre-Cretaceous fossil record and the presence of extensive ghost lineages for maniraptoran subclades render much of their early diversity unknown and act as a serious impediment to reconstructing macroevolutionary patterns within the group. Nonetheless, if correctly hypothesized to have occurred penecontemporaneously with the origin of Maniraptora, the advent of dietary plasticity offers a testable mechanism for: (i) the invasion of new eco- and morphospace; and (ii) high rate of cladogenesis exhibited by the group. In short, ecomorphological diversification presents as a valuable consideration for future macroevolutionary and palaeobiological studies of Coelurosauria.
Acknowledgements
We thank the Museum of Northern Arizona, Arizona Museum of Natural History, Utah Museum of Natural History, Northern Arizona University, Utah Geological Survey, Bureau of Land Management (GSENM), Glen Canyon National Recreation Area (NPS), Utah School and Institutional Trust Lands Administration; S. Sampson, J. Kirkland, A. Ekdale, F, Brown and P. Makovicky for review and discussion; K. Tsogtbaatar, C. Tsogtbaatar, R. Barsbold, Y. Hailu, X. Xu, P. Sereno, M. Norell, P. Currie, J. Gardner, K. Seymour, D. Brinkman, W. Joyce, J. Horner, R. Barrick, J. Bartlett and J. Bird for access to specimens; A. Turner for matrix files; and M. Graffam, R. Breunig, R. Banfiel, M. Eaton, R. Gaston, E. Lund, M. Getty, J. Gillette, D. Hunsaker, J. Kirkland, R. McCord, L. Newcomb, D. Rankin, V. Wallis, D. Wolfe, 2001 and 2002 field crews and laboratory volunteers. Financial support provided by the Jurassic Foundation, Palaeontological Association, Paleontological Society, MNA Colbert Endowment, MNA Science Education Endowment, University of Utah GR Fellowship (L.E.Z.) and NSF GK-12 Fellowship (L.E.Z.). Free online versions of TNT and Mesquite were generously provided by the Willi Hennig Society and the Free Software Foundation Inc.
References
- Albright L. B., III, Gillette D. D., Titus A. L.2007Plesiosaurs from the Upper Cretaceous (Cenomanian–Turonian) Tropic Shale of southern Utah, part 2: Polycotylidae. J. Vertebr. Paleontol. 27, 41–58 (doi:10.1671/0272-4634(2007)27[41:PFTUCC]2.0.CO;2) [Google Scholar]
- Barrett P. M.2005The diet of ostrich dinosaurs (Theropoda: Ornithomimosauria). Palaeontology 48, 347–358 (doi:10.1111/j.1475-4983.2005.00448.x) [Google Scholar]
- Barrett P. M., Rayfield E. J.2006Ecological and evolutionary implications of dinosaur feeding behavior. Trends Ecol. Evol. 21, 217–224 (doi:10.1016/j.tree.2006.01.002) [DOI] [PubMed] [Google Scholar]
- Chen P., Dong Z., Zhen S.1998An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature 391, 147–152 (doi:10.1038/34356) [Google Scholar]
- Chin K., Tokaryk T. T., Erickson G. M., Calk L. C.1998A king-sized theropod coprolite. Nature 393, 680–682 (doi:10.1038/31461) [Google Scholar]
- Farris J. S.1970Methods of computing Wagner trees. Syst. Zool. 19, 82–83 (doi:10.2307/2412028) [Google Scholar]
- Fitch W. M.1971Toward defining the course of evolution: minimum change for a specific tree topology. Syst. Zool. 20, 406–426 (doi:10.2307/2412116) [Google Scholar]
- Gauthier J. A.1986Saurischian monophyly and the origin of birds. In The origin of birds and the evolution of flight. Memoirs of the California Academy of Sciences (ed. Padian K.), pp. 1–55 San Francisco, CA: California Academy of Sciences [Google Scholar]
- Holliday J. A., Steppan S. J.2004Evolution of hypercarnivory: the effect of specialization on morphological and taxonomic diversity. Paleobiology 30, 108–128 (doi:10.1666/0094-8373(2004)030<0108:EOHTEO>2.0.CO;2) [Google Scholar]
- Holtz T. R., Jr, Brinkman D. L., Chandler C. L.1998Denticle morphometrics and a possibly omnivorous feeding habit for the theropod dinosaur Troodon. Gaia 15, 159–166 [Google Scholar]
- Ji Q., Ji A.1997Protarchaeopterygid bird (Protarchaeopteryx gen. nov.): fossil remains of archaeopterygids from China. Chin. Geol. 238, 38–41 [Google Scholar]
- Ji Q., Currie P. J., Norell M. A., Ji A.1998Two feathered dinosaurs from northeastern China. Nature 393, 753–761 (doi:10.1038/31635) [Google Scholar]
- Ji Q., Ji S., Lu J., You H., Chen W., Liu Y., Liu Y.2005First avialan bird from China (Jinfengopteryx elegans gen. et sp. nov.). Geol. Bull. China 24, 197–205 [Google Scholar]
- Kirkland J. I., Wolfe D. G.2001First definitive Therizinosaurid (Dinosauria; Theropoda) from North America. J. Vertebr. Paleontol. 21, 410–414 (doi:10.1671/0272-4634(2001)021[0410:FDTDTF]2.0.CO;2) [Google Scholar]
- Kirkland J. I., Zanno L. E., Sampson S. D., Clark J. M., DeBlieux D. D.2005A primitive therizinosauroid dinosaur from the Early Cretaceous of Utah. Nature 435, 84–87 (doi:10.1038/nature03468) [DOI] [PubMed] [Google Scholar]
- Kluge A. G., Farris J. S.1969Quantitative phyletics and the evolution of anurans. Syst. Zool. 18, 1–32 (doi:10.2307/2412407) [Google Scholar]
- Kobayashi Y., Lu J.-C., Dong Z.-M., Barsbold R., Azuma Y., Tomida Y.1999Herbivorous diet in an ornithomimid dinosaur. Nature 402, 480–481 (doi:10.1038/44999)10591205 [Google Scholar]
- Li D., Peng C., You H., Lamanna M. C., Harris J. D., Lacovara K. J., Zhang J.2007A large therizinosauroid (Dinosauria: Theropoda) from the Early Cretaceous of northwestern China. Acta Geol. Sin. 81, 539–549 [Google Scholar]
- Li D.-Q., You H.-L., Zhang P.2008A new specimen of Suzhousaurus megatherioides (Dinosauria: Therizinosauroidea) from the Early Cretaceous of northwestern China. Can. J. Earth Sci. 45, 769–779 (doi:10.1139/E08-021) [Google Scholar]
- Maddison W. P.1990A method for testing the correlated evolution of two binary characters: are gains or losses concentrated on certain branches of a phylogenetic tree? Evolution 44, 539–557 (doi:10.2307/2409434) [DOI] [PubMed] [Google Scholar]
- Makovicky P. J., Sues H.-D.1998Anatomy and phylogenetic relationships of the theropod dinosaur Microvenator celer from the lower Cretaceous of Montana. Am. Mus. Novit. 3240, 1–27 [Google Scholar]
- Maleev E. A.1954New turtle-like reptile in Mongolia (Russian). Priroda 3, 106–108 [Google Scholar]
- Marsh O. C.1881Principal characters of American Jurassic dinosaurs, Part V. Am. J. Sci. 21, 417–423 [Google Scholar]
- Norell M. A., Makovicky P. J., Currie P. J.2001The beaks of ostrich dinosaurs. Nature 12, 873–874 (doi:10.1038/35091139) [DOI] [PubMed] [Google Scholar]
- Ostrom J. H.1978The osteology of Compsognathus longipes Wagner. Zitteliana 4, 73–118 [Google Scholar]
- Paul G. S.1984The segnosaurian dinosaurs: relics of the Prosauropod—Ornithischian transition? J. Vertebr. Paleontol. 4, 507–515 [Google Scholar]
- Rauhut O. W. M.2003. In The interrelationships and evolution of basal theropod dinosaurs London, UK: Palaeontological Association [Google Scholar]
- Russell D. A., Dong M.1993The affinities of a new theropod from the Alxa Desert, Inner Mongolia, People's Republic of China. Can. J. Earth Sci. 30, 2107–2127 [Google Scholar]
- Russell D. A., Russell D. E.1993Mammal–dinosaur convergence: evolutionary convergence between a mammalian and dinosaurian clawed herbivore. Nat. Geogr. Res. 9, 70–79 [Google Scholar]
- Senter P.2007A new look at Coelurosauria (Dinosauria: Theropoda). J. Syst. Paleontol. 5, 429–463 [Google Scholar]
- Sues D.1997On Chirostenotes, an oviraptorosaur (Dinosauria: Theropoda) from the Upper Cretaceous of western North America. J. Vertbr. Paleontol. 17, 698–716 [Google Scholar]
- Turner A. H., Hwang S. H., Norell M. A.2007aA small derived theropod from Öösh, Early Cretaceous, Baykhangor Mongolia. Am. Mus. Novit. 3557, 1–27 (doi:10.1206/0003-0082(2007)3557[1:ASDTFS]2.0.CO;2) [Google Scholar]
- Turner A. H., Pol D., Clarke J. A., Erickson G. M., Norell M. A.2007bA basal dromaeosaurid and size evolution preceding avian flight. Science 317, 1378–1381 (doi:10.1126/science.1144066) [DOI] [PubMed] [Google Scholar]
- Weishampel D. B., Norman D. B.1989Vertebrate herbivory in the Mesozoic; jaws, plants, and evolutionary metrics. In Paleobiology of the dinosaurs (ed. Farlow J. O.), pp. 87–100 Boulder, CO: Geological Society of America [Google Scholar]
- Wings O., Sander P. M.2007No gastric mill in sauropod dinosaurs: new evidence from analysis of gastrolith mass and function in ostriches. Proc. R. Soc. B 274, 635–640 (doi:10.1098/rspb.2006.3763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Tang Z.-L., Wang L.1999A therizinosauroid dinosaur with integumentary structures from China. Nature 399, 350–354 (doi:10.1038/20670) [Google Scholar]
- Xu X., Cheng Y.-N., Wang X.-L., Chang H.2002aAn unusual oviraptorosaurian dinosaur from China. Nature 419, 291–293 (doi:10.1038/nature00966) [DOI] [PubMed] [Google Scholar]
- Xu X., Zhang X.-H., Sereno P. C., Zhao X.-L., Kuang X.-W., Han J., Tan L.2002bA new therizinosauroid (Dinosauria, Theropoda) from the Upper Cretaceous Iren Dabasu Formation of Nei Mongol. Vertebr. PalAsiat. 40, 228–240 [Google Scholar]
- Zhang X.-H., Xu X., Zhao X.-J., Sereno P. C., Kuang X.-W., Tan L.2001A long-necked Therizinosauroid dinosaur from the Upper Cretaceous Iren Dabasu Formation of Nei Mongol, People's Republic of China. Vertebr. PalAsiat. 10, 282–290 [Google Scholar]
- Zhou Z., Zhang F.2002A long-tailed, seed-eating bird from the Early Cretaceous of China. Nature 418, 405–409 (doi:10.1038/nature00930) [DOI] [PubMed] [Google Scholar]
- Zhou Z.-H., Zhang F. C.2006Mesozoic birds of China: a synoptic review. Vertebr. PalAsiat. 44, 74–98 [Google Scholar]
- Zhou Z., Clarke J., Zhang F.2004Gastroliths in Yanornis: an indication of the earliest radical diet-switching and gizzard plasticity in the lineage leading to living birds? Naturwissenschaften 91, 571–574 (doi:10.1007/s00114-004-0567-z) [DOI] [PubMed] [Google Scholar]