Abstract
Many songbirds learn their songs early in life from a song model. In the absence of such a model, they develop an improvised song that often lacks the species-typical song structure. Open-ended learners, such as the domesticated canary, are able to modify their songs in adulthood, although the mechanisms that guide and time the song-learning process are still not fully understood. In a previous study, we showed that male domesticated canaries lacking an adult song model in their first year substantially change their song repertoire and composition when exposed to normally reared conspecifics in their second year. Here, we investigate song development in descendants of canaries that were raised and kept as a peer group without a song model. Such males represent tutors with abnormal song characteristics. Interestingly, the F1 generation developed quite normal song structure, and when brought into an environment with normally raised canaries in their second year, they did not modify their songs substantially. These results suggest that contact with an adult song model early in life is crucial for song crystallization, but also that song development is at least partly guided by innate rules. They also question the existing classification of canaries as open-ended learners.
Keywords: vocal communication, song learning, song model, domesticated canaries, acoustic isolation
1. Introduction
Experience of environmental and social cues during a discrete period after hatching strongly influences the development of song in many birds (Catchpole & Slater 2008). Some species typically acquire new songs only during a restricted period early in life (i.e. zebra finch (Taeniopygia guttata; Eales 1985) or chaffinch (Fringilla coelebs; Slater & Ince 1982). In contrast, open-ended learners, such as the domesticated canary (Serinus canaria), are thought to maintain the ability to modify the content of their song throughout their lives (Nottebohm & Nottebohm 1978; Nottebohm et al. 1986).
The social environment for song learning is extremely important. For example, some species learn better from live tutors than from tapes (Baptista & Petrinovitch 1984; Chaiken et al. 1993). Poirier et al. (2004) found that, in starlings (Sturnus vulgaris), direct social contact is necessary for song learning and overrides auditory information acquired earlier in life. Field studies also suggest that several songbirds learn their songs from neighbours in a new area after dispersal from their natal territory (e.g. marsh wrens Cistothorus palustris; white-crowned sparrows Zonotrichia leucophrys; reviewed in Payne & Payne 1997). Other species frequently copy their father's song (e.g. medium ground finch Geospiza fortis, Millington & Price 1985; zebra finch, Zann 1996). Young song sparrows (Melospiza melodia) build their repertoire by preferentially selecting song types that are shared by three or four adult birds from neighbouring territories (Beecher et al. 1994). Thus, under natural conditions, a juvenile bird is able to choose one or more tutors to follow an adaptive learning strategy.
The domesticated canary is one of the best-studied species regarding song development and its underlying neuroendocrine substrates (Nottebohm & Nottebohm 1978; Nottebohm et al. 1986; Ball et al. 2003). Song is normally only produced by males (Nottebohm & Arnold 1976, but see Pesch & Güttinger 1985). First investigations were conducted more than 70 years ago with roller canaries (Metfessel 1935). If reared in acoustic isolation, male birds still develop the characteristic rolls and tours. Similar results were reported by Poulsen (1959) from a different canary strain. However, these data are limited because they were based solely on hearing, as sonographic analysis was only implemented in the 1960s. Since then, substantial insights into the song-learning process have been achieved from experiments with cross-fostered, and normally reared male canaries (Waser & Marler 1977; Nottebohm & Nottebohm 1978; Güttinger 1979; Nottebohm et al. 1986; Mundinger 1995; Leitner & Catchpole 2004). More recently, male canary song development has been investigated in restricted acoustic environments (Lehongre et al. 2006; Leitner & Catchpole 2007). However, the exact mechanisms that guide and constrain vocal development remain elusive. Gardner et al. (2005) suggest that canaries have an innate disposition to develop the species-typical phrased song when reaching sexual maturity and that they are able to rearrange previously acquired songs from a model that lacked these features. The authors therefore propose different learning strategies in juvenile and adolescent canaries. Yet other mechanisms must be present when birds modify their songs later in life. Leitner & Catchpole (2007) conducted an experiment with male canaries that experienced different social and auditory conditions before and after sexual maturation. Males in one group were raised individually in acoustic isolation, males in another group were raised together as peers but without an adult song model, and control males were raised in a communal aviary with adult conspecifics. At the age of 1 year, all groups were transferred into a large mixed-sex colony of conspecifics. The results showed that, at sexual maturity, males from both experimental groups had developed abnormal song characteristics, with isolate-raised males having very large and peer group males having very small repertoires compared with controls. Further, both groups of males sang with a lower syllable repetition rate than control males. In the second year, after introduction into the large colony, isolates and peers adjusted their syllable repertoire size and composition to that of control males, but syllable repetition rate remained unchanged. These data reveal the importance of social and auditory stimuli for song development and the crystallization process.
Based on these results, we aimed to investigate, in more detail, how the timing of the song crystallization process is affected by the presence of an adult song model. We studied song development in the F1 generation of canaries that were raised as a peer group in isolation from an adult song model. As described above, under these conditions males develop abnormal song and hence represent an abnormal song model to their descendants. Here we analyse the songs of the latter, when 1 year old, and compare them with their tutors’ songs. Further, we monitor in their second year of life in comparison with control males, any changes after being introduced into a new acoustic environment with normally reared canaries.
2. Material and methods
(a). Tutors
We established a peer group of common domesticated canaries (n = 6 males, n = 3 females) that consisted of birds raised in isolation from adult male song. These were the offspring of pairs bred in cages (56 × 28 × 37 cm) inside sound-proof chambers (65 × 43 × 56 cm inside, 103 × 57 × 101 cm outside) on a 14 : 10 L/D photoperiod. Each pair was provided with plastic nest bowls and nesting material that allowed females to build a nest and lay eggs. One to two days after hatching, the male was removed from the cage, and females raised the young on their own. Female canaries do not sing, and thus can be eliminated as possible song models. Furthermore, previous experiments have shown that song learning of young males is not biased by the presence of the rearing female or female peers (Güttinger et al. 1990; Mundinger 1995). From the age of six weeks on, the juveniles were kept together in an indoor aviary without acoustic contact with other birds. This aviary had normal acoustic properties, and was lacking only the vocalizations of other species and adult canaries. We adjusted the L/D cycle to the seasonal photoperiod that was gradually altered from 14 : 10 L/D in spring when birds were juvenile to 9 : 15 L/D in winter and back to 14 : 10 L/D in the following spring when birds were adult, thus simulating a natural seasonal change in photoperiod. Song recordings of males were made using a tape recorder (Sony Walkman WM-D6C) and a directional microphone (Sennheiser ME-67). In the following text, we refer to the males (n = 6) of this peer group as ‘tutors’.
(b). F1 generation
These birds represent the offspring of the tutors. When the tutor birds reached sexual maturity, they were allowed to breed by equipping the aviary with nest bowls and nesting material. The photoperiod was gradually altered from 14 : 10 L/D in spring when eggs hatched, to 9 : 15 L/D in winter and back to 14 : 10 L/D in the following spring. Tutors were removed from the aviary when the F1 generations (n = 6 males, n = 6 females) were 60–70 days old. At that time, juvenile males were in the transition from the subsong to the plastic song (Nottebohm 1999). In the following year, when these males reached the age of 17–18 months, their songs were recorded. Afterwards, in late autumn, they were introduced into a large communal aviary with normally reared canaries that were kept on 9 : 15 L/D in winter with a gradual alteration to 14 : 10 L/D towards the next spring. The songs of the males were recorded at 9 and 20 weeks after being transferred into the new environment. At the sampling point of 20 weeks, birds were in breeding condition and had reached the age of 23–24 months.
(c). Controls
We selected adult males (n = 4) from our colony of common outbred domesticated canaries (n = 60) kept at Seewiesen/Germany (47°58′ N, 11°14′ E) in a large aviary on the same natural photoperiod. The songs of the males were first recorded in late autumn and then after 9 and 20 weeks. At the sampling point of 20 weeks, the birds were in breeding condition.
(d). Song analysis
Sonographic analysis used Canary 1.2.4 software (Cornell Laboratory of Ornithology, Ithaca, NY, USA) on a Power Macintosh computer. Sampling rate was at a frequency of 22 kHz with a 16-bit sample size. The frequency/time resolution was set at 342 Hz with a frame length of 256 fast Fourier transform. Song analysis was performed on spectrograms by visual inspection and by using the built-in functions of the software. We focused on measuring song length, syllable repertoire size, syllable composition and syllable repetition rate. In addition, a catalogue of the different syllables of each bird was prepared in order to identify syllable turnover during the different recording weeks. To obtain the syllable repertoire size of each bird and the number of identical syllables between birds (syllable sharing), respectively, our protocol followed previously described methods to quantify canary songs (Leitner et al. 2001a; Leitner & Catchpole 2004, 2007). The syllable repertoire is the number of different syllables that a bird uses to construct songs. We obtained a minimum sample of 390 s of song from each bird for each time point. This is sufficient to estimate the repertoire size, as a cumulative plot of new syllables reaches an asymptote well before this (Halle et al. 2003). A song is defined as a syllable sequence longer than 1.5 s that contains intervals that are not longer than 0.4 s (see also Leitner et al. 2001a). Single syllables are syllables that occur without repetition. The proportion of single syllables was defined as the number of these syllables divided by the total syllable repertoire size. Syllable repetition rate (produced in a tour) was calculated as the number of syllable repetitions per second (Hz). Song analysis was carried out blind to rearing condition.
(e). Statistical analysis
We used non-parametric statistics for all analyses. Song characteristics of tutors and the offspring generation were compared with the Mann–Whitney U-test. To analyse the changes of offspring song at different time points after transfer into a new environment we performed Friedman tests with post hoc comparisons. All tests were two-tailed and the significance level was set at p < 0.05 for all comparisons. If not otherwise stated, values represent medians and quartiles.
3. Results
(a). Songs of tutors and F1 generation differ
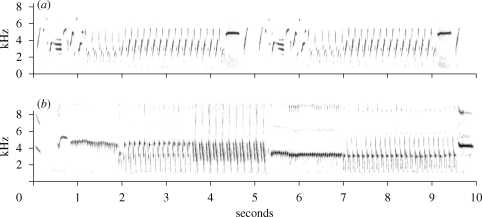
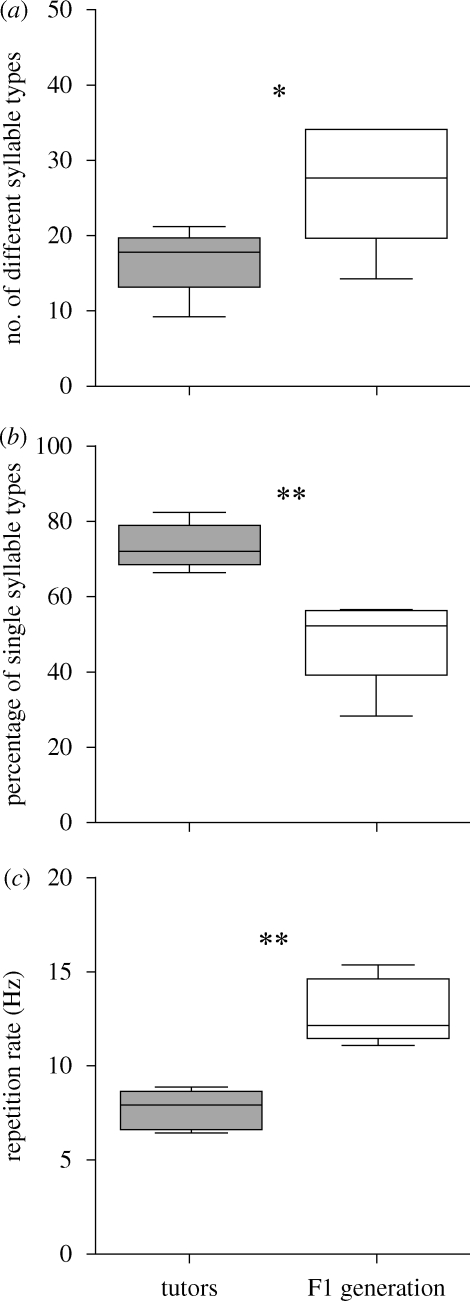
The tutors were raised without having ever heard an adult song model and spent their life as a peer group. Their song differs from that of normally reared males in having a smaller repertoire size, a higher proportion of single syllables and a reduced repetition rate, similar to the study of Leitner & Catchpole (2007). A typical song of a tutor is shown in figure 1a. All males of the F1 generation developed song and recordings were made when 17–18 months old (figure 1b). Their song length was similar to that of the tutors (U = 12.0, N1 = 6, N2 = 6, p = 0.394). However, the F1 generation had acquired a significantly higher syllable repertoire size (U = 5.0, N1 = 6, N2 = 6, p = 0.041; figure 2a). Whereas the repertoires of the tutors ranged from 9 to 21 different syllables, those of the F1 generation were between 14 and 34 different syllables. Both groups not only differed in repertoire size but also in its composition, with the F1 generation having fewer single syllable types in their songs compared with tutors (U = 0.0, N1 = 6, N2 = 6, p = 0.002; figure 2b). Also, repetition rate of repeated syllable types was higher in the F1 generation (U = 0.0, N1 = 6, N2 = 6, p = 0.002; figure 2c). Therefore, in all the characteristics measured, the songs of the F1 generation were more similar to those of normal canaries than to those of their tutors. Furthermore, tutors and the F1 generation shared on average only 5.72 ± 1.9% (mean ± s.d.) of their syllables. However, those syllables were the ones most frequently used in the tutors' songs (63.3% of total song length).
Figure 1.
Sonagrams of songs from (a) a tutor male and (b) a male of the F1 generation at the age of 1 year.
Figure 2.
(a) Repertoire size, (b) percentage of single syllable types and (c) syllable repetition rate of songs from tutors and the F1 generation (*p < 0.05, **p < 0.01).
(b). F1 generation do not change their songs in a new acoustic environment
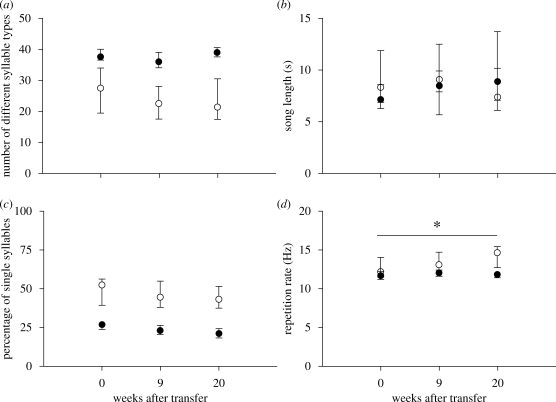
After being transferred from their original environment into a communal aviary with normally reared canaries, the songs of the F1 generation were analysed at three time points: at the time of transfer (w0), after 9 weeks (w9) and after 20 weeks (w20). Over the entire period, no changes in repertoire size (Fr = 2.94, p = 0.57; figure 3a), song length (Fr = 4.13, p = 0.39; figure 3b) and the percentage of single syllable types could be detected (Fr = 4.27, p = 0.37; figure 3c). However, males significantly increased their syllable repetition rate (Fr = 15.07, p = 0.005; figure 3d). None of these parameters changed in the songs of control males over the same time period (p > 0.05 for all tests). Comparing both groups with each other revealed that the F1 generation, although possessing more ‘normal’ characteristics than their tutors, had acquired songs that still could be clearly distinguished from normal canary song in terms of repertoire size (w20: U = 0.0, p = 0.009; figure 3a) and composition (w20: U = 0.0, p = 0.009; figure 3c). Interestingly, repetition rate of the F1 generation at the time of transfer was similar to that of controls but increased constantly in the new environment, and at w20 the difference became significant (w20: U = 0.0, p = 0.009; figure 3d). Song length was similar in both groups (w20: U = 9.0, p = 0.61; figure 3b). Also, syllable turnover (i.e. the number of lost syllable types) between sampling points was in the same range for the experimental group (w0–w9: 22.3%; w9–w20: 18.2%) and the control group (w0–w9: 30.6%; w9–w20: 12.7%) and did not change significantly over time (Wilcoxon test, F1 generation: W = 3.0, p = 0.84; controls: W = 10.0, p = 0.13). Furthermore, at w20 both groups retained about the same percentage of syllable types that were present in their original repertoire at w0 (F1 generation: 77.3 ± 11.0%; controls: 71.3 ± 4.5% (mean ± s.d.)).
Figure 3.
Changes in (a) repertoire size, (b) song length, (c) percentage of single syllables and (d) syllable repetition rate in songs of the F1 generation (open circles) at different time points after transfer into a new acoustic environment in comparison to control males (filled circles) (*p < 0.05). Dots represent the median and the error bars indicate the interquartile range.
4. Discussion
Our study reveals two rather striking results. First, early social contact with a live tutor is sufficient to lead to song crystallization, even though the song model exhibited abnormal song properties. Second, although the presence of the tutors was crucial, the F1 generation did not simply copy the ‘abnormal’ song model but developed rather species-typical song properties.
A previous experiment on peer group male canaries, raised without an adult song model, had shown that these males adjust their song properties towards those of control males when exposed to conspecific tutors in their second year of life (Leitner & Catchpole 2007). Furthermore, they retained only approximately 20 per cent of their original syllables after transfer to the new environment. In the present study, the experimental group (i.e. the F1 generation) neither changed repertoire size nor repertoire composition and kept approximately 77 per cent from their original syllable repertoire, which is similar to control males, when brought into the new environment. This is clearly evidence that no further learning took place in these males. The difference between the two studies can be attributed to the presence of tutors with the F1 generation during the first 30 days of the song development phase, which in canaries lasts from about post-hatching days 40–240 (Nottebohm et al. 1986; Weichel et al. 1986). Previous experiments revealed that the presence of a tutor earlier than day 40 has no influence on song learning (Waser & Marler 1977). The lack of a song model in the peer group males in the first study (Leitner & Catchpole 2007) may have delayed their song crystallization process until an appropriate model was found in the second year of life. By contrast, for the males of the F1 generation in the current study, the auditory experience obtained during the period when the tutors were present (post-hatching days 40–70) was obviously sufficient to induce the normal timing of song development, with songs being crystallized at sexual maturity.
The only characteristic that the F1 generation changed in their second year was syllable repetition rate. At the time of transfer, it was similar to control males but then increased steadily afterwards. This was not due to incorporation of new faster-sung syllable types but to an increase in the repetition rate of syllables already present within the repertoire. High repetition rates are thought to be an androgen-dependent feature (Heid et al. 1985), constrained by motor abilities. For example, free-living island canaries (S. canaria) modulate syllable repetition rates and circulating testosterone levels on a seasonal basis with an increase of both during the breeding season (Leitner et al. 2001b). That the male offspring in our study experienced a rise in circulating testosterone levels is unlikely as the transfer took place in late autumn when testosterone levels are low (Fusani et al. 2000; Voigt & Leitner 2008). Alternatively, it is possible that the singing rate of the transferred males had drastically increased in the new environment, due to the increase of stimuli, and consequently motor learning was induced. The peer group males in the study of Leitner & Catchpole (2007) also increased repetition rate after being brought into a new environment, although the differences were not significant.
The fact that early social contact with a tutor prevented the males from substantial learning later in life is especially interesting as domesticated canaries are classified as open-ended learners (Nottebohm & Nottebohm 1978; Nottebohm et al. 1986). However, the exchange of syllables on a seasonal or annual basis does not necessarily involve learning, as birds might simply have acquired a large syllable pool during the song-learning phase from which they choose syllable types to sing at different times later in life. In Island canaries, approximately 50 per cent of the syllable types that are lost after one breeding season reappear in the song repertoire of the following breeding season (Leitner et al. 2001a,b). Playback experiments with nightingales (Luscinia megarhynchos), also considered an open-ended learner, have shown that adult males not only retain auditory memories of song types that they discarded from their overt repertoire after song crystallization, but also of those that were acquired during song learning but never produced (Geberzahn et al. 2002). Further evidence against learning in adult canaries comes from a recent study, which investigated the effect of age on song structure and the neural substrate. It was found that older males had neither higher repertoire sizes than younger males nor were their song control areas larger (Leitner & Catchpole 2004).
Most songbirds are generally thought to have an innate preference for learning species-specific song, which enables them to choose the correct song model (Marler & Peters 1977, 1988; Güttinger 1979; Mundinger 1995). Although the presence of the tutors in our study had a crucial influence on the timing of the song crystallization process, the F1 generation did not imitate the tutors' song characteristics and copied only a small percentage from their syllable repertoire (figure 2). Those copied syllables were the ones most frequently sung by the tutors. This result clearly shows that birds must possess a template for guidance towards normal species-specific or even strain-specific song development, which is compatible with the selection-based model of song learning (Marler 1997). Cross-fostering experiments with roller and border canaries revealed similar results that males copied very little from an alien-strain tutor but had a strong preference for learning from their own strain's tutors (Mundinger 1995). That innate rules of song development override abnormal auditory experience during song ontogeny was recently also found in Belgian Waterslager canaries (Gardner et al. 2005). On the contrary, in zebra finches, sons of untutored males copy a higher percentage of syllables from their father's abnormal song than from other males' song compared with sons of normally reared males (Williams et al. 1993). This suggests that unlike canaries, the songs of untutored zebra finch males still retain the species-specific characteristics that match a young male's template.
In our study, the F1 generation failed to develop fully normal canary song as they differed in several song parameters from control males (figure 3). This might have happened because the auditory stimulus did not match the template completely and therefore a song was crystallized that constitutes an intermediate version between the songs of the tutors and the controls. Alternatively, it is conceivable that the peers had a strong acoustic influence on each other during the sensory-motor phase of learning, which guided the crystallization process towards the formation of a group song. Such a process resembles ‘action-based learning’, which refers to the attrition of an overproduced repertoire during song crystallization based on the influence of social factors (Marler 1997; Nelson 1997). Further experiments are needed to distinguish between the two possibilities.
In conclusion, our data provide evidence that male domesticated canaries crystallize song upon early exposure to an adult song model. This probably happens through auditory stimulation of the templates that guide the motor-learning process. The lack of such stimulation early in life probably leaves the sensitive phase for song learning open in order for the bird to make substantial modifications to its song later in life when encountering an appropriate model.
Acknowledgements
The experiments were in compliance with the German animal protection law.
We thank Roswitha Brighton for help in animal care and the experiment. The study was funded by BBSRC grant BBC5002601.
References
- Ball G. F., Castelino C. B., Maney D. L., Appeltants D., Balthazart J.2003The activation of birdsong by testosterone. Multiple sites of action and role of ascending catecholamine projections. Ann. NY Acad. Sci. 1007, 211–231 (doi:10.1196/annals.1286.021) [DOI] [PubMed] [Google Scholar]
- Baptista L. F., Petrinovitch L.1984Social interaction, sensitive phases and the song template hypothesis in the white-crowned sparrow. Anim. Behav. 32, 1359–1371 [Google Scholar]
- Beecher M. D., Campbell S. E., Stoddard P. K.1994Correlation of song learning and territory establishment strategies in the song sparrow. Proc. Natl Acad. Sci. USA 91, 1450–1454 (doi:10.1073/pnas.91.4.1450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole C. K., Slater P. J. B.2008Bird song: biological themes and variations, 2nd edn.Cambridge, UK: Cambridge University Press [Google Scholar]
- Chaiken M., Böhner J., Marler P.1993Song acquisition in European starlings Sturnus vulgaris. A comparison of the songs of live-tutored, tape-tutored, untutored, and wild males. Anim. Behav. 46, 1079–1090 (doi:10.1006/anbe.1993.1298) [Google Scholar]
- Eales L. A.1985Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim. Behav. 33, 1293–1300 (doi:10.1016/S0003-3472(85)80189-5) [Google Scholar]
- Fusani L., Van’t Hof T., Hutchison J. B., Gahr M.2000Seasonal expression of androgen receptors, estrogen receptors, and aromatase in the canary brain in relation to circulating androgens and estrogens. J. Neurobiol. 43, 254–268 (doi:10.1002/(SICI)1097-4695(20000605)43:3<254::AID-NEU4>3.0.CO;2-W) [PubMed] [Google Scholar]
- Gardner T. J., Naef F., Nottebohm F.2005Freedom and rules: the acquisition and reprogramming of a bird's learned song. Science 308, 1046–1049 (doi:10.1126/science.1108214) [DOI] [PubMed] [Google Scholar]
- Geberzahn N., Hultsch H., Todt D.2002Latent song type memories are accessible through auditory stimulation in a hand-reared songbird. Anim. Behav. 64, 783–790 (doi:10.1006/anbe.2002.3099) [Google Scholar]
- Güttinger H. R.1979The integration of learned and genetically programmed behaviour: a study of hierarchical organization in songs of canaries, greenfinches and their hybrids. Z. Tierpsychol. 49, 285–303 [Google Scholar]
- Güttinger H. R., Fuchs H., Schwager G.1990Das Gesangslernen und seine Beziehung zur Gehirnentwicklung beim Kanarienvogel (Serinus canaria). Die Vogelwarte 35, 287–300 [Google Scholar]
- Halle F., Gahr M., Kreutzer M.2003Effects of unilateral lesions of HVC on song patterns of male domesticated canaries. J. Neurobiol. 56, 303–314 (doi:10.1002/neu.10230) [DOI] [PubMed] [Google Scholar]
- Heid P., Güttinger H. R., Pröve E.1985The influence of castration and testosterone replacement on the song architecture of canaries. Z. Tierpsychol. 69, 224–237 [Google Scholar]
- Lehongre K., Lenouvel P., Draganoiu T., Del Negro C.2006Long-term effect of isolation rearing conditions on songs of an ‘open-ended’ song learner species, the canary. Anim. Behav. 72, 1319–1327 (doi:10.1016/j.anbehav.2006.03.025) [Google Scholar]
- Leitner S., Catchpole C. K.2004Syllable repertoire and the size of the song control system in captive canaries (Serinus canaria). J. Neurobiol. 60, 21–27 (doi:10.1002/neu.10331) [DOI] [PubMed] [Google Scholar]
- Leitner S., Catchpole C. K.2007Song and brain development in canaries raised under different conditions of acoustic and social isolation over two years. Dev. Neurobiol. 67, 1478–1487 (doi:10.1002/dneu.20521) [DOI] [PubMed] [Google Scholar]
- Leitner S., Voigt C., Gahr M.2001aSeasonal changes in the song pattern of the non-domesticated island canary (Serinus canaria), a field study. Behaviour 138, 885–904 (doi:10.1163/156853901753172700) [Google Scholar]
- Leitner S., Voigt C., Garcia-Segura L. M., Van't Hof T., Gahr M.2001bSeasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Horm. Behav. 40, 160–168 (doi:10.1006/hbeh.2001.1700) [DOI] [PubMed] [Google Scholar]
- Marler P.1997Three models of song learning: evidence from behavior. J. Neurobiol. 33, 501–516 (doi:10.1002/(SICI)1097-4695(19971105)33:5<501::AID-NEU2>3.0.CO;2-8) [PubMed] [Google Scholar]
- Marler P., Peters S.1977Selective vocal learning in a sparrow. Science 198, 519–521 (doi:10.1126/science.198.4316.519) [DOI] [PubMed] [Google Scholar]
- Marler P., Peters S.1988The role of phonology and syntax in vocal learning preferences in the song sparrow, Melospiza melodia. Ethology 77, 125–149 [Google Scholar]
- Metfessel M.1935Roller canary song produced without learning from external sources. Science 81, 470 (doi:10.1126/science.81.2106.470) [DOI] [PubMed] [Google Scholar]
- Millington S. J., Price T. D.1985Song inheritance and mating patterns in Darwin's finches. Auk 102, 342–346 [Google Scholar]
- Mundinger P. C.1995Behaviour-genetic analysis of canary song: inter-strain differences in sensory learning, and epigenetic rules. Anim. Behav. 50, 1491–1511 (doi:10.1016/0003-3472(95)80006-9) [Google Scholar]
- Nelson D. A.1997Social interaction and sensitive phases for song learning: a critical review. In Social influences on vocal development (eds Snowdon C. T., Hausberger M.), pp. 7–22.Cambridge, UK: Cambridge University Press [Google Scholar]
- Nottebohm F.1999The anatomy and timing of vocal learning in birds. In The design of animal communication (eds Hauser M. D., Konishi M.), pp. 63–110.Cambridge, MA: MIT Press [Google Scholar]
- Nottebohm F., Arnold A. P.1976Sexual dimorphism in vocal control areas of the songbird brain. Science 194, 211–213 (doi:10.1126/science.959852) [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Nottebohm M. E.1978Relationship between song repertoire and age in the canary, Serinus canarius. Z. Tierpsychol. 46, 298–305 [Google Scholar]
- Nottebohm F., Nottebohm M. E., Crane L.1986Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav. Neural Biol. 46, 445–471 (doi:10.1016/S0163-1047(86)90485-1) [DOI] [PubMed] [Google Scholar]
- Payne R. B., Payne L. L.1997Field observations, experimental design, and the time and place of learning bird songs. In Social influences on vocal development (eds Snowdon C. T., Hausberger M.), pp. 57–84 Cambridge, UK: Cambridge University Press [Google Scholar]
- Pesch A., Güttinger H. R.1985Der Gesang des weiblichen Kanarienvogels. J. Ornithol. 126, 108–110 (doi:10.1007/BF01640450) [Google Scholar]
- Poirier C., Henry L., Mathelier M., Lumineau S., Cousillas H., Hausberger M.2004Direct social contacts override auditory information in the song-learning process in starlings (Sturnus vulgaris). J. Comp. Psychol. 118, 179–193 (doi:10.1037/0735-7036.118.2.179) [DOI] [PubMed] [Google Scholar]
- Poulsen H.1959Song learning in the domestic canary. Z. Tierpsychol. 16, 173–178 [Google Scholar]
- Slater P. J. B., Ince S. A.1982Song development in chaffinches: what is learnt and when? Ibis 124, 21–26 (doi:10.1111/j.1474-919X.1982.tb03737.x) [Google Scholar]
- Voigt C., Leitner S.2008Seasonality in song behaviour revisited: seasonal and annual variants and invariants in the song of the domesticated canary (Serinus canaria). Horm. Behav. 54, 373–378 (doi:10.1016/j.yhbeh.2008.05.001) [DOI] [PubMed] [Google Scholar]
- Waser M. S., Marler P.1977Song learning in canaries. J. Comp. Physiol. Psychol. 91, 1–7 (doi:10.1037/h0077299) [DOI] [PubMed] [Google Scholar]
- Weichel K., Schwager G., Heid P., Güttinger H. R., Pesch A.1986Sex differences in plasma steroid concentrations and singing behaviour during ontogeny in canaries (Serinus canaria). Ethology 73, 281–294 [Google Scholar]
- Williams H., Kilander K., Sotanski M. L.1993Untutored song, reproductive success and song learning. Anim. Behav. 45, 695–705 (doi:10.1006/anbe.1993.1084) [Google Scholar]
- Zann R. A.1996The zebra finch: a synthesis of field and laboratory studies Oxford, UK: Oxford University Press [Google Scholar]