Abstract
Angiosperm diversification has resulted in a vast array of plant morphologies. Only recently has it been appreciated that diversification might have proceeded quite differently for the two key diagnostic structures of this clade, flowers and fruits. These structures are hypothesized to have experienced different selective pressures via their interactions with animals in dispersal mutualisms, resulting in a greater amount of morphological diversification in animal-pollinated flowers than in animal-dispersed fruits. I tested this idea using size and colour traits for the flowers and fruits of 472 species occurring in three floras (St John, Hawaii and the Great Plains). Phylogenetically controlled analyses of nearest-neighbour distances in multidimensional trait space matched the predicted pattern: in each of the three floras, flowers were more divergent from one another than were fruits. In addition, the spacing of species clusters differed for flowers versus fruits in the flora of St John, with clusters in flower space more divergent than those in fruit space. The results are consistent with the idea that a major driver of angiosperm diversification has been stronger selection for divergent floral morphology than for divergent fruit morphology, although genetic, physiological and ecological constraints may also play a role.
Keywords: angiosperm radiation, floral character displacement, ecological sorting, pollinator constancy, frugivory, seed dispersal and pollination syndromes
1. Introduction
Angiosperm diversity, encompassing richness of both species and morphologies, has been an obsession of biologists since before the time Darwin termed it an ‘abominable mystery’ (Darwin 1879). Over 250 000 species arose in less than 125 Myr (De Bodt et al. 2005), and with this radiation came tremendous phenotypic variation. For example, flower diameters of modern species span four orders of magnitude, from <0.3 mm to >1 m (Bernard et al. 1990; Barkman et al. 2004). Yet only recently has it been appreciated that morphological diversification might have proceeded quite differently for the clade's two key diagnostic structures, flowers and fruits. The expectation, articulated by Schaefer et al. (2004), is that flowers and fruits have been exposed to quite different selective pressures via their interactions with animal associates. As I elaborate below, these selective pressures are expected to have driven a greater amount of morphological diversification in animal-pollinated flowers than in animal-dispersed fruits. This idea, however, has never been rigorously tested.
Sharing pollinators can lead to substantial reproductive costs. At early stages of the divergence between two plant lineages, shared pollinators can lead to unfit hybrid progeny and can trigger reinforcement, the enhancement of prezygotic isolation by natural selection (reviewed in Coyne & Orr 2004). Even among ‘good’ species (fully diverged lineages) that do not produce hybrid progeny, sharing pollinators can lead to reproductive costs via two processes, termed conspecific pollen loss (CPL) and heterospecific pollen deposition (HPD) (Morales & Traveset 2008). CPL results in reduced donation and receipt of conspecific pollen and occurs because pollen grains from a given donor are increasingly lost as a pollinator visits other species en route to the next conspecific individual. HPD can result in stigma clogging or closure, allelopathic inhibition of conspecific pollen grain germination or pollen tube growth, and/or usurpation of ovules. Both CPL and HPD can lead to substantial fitness losses in plants, as determined in experimental studies (reviewed in Morales & Traveset 2008). Thus, we expect that both reinforcement and reproductive character displacement may have played a large role in floral evolution.
Reinforcement, character displacement, ecological sorting or any of these processes in combination may result in high diversity and low overlap of floral morphologies across species in a community. During reinforcement or character displacement, floral traits may evolve to increase pollinator constancy (the tendency of a pollinator to move between conspecific plants, which increases as flower signals diverge; Chittka et al. 1999, 2001; Gumbert et al. 1999; Gegear & Laverty 2001), to limit the diversity of visitors (Altshuler 2003), and/or to place pollen on unique parts of a pollinator's body (Feinsinger 1987; Armbruster et al. 1994). Experimental studies (Fishman & Wyatt 1999; Caruso 2000; Smith & Rausher 2008) have confirmed that the patterns of natural selection on floral traits of a focal species can depend on the presence or absence of sympatric species with similar floral morphologies. Alternatively, species assemblages may be shaped by ecological sorting, in which species experiencing large reproductive costs due to shared pollinators may either fail to colonize or be driven extinct in a given community (Armbruster et al. 1994; Morales & Traveset 2008). Using null-model approaches, studies at both the guild-level (Armbruster et al. 1994; but see Murray et al. 1987) and the community-level (Gumbert et al. 1999) have found evidence that floral morphologies are sometimes more divergent than expected by chance alone.
Fruits, in contrast to flowers, have far less-specialized relationships with their animal associates (Blüthgen et al. 2007). Sharing seed dispersers should not lead to unfit hybrids nor engender costs analogous to either CPL or HPD, suggesting that natural selection would not drive a similar amount of diversification in fruit traits across species. Furthermore, a type of Müllerian mimicry, in which morphologies of different species converge in order to attract a shared set of high-quality dispersers, could be prevalent among fruits (Burns 2005). Finally, traits encouraging a restricted set of seed dispersal agents (or disperser constancy) might actually be selected against, if such patterns resulted in extensive co-deposition of conspecific seeds and thus high levels of intraspecific competition during seed germination and growth (Schaefer et al. 2004). All these processes could limit fruit diversification, reinforce convergence of fruit traits across species and/or allow the retention of morphologically similar species in communities via a lack of ecological sorting. Thus, we expect fruit traits in animal-dispersed plant species to exhibit low levels of diversification relative to flowers. Consistent with this expectation, a large percentage of animal-dispersed plant species have either red or black fruits (62–66%, Wheelwright & Janson 1985) despite a wide range of possible colours.
Reduced diversification in fruits relative to flowers is also predicted if the former suffer more from genetic, physiological or ecological constraints. Special constraints on fruit traits might arise via their non-modular design, their high energy density relative to flowers and/or their interactions with gape-limited frugivores (see §4). However, there are at least four non-mutually exclusive reasons why the expected pattern (greater flower than fruit divergence) may not have been generated over evolutionary time (box 1). Given these considerations, it remains unclear whether flowers have achieved greater morphological diversification than fruits, and if so, whether the pattern has been driven more by differential constraints or by differential signalling/reward interactions with mutualists.
Box 1. Four reasons why flowers may not have diverged more than fruits over evolutionary time.
The negative effects of sharing pollinators could result in the temporal displacement of flowering times or the spatial segregation of morphologically similar species into different habitats, rather than the displacement of floral morphologies (Waser 1978; Feinsinger 1987; Morales & Traveset 2008). Evidence consistent with both temporal displacement and spatial segregation has been found (Kephart 1983; Stone et al. 1998; Aizen & Vazquez 2006; but see Murray et al. 1987).
Mimicry could reduce the realized floral diversification. Examples consistent with mimicry exist for both flowers and fruits, but the (admittedly limited) documentation appears to suggest many more cases of the former. Roy & Widmer (1999) describe cases of potential flower mimics from many plant families, including an estimated 10 000 species of orchids (see also Johnson et al. 2003; Galizia et al. 2005; Benitez-Vieyra et al. 2007), whereas Burns (2005) provides apparently the sole example consistent with mimicry in fleshy fruits. If floral mimicry is indeed more common than fruit mimicry, the resulting patterns of diversification could be skewed towards equal or lesser diversification of flowers relative to fruits.
If seed dispersers are limiting, competition for dispersers may have driven a high degree of divergence in fruit traits; highly rewarding species may have undergone selection to distinguish themselves from mimics and/or low-reward species.
Flower and fruit traits serve not only as signals and rewards to mutualists, but also play other roles as attractants to or defences against antagonists (Willson & Whelan 1990; Cipollini & Levey 1997; Schaefer et al. 2004). For example, flower and fruit pigments such as anthocyanins and carotenoids have been shown to be influenced by a variety of selective agents such as floral herbivores (Irwin et al. 2003) and seed predators (Whitney & Stanton 2004). Thus, agents of selection other than mutualists might override the expected pattern of diversification.
Here, I present, to my knowledge, the first comparisons of morphological (colour and size) dispersion for flowers versus fruits, using data from three floras. First, I test the hypothesis that the nearest–neighbour (NN) distance in trait space for flowers will be greater than the NN distance for fruits of the same set of species within a species assemblage. Second, I examine whether the clustering of species in trait space differs for flowers versus fruits, which may be relevant to understanding similarities and differences in flower and fruit syndromes (suites of traits associated with the attraction and utilization of specific groups of animals, Gautier-Hion et al. 1985; Fenster et al. 2004). Because I measure flower and fruit traits on common scales, the patterns detected are directly comparable, allowing insight into whether morphological evolution has played out differently for flowers and fruits.
2. Material and methods
(a). Approach and data sources
I compare morphological diversification of floral versus fruit structures across animal-pollinated, animal-dispersed species living within three regions. Data were extracted from published floras of the Caribbean island of St John, US Virgin Islands (Acevedo-Rodriguez 1996); Hawaii (Wagner et al. 1999) and the Great Plains of the USA (Great Plains Flora Association 1986). These floras were chosen because their standardized species descriptions allowed trait data to be extracted consistently across species. There is a trade-off between the ability to obtain highly detailed data for a particular species assemblage and the ability to collect less detailed data on several assemblages. Replicating at the level of assemblages with coarser data, as I do here, arguably provides more insight into the overall pattern of evolution in the angiosperms.
For each flora, I generated a list of animal-pollinated, fleshy-fruited (presumably endozoochorously dispersed) species. Species described as having small flowers lacking display structures (e.g. bracts, sepals, petals), and belonging to groups described as anemophilous (Zomlefer 1994), were classified as wind-pollinated and excluded. Species for which the fruit is not the unit of dispersal (e.g. species with dehiscent fruits and arillate seeds) were rare and were excluded. Introduced (non-native) species present in the floras were excluded from the datasets, reflecting the assumption that processes generating morphological diversification occur within communities of species that interact over long periods of time. In addition, figs were excluded as no quantitative size and colour data were available for their tiny, specialized flowers.
Flower and fruit information for each of the species was then extracted from the flora. Data on length and colour of both fruits and flowers were consistently available. These traits formed the bases of the analyses (see below). Unfortunately, I was forced to ignore parts of the floral and fruit displays that were not consistently quantified in the floras. For example, traits of bracts and sepals (both flowers and fruits) were not consistently described across species and could not be included. An exception is that traits of sepals were substituted for those of petals in species where the corolla is missing and display function is carried out by the calyx (e.g. Wikstroemia, Thymelaeaceae, Hawaii; Euphorbiaceae, Hawaii). Data were recorded for 132, 189 and 151 animal-pollinated and animal-dispersed angiosperm species from the Great Plains, Hawaii and the island of St John, respectively.
(b). Trait coding
Length (millimetre) of each structure (flower or fruit) was extracted from the floras for each species. When lengths were given as a range, I took the midpoint value to represent the species. For flowers, length represented petal length for species without a floral tube, or tube + lobe length for species with a floral tube. For species with different floral morphs (e.g. both staminate and pistillate flowers), I used the midpoint of the combined range. For fruits, lengths represented the largest dimension given.
Colour traits were extracted from the floras in the following manner. Each written description of a colour (e.g. ‘violet’) was converted to the three-axis red, green, blue (RGB) colour scheme by selecting the colour from a colour chart and recording the corresponding RGB values (see appendix 1, electronic supplementary material). While this process is arbitrary by necessity (written colours do not map one-to-one to any multivariate colour scheme), assignments were consistent for flowers and fruits and therefore should not introduce biases into the analyses. For example, the distance in colour space between a red and a white flower would be exactly the same as the distance between a red and a white fruit. A primary and a secondary colour were recognized for each structure (flower or fruit); thus each structure was described by six colour axes. Unicoloured structures were represented by the same three RGB values repeated twice, while bicoloured (e.g. striped, spotted) structures were represented by two unique sets of three RGB values.
Roughly one-third of the species in the floras were described as having intraspecific colour variation in fruits and/or flowers. In some cases, these represented true (discrete) colour morphs, but in most cases they represented bounds of continuous variation, e.g. ‘petals white to lavender’. I included both types of variation in the same way, by representing a species multiple times in the dataset, once for each described colour up to a maximum of three ‘morphs’.
(c). Data analysis
All programming and analyses were performed in SAS macro language (SAS Institute 2003). Data from each flora were analysed separately. Within a flora, each of the seven trait axes (length, colour axes 1–6) was standardized to mean 0 and standard deviation 1. This standardization was performed on the flower and fruit data together so that interspecific distances would be comparable in flower versus fruit space. The standardized flower and fruit data were then considered separately. For the analyses presented here, size and colour traits were weighted equally, that is the single size axis was given the same weight as that of the six colour axes combined. Alternative weightings were analysed, and included (i) equal weightings for all seven axes, and (ii) equal weightings of the size axis, the three primary colour axes combined and the three secondary colour axes combined. The alternative weightings did not result in any qualitative shifts in the patterns or conclusions (data not shown).
Morphological divergence between species was assessed using NN distances. For each colour morph within a species, Euclidean distances to all other species in seven-dimensional trait space were calculated using SAS Proc Distance, and the NN distance was retained. The minimum NN distance was then determined for each set of morphs to provide a single NN distance for each species within each trait space. NN distances in flower space versus fruit space were then compared using ANOVA with structure (flower versus fruit), flora and their interaction as fixed factors. Because NN distances were roughly Poisson-distributed and generated non-normality in the residuals, I used a randomization procedure (Cassell 2002) with 10 000 replicates to generate significance levels.
With regard to phylogenetic controls, the critical issue is that the trait spaces for flowers and fruits, respectively, be constructed from the same set of species so that phylogenetic inertia applies equally to the two datasets. By examining both flowers and fruits for each species in the three datasets, I account for any influence of phylogeny on the evolution of these structures. Note that methods such as Phylogenetically Independent Contrasts (Felsenstein 1985) are inappropriate for our purposes because (i) the pattern of interest is not a correlation between two traits and (ii) distances between species, not species themselves, are used as data points.
To examine whether patterns of clustering differed for species in flower space versus fruit space, I used a hierarchical agglomerative clustering algorithm (SAS Proc Cluster, method = centroid) to assign species to clusters. The algorithm begins with each species assigned to its own cluster and merges them into successively larger clusters. Because I was interested in relatively inclusive groupings that might correspond to broad ‘pollination syndromes’ or ‘dispersal syndromes,’ I focused on arrangements of species into two, three, four, five and six clusters. For each arrangement, I calculated the centroid of each cluster and then the Euclidean centroid-to-centroid distance to all other clusters. Mean distances between clusters were examined via ANOVA with structure (flower versus fruit), flora, number of clusters and all two- and three-way interactions as fixed effects in the model. Data met the assumptions of normality and homogeneity of variances without transformation.
3. Results
(a). Divergence of species: NN distances
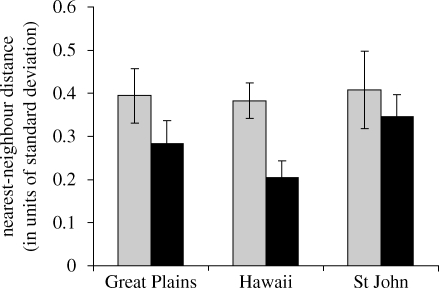
Flowers showed significantly higher NN distances than fruits (figure 1; df = 2,938; p = 0.007), indicative of more morphological dispersion. Patterns of variation were similar across floras, as neither effects of flora nor a flora × structure type interaction were detected (df = 1,938 and 2,938; p = 0.314 and 0.582, respectively).
Figure 1.
Mean nearest-neighbour distances (±s.e.) in trait space for flowers (grey bars) and fruits (black bars) in three floras. ANOVA results: flora p = 0.31; structure (flower versus fruit) p = 0.007; flora × structure p = 0.58. n = 132, 189 and 151 species, respectively.
(b). Divergence of morphological clusters
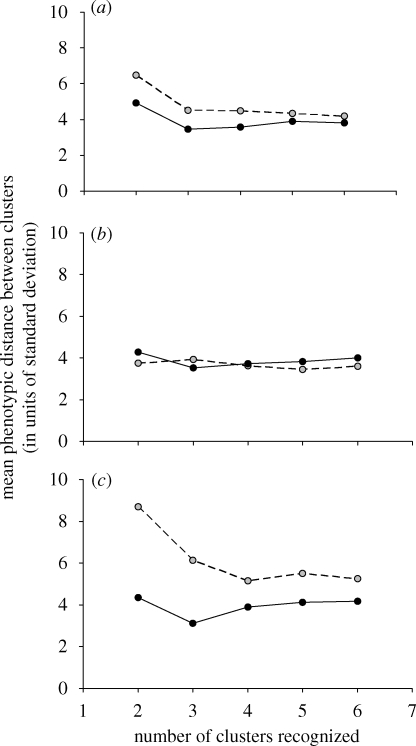
Structure type (flower versus fruit) influenced the spacing of species clusters, but patterns differed by flora and by the number of clusters recognized (significant structure × flora and structure × number of clusters interactions; table 1, figure 2). Clusters in flower space were more dispersed than clusters in fruit space in the flora of St John (Tukey tests within floras, structure effect, p = 0.0002; figure 2c). In contrast, neither the Great Plains flora nor the Hawaiian flora showed significant differences in cluster spacing for flowers versus fruits (Tukey tests within floras, structure effect, p = 0.260 and 0.994, respectively; figure 2a,b). Note, however, that the trend in the Great Plains is similar to that in St John, with larger intercluster distances for flowers relative to fruits (figure 2a).
Table 1.
ANOVA results for the effects of structure (flower versus fruit), flora (Great Plains, Hawaii or St John) and number of clusters on the spacing of species clusters in trait space. Italics indicate p-values significant at p < 0.05.
| effect | df | F | p |
|---|---|---|---|
| flora | 2,18 | 4.66 | 0.023 |
| structure | 1,18 | 14.27 | 0.002 |
| number of clusters | 1,18 | 9.34 | 0.007 |
| flora × structure | 2,18 | 5.79 | 0.012 |
| flora ×number of clusters | 2,18 | 1.44 | 0.263 |
| structure × number of clusters | 1,18 | 6.14 | 0.023 |
| flora × structure × number of clusters | 2,18 | 2.08 | 0.154 |
Figure 2.
Mean distances between clusters in trait space for flowers (grey circles) and fruits (black circles) in three floras, (a) Great Plains, (b) Hawaii and (c) St John. Cluster analysis was used to sort species into sequentially larger clusters, the final six clusters were retained and Euclidean distances between centroids were then calculated for each level of clustering.
4. Discussion
(a). Divergence of species
Overall, the results are consistent with the hypotheses that a major driver of angiosperm diversification has been stronger selection for divergent floral morphology than for divergent fruit morphology, and/or that ecological sorting filters out species with similar floral morphologies more strongly than those with similar fruit morphologies. The patterns detected in three regional floras were highly consistent: in each case, flowers of animal-pollinated, animal-dispersed species were more dispersed in size/colour space than were fruits of the same species. Distances between neighbouring species were on average 48 per cent larger in flower space than in fruit space. These patterns suggest that other factors, such as temporal or spatial displacement of species with similar floral morphologies, floral mimicry, competition for seed dispersers and effects of selective agents other than mutualists (box 1), are not so strong as to subvert or obscure processes generating greater spacing between flowers than fruits.
The strength of the patterns detected was somewhat surprising, given that the analysis was expected to have low power for several reasons. Only a single size axis was considered, and quantification of colour was coarse. No information could be incorporated on many components of the floral display (e.g. bracts, sepals, coloured or ornamented stamens or pistils), on display orientation (e.g. vertical versus horizontal) or on the orientation or amount of fusion of floral parts, all of which are traits that may have responded to selection by pollinators. Including these additional axes of variation in a similar analysis would presumably increase the measured floral divergence between species. While some fruit displays vary along similar axes, there appears to be much less variation than that in flowers (2008, personal observation). In addition, for structure length, the lower end of floral (but not the fruit) size range tends to be missing; when flowers are minute, a general trend across floras is to not give dimensions (e.g. Vitex, Morus). The absence of such species from the analysis again reduces the estimated floral variation and results in a conservative test for differences between flower and fruit divergence.
(b). Clustering of species in trait space
Despite the presence of morphological divergence between species for both fruits and flowers at the level of NN pairs, analyses at larger scales typically show evidence for clustering of species (e.g. this study; Gautier-Hion et al. 1985; Wilson et al. 2004). The clusters themselves may reflect phylogenetic inertia or constraints, and/or convergent evolution onto suites of traits associated with the attraction and use of specific groups of animals (syndromes, Fenster et al. 2004). Because we know little about the pollinators and frugivores visiting the plants in each flora, the clusters identified in the current analysis have an unknown relationship to pollination and dispersal syndromes. However, the main focus here was the novel question of whether the relationships between clusters differ for flowers and fruits. One of the three floras (St John) showed evidence that cluster spacing differed significantly, with clusters of flowers more dispersed in trait space than those of fruit. This suggests that morphological diversification has proceeded differently for flowers and fruits across not just one, but multiple evolutionary scales.
(c). Special constraints on fruit evolution?
However, at least two alternative mechanisms aside from differential selection pressures and differential ecological sorting may also contribute to the observed patterns. First, genetic constraints may be more limiting for fruits than flowers. Schaefer et al. (2004) suggest that such constraints may exist because fruits develop from a single structure (usually, the ovary), while flowers are modular, with petals, stamens and pistils arising from ontogenetically distinct pathways. Such architecture may make it easier for flowers to diverge in one or more modules without negatively impacting essential functions in other modules. For example, in flowers, corolla pigmentation is physically and developmentally decoupled from the nectar, pollen or resin rewards produced elsewhere in the flower. This is not generally true in fruits, where the pigments in fruit tissues serve as both signals and rewards (Schaefer et al. 2004). Because carotenoids and anthocyanins are dietary requirements and have important physiological functions in animals (Feltwell 1978; Olson & Owens 1998; Schaefer et al. 2008), the evolution of visual signal diversity in fruits may be constrained by the necessity of producing certain types or amounts of pigments to remain nutritionally attractive to frugivores.
Second, physiological and ecological constraints may limit fruit sizes more than flower sizes. At the lower end of the size spectrum, animal-dispersed fruits must be large enough to attract large frugivores capable of carrying seeds. Indeed, the sizes of fruit and the frugivores that consume them are positively correlated (summarized by Herrera 2002). Because pollen grains are much lighter than seeds, smaller animal associates can serve as effective pollen vectors and, presumably, floral rewards and associated floral structures can be correspondingly smaller. At the upper end of the size spectrum, because fruits have greater energy density than flowers, one might hypothesize that resource limitation and size-number trade-offs limit maximum fruit size more than flower size. Also, frugivore gape constraints (Wheelwright 1985) mean that opportunities for dispersal decrease with fruit size, potentially limiting maximum fruit sizes. There is evidence that fruit size has coevolved with body and gape sizes of frugivores: plant species in New Zealand, where avian frugivores are small and native mammals are absent, have smaller fruits than congeners in Australia and South America, where frugivores are larger and include both birds and mammals (Lord 2004).
(d). Future directions
Better estimates of the relative amount of floral and fruit diversification could be obtained via more complete trait measurements. Candidate traits include bracts, sepals, coloured or ornamented stamens or pistils, display orientation and the orientation or amount of fusion of floral parts. In addition, it will be important to include spectral colour measurements that include all wavelengths that are perceived by animal associates (e.g. ultraviolet for birds and some insects, Siitari et al. 1999), and to then measure the divergence between species in units of the perceptual space of the relevant animals (Gumbert et al. 1999; Schaefer et al. 2007). For many species with clustered flowers or fruits, overall display size may matter more to animal perception and behaviour than the size of an individual flower or fruit (Ohashi & Yahara 2001). In addition, the composition of nutritional rewards (nectar, pollen, fruit tissue), olfactory cues (e.g. floral fragrances) and flowering or fruiting phenology represents other important axes of variation that are relevant for understanding plant–animal interactions and the divergence of fruit and flower structures.
Overall, the presence of predicted differences in fruit and flower morphologies argues that our understanding of the evolutionary consequences of plant-pollinator and plant-seed disperser mutualisms is broadly correct. A remaining problem is distinguishing the relative contributions of selective pressures versus genetic constraints on the differences in divergence between flowers and fruits. Experimental studies of selection and response could shed some light here. For example, selection on floral and fruit traits could be measured for focal species alone or in the presence of another species chosen based on a standardized amount of morphological similarity. The resulting changes in coefficients of selection could be used to assess whether the strength of selection for divergence differs for flower versus fruit traits. Further work on response to selection and genetic architecture would be needed to establish the presence of differential genetic constraints.
Acknowledgements
Many thanks to Pedro Jordano, Jennifer Rudgers, the Rudgers and Whitney laboratory groups and especially to Martin Schaefer for discussion and advice during the design and analysis of the project. Loren Albert and Matt King were instrumental in data collection.
References
- Acevedo-Rodriguez P.1996Flora of St. John. Memoirs of the New York Botanical Garden, vol. 78 New York, NY: The New York Botanical Garden [Google Scholar]
- Aizen M. A., Vazquez D. P.2006Flowering phenologies of hummingbird plants from the temperate forest of southern South America: is there evidence of competitive displacement? Ecography 29, 357–366 (doi:10.1111/j.2006.0906-7590.04552.x) [Google Scholar]
- Altshuler D. L.2003Flower color, hummingbird pollination, and habitat irradiance in four neotropical forests. Biotropica 35, 344–355 [Google Scholar]
- Armbruster W. S., Edwards M. E., Debevec E. M.1994Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium). Ecology 75, 315–329 (doi:10.2307/1939537) [Google Scholar]
- Barkman T. J., Lim S. H., Salleh K. M., Nais J.2004Mitochondrial DNA sequences reveal the photosynthetic relatives of Rafflesia, the world's largest flower. Proc. Natl Acad. Sci. USA 101, 787–792 (doi:10.1073/pnas.0305562101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Vieyra S., de Ibarra N. H., Wertlen A. M., Cocucci A. A.2007How to look like a mallow: evidence of floral mimicry between Turneraceae and Malvaceae. Proc. R. Soc. B 274, 2239–2248 (doi:10.1098/rspb.2007.0588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard F. A., Bernard J. M., Denny P.1990Flower structure, anatomy and life-history of Wolffia australiana (Benth) Denhartog and Vanderplas. Bull. Torrey Bot. Club 117, 18–26 (doi:10.2307/2997125) [Google Scholar]
- Blüthgen N., Menzel F., Hovestadt T., Fiala B., Blüthgen N.2007Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 17, 341–346 (doi:10.1016/j.cub.2006.12.039) [DOI] [PubMed] [Google Scholar]
- Burns K. C.2005Does mimicry occur between fleshy-fruits? Evol. Ecol. Res. 7, 1067–1076 [Google Scholar]
- Caruso C. M.2000Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution 54, 1546–1557 [DOI] [PubMed] [Google Scholar]
- Cassell D. L.2002. A randomization-test wrapper for SAS PROCs. In Proc. of the 27th Annual SAS User's Group Int. Conf. Paper 251 Orlando, FL: SAS Institute [Google Scholar]
- Chittka L., Thomson J. D., Waser N. M.1999Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86, 361–377 (doi:10.1007/s001140050636) [Google Scholar]
- Chittka L., Spaethe J., Schmidt A., Hickelsberger A.2001Adaptation, constraint, and chance in the evolution of flower color and pollinator color vision. In Cognitive ecology of pollination: animal behaviour and floral evolution (eds Chittka L., Thompson J. D.), pp. 106–126 Cambridge, UK: Cambridge University Press [Google Scholar]
- Cipollini M. L., Levey D. J.1997Secondary metabolites of fleshy vertebrate-dispersed fruits: adaptive hypotheses and implications for seed dispersal. Am. Nat. 150, 346–372 (doi:10.1086/286069) [DOI] [PubMed] [Google Scholar]
- Coyne J. A., Orr H. A.2004Speciation Sunderland, MA: Sinauer Associates [Google Scholar]
- Darwin C.1879Letter to J. D. Hooker, 22 July 1879. In More letters of Charles Darwin, vol 2. 1903 (ed. Darwin F.). New York, NY: D. Appleton and Company [Google Scholar]
- De Bodt S., Maere S., Van de Peer Y.2005Genome duplication and the origin of angiosperms. Trends Ecol. Evol. 20, 591. [DOI] [PubMed] [Google Scholar]
- Feinsinger P.1987Effects of plant species on each others pollination: is community structure influenced? Trends Ecol. Evol. 2, 123–126 (doi:10.1016/0169-5347(87)90052-8) [DOI] [PubMed] [Google Scholar]
- Felsenstein J.1985Phylogenies and the comparative method. Am. Nat. 125, 1–15 (doi:10.1086/284325) [Google Scholar]
- Feltwell J.1978The distribution of carotenoids in insects. In Biochemical aspects of plant and animal coevolution (ed. Harborne J. B.), pp. 277–293 London, UK: Academic Press [Google Scholar]
- Fenster C. B., Armbruster W. S., Wilson P., Dudash M. R., Thomson J. D.2004Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403 (doi:10.1146/annurev.ecolsys.34.011802.132347) [Google Scholar]
- Fishman L., Wyatt R.1999Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae). Evolution 53, 1723–1733 (doi:10.2307/2640435) [DOI] [PubMed] [Google Scholar]
- Galizia C. G., Kunze J., Gumbert A., Borg-Karlson A. K., Sachse S., Markl C., Menzel R.2005Relationship of visual and olfactory signal parameters in a food-deceptive flower mimicry system. Behav. Ecol. 16, 159 (doi:10.1093/beheco/arh147) [Google Scholar]
- Gautier-Hion A., et al. 1985Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia 65, 324–337 (doi:10.1007/BF00378906) [DOI] [PubMed] [Google Scholar]
- Gegear R. J., Laverty T. M.2001The effect of variation among floral traits on the flower constancy of pollinators. In Cognitive ecology of pollination: animal behaviour and floral evolution (eds Chittka L., Thompson J. D.), pp. 1–20 Cambridge, UK: Cambridge University Press [Google Scholar]
- Great Plains Flora Association 1986Flora of the Great Plains Lawrence, KS: University Press of Kansas [Google Scholar]
- Gumbert A., Kunze J., Chittka L.1999Floral colour diversity in plant communities, bee colour space and a null model. Proc. R. Soc. Lond. B 266, 1711–1716 (doi:10.1098/rspb.1999.0836) [Google Scholar]
- Herrera C. M.2002Correlated evolution of fruit and leaf size in bird-dispersed plants: species-level variance in fruit traits explained a bit further? Oikos 97, 426–432 (doi:10.1034/j.1600-0706.2002.970312.x) [Google Scholar]
- Irwin R. E., Strauss S. Y., Storz S., Emerson A., Guibert G.2003The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology 84, 1733–1743 (doi:10.1890/0012-9658(2003)084[1733:TROHIT]2.0.CO;2) [Google Scholar]
- Johnson S. D., Peter C. I., Nilsson L. A., Agren J.2003Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84, 2919–2927 (doi:10.1890/02-0471) [Google Scholar]
- Kephart S. R.1983The partitioning of pollinators among three species of Asclepias. Ecology 64, 120–133 (doi:10.2307/1937335) [Google Scholar]
- Lord J. M.2004Frugivore gape size and the evolution of fruit size and shape in southern hemisphere floras. Austral. Ecol. 29, 430–436 (doi:10.1111/j.1442-9993.2004.01382.x) [Google Scholar]
- Morales C. L., Traveset A.2008Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Crit. Rev. Plant Sci. 27, 221–238 (doi:10.1080/07352680802205631) [Google Scholar]
- Murray K. G., Feinsinger P., Busby W. H., Linhart Y. B., Beach J. H., Kinsman S.1987Evaluation of character displacement among plants in two tropical pollination guilds. Ecology 68, 1283–1293 (doi:10.2307/1939213) [DOI] [PubMed] [Google Scholar]
- Ohashi K., Yahara T.2001Behavioural responses of pollinators to variation in floral display size, their influences on the evolution of floral traits. In Cognitive ecology of pollination: animal behaviour and floral evolution (eds Chittka L., Thompson J. D.), pp. 274–296 Cambridge, UK: Cambridge University Press [Google Scholar]
- Olson V. A., Owens I. P. F.1998Costly sexual signals: are carotenoids rare, risky, or required? Trends Ecol. Evol. 13, 510–514 (doi:10.1016/S0169-5347(98)01484-0) [DOI] [PubMed] [Google Scholar]
- Roy B. A., Widmer A.1999Floral mimicry: a fascinating yet poorly understood phenomenon. Trends Plant Sci. 4, 325–330 (doi:10.1016/S1360-1385(99)01445-4) [DOI] [PubMed] [Google Scholar]
- SAS Institute 2003The SAS system for Windows, release 9.1. Cary, NC: SAS Institute [Google Scholar]
- Schaefer H. M., Schaefer V., Levey D. J.2004How plant–animal interactions signal new insights in communication. Trends Ecol. Evol. 19, 577 (doi:10.1016/j.tree.2004.08.003) [Google Scholar]
- Schaefer H. M., Schaefer V., Vorobyev M.2007Are fruit colors adapted to consumer vision and birds equally efficient in detecting colorful signals? Am. Nat. 169, S159–S169 [DOI] [PubMed] [Google Scholar]
- Schaefer H. M., McGraw K., Catoni C.2008Birds use fruit colour as honest signal of dietary antioxidant rewards. Funct. Ecol. 22, 303–310 (doi:10.1111/j.1365-2435.2007.01363.x) [Google Scholar]
- Siitari H., Honkavaara J., Viitala J.1999Ultraviolet reflection of berries attracts foraging birds. A laboratory study with redwings (Turdus iliacus) and bilberries (Vaccinium myrtillus). Proc. R. Soc. Lond. B 266, 2125–2129 (doi:10.1098/rspb.1999.0897) [Google Scholar]
- Smith R. A., Rausher M. D.2008Experimental evidence that selection favors character displacement in the ivyleaf morning glory. Am. Nat. 171, 1–9 (doi:10.1086/523948) [DOI] [PubMed] [Google Scholar]
- Stone G. N., Willmer P., Rowe J. A.1998Partitioning of pollinators during flowering in an African Acacia community. Ecology 79, 2808–2827 [Google Scholar]
- Wagner W. L., Herbst D. R., Somer S. H.1999Manual of the flowering plants of Hawai'i Honolulu, HI: University of Hawai'i Press [Google Scholar]
- Waser N. M.1978Interspecific pollen transfer and competition between co-occurring plant species. Oecologia 36, 223–236 (doi:10.1007/BF00349811) [DOI] [PubMed] [Google Scholar]
- Wheelwright N. T.1985Fruit size, gape width, and the diets of fruit-eating birds. Ecology 66, 808–818 (doi:10.2307/1940542) [Google Scholar]
- Wheelwright N. T., Janson C. H.1985Colors of fruit displays of bird-dispersed plants in two tropical forests. Am. Nat. 126, 777–799 (doi:10.1086/284453) [Google Scholar]
- Whitney K. D., Stanton M. L.2004Insect seed predators as novel agents of selection on fruit color. Ecology 85, 2153–2160 (doi:10.1890/03-3138) [Google Scholar]
- Willson M. F., Whelan C. J.1990The evolution of fruit color in fleshy-fruited plants. Am. Nat. 136, 790–809 (doi:10.1086/285132) [Google Scholar]
- Wilson P., Castellanos M. C., Hogue J. N., Thomson J. D., Armbruster W. S.2004A multivariate search for pollination syndromes among penstemons. Oikos 104, 345–361 (doi:10.1111/j.0030-1299.2004.12819.x) [Google Scholar]
- Zomlefer W. B.1994Guide to flowering plant families Chapel Hill, NC: University of North Carolina Press [Google Scholar]