Abstract
Bet-hedging theory addresses how individuals should optimize fitness in varying and unpredictable environments by sacrificing mean fitness to decrease variation in fitness. So far, three main bet-hedging strategies have been described: conservative bet-hedging (play it safe), diversified bet-hedging (don’t put all eggs in one basket) and adaptive coin flipping (choose a strategy at random from a fixed distribution). Within this context, we analyse the trade-off between many small eggs (or seeds) and few large, given an unpredictable environment. Our model is an extension of previous models and allows for any combination of the bet-hedging strategies mentioned above. In our individual-based model (accounting for both ecological and evolutionary forces), the optimal bet-hedging strategy is a combination of conservative and diversified bet-hedging and adaptive coin flipping, which means a variation in egg size both within clutches and between years. Hence, we show how phenotypic variation within a population, often assumed to be due to non-adaptive variation, instead can be the result of females having this mixed strategy. Our results provide a new perspective on bet-hedging and stress the importance of extreme events in life history evolution.
Keywords: bet-hedging, adaptive coin flipping, life-history theory, clutch size, environmental stochasticity
1. Introduction
Bet-hedging theory addresses how individuals should optimize their fitness in a variable and unpredictable environment. The idea behind a bet-hedging strategy is that an individual has to lower its variance in fitness between years in order to maximize its long-term fitness (e.g. Slatkin 1974; Seger & Brockmann 1987; Philippi & Seger 1989). In a variable, unpredictable environment (e.g. some years are dry while others are wet), individuals should, according to standard bet-hedging theory, avoid specialization on a specific environmental condition. As an example, a dry year specialist will have a much higher fitness in dry years compared with wet years, resulting in highly varying fitness contributions between years. A generalist, on the other hand, has about the same, intermediate, fitness contributions from both wet and dry years. Even when the specialist has the higher arithmetic mean fitness, the generalist strategy can still win in the long run. Long-term fitness is measured as the geometric mean of the yearly fitness contributions, which is sensitive to large fitness variations; thus, the generalist has adopted a successful (bet-hedging) strategy despite the sacrifice in fitness from dry years.
There are, in principle, two ways an individual can lower its variation in fitness. The first, called conservative bet-hedging, is to ‘always play it safe’ (Seger & Brockmann 1987; Philippi & Seger 1989), which means that an organism always uses exactly the same, low-risk strategy. The alternative is the diversified bet-hedging strategy, ‘don't put all your eggs in one basket’, where an individual invests in several strategies at once, with low variation in total success as a result. However, the most successful bet-hedging strategy is not always to minimize the variation in fitness between years. It has been shown that a strategy called ‘adaptive coin flipping’ can be more successful than a generalist strategy with minimal variation in fitness between years (Cooper & Kaplan 1982; Kaplan & Cooper 1984; for a numerical example, see Seger & Brockmann 1987). Cooper and Kaplan show that by mixing two pure strategies randomly, a third and better strategy can arise even if one of the strategies has a lower geometric mean fitness than the other. This means that an individual with an ‘adaptive coin flipping’ strategy will ‘flip a coin’ every year, and thereby determine whether to behave according to, for example, a dry year or a wet year specialist. The probability for the individual to use a specific strategy evolves to match the probability for that kind of weather. If the same strategy is represented in several individuals the strategy can be successful even if a specific individual in a given year makes the wrong choice.
Bet-hedging theory has a long tradition of primarily concerning the trade-off between adult survival and reproduction, but can involve, in principle, any life history trait. As an example, dormancy in one or several life stages is commonly thought of as an adaptation to variable environments (e.g. Cohen 1966; Brown & Venable 1986; Hopper 1999; Ivarsson et al. 2005). The size of offspring at birth is another important trait since it has a great influence on offspring survival chances (Roff 1992 and references therein). Given a limited amount of resources, should a female produce several small, at risk, or few large, with improved survivorship, offspring? If conditions during offspring development and maturation are benign, many small offspring are most probably the best option, but if conditions are harsh the whole clutch can be lost. In this paper, we focus on this trade-off between offspring number and quality, given a variable, unpredictable environment during offspring maturation.
Previous studies of optimal variation in egg sizes and numbers in a clutch (e.g. Cooper & Kaplan 1982; McGinley et al. 1987; Forbes 1991; Einum & Fleming 2004, 2007) have reached somewhat conflicting conclusions on what the most adaptive strategy looks like. Cooper & Kaplan (1982) show how it can be adaptive for a specific individual experiencing a totally random and unpredictable environment to have variation in, for example, egg size between years. McGinley et al. (1987) and Einum & Fleming (2004) show how variation within a clutch can be an adaptive strategy, but only for annuals in a highly variable environment. The general conclusion from previous studies is, however, that selection will favour uniform clutches in most cases (McGinley et al. 1987; Forbes 1991; Einum & Fleming 2004).
All studies mentioned above have differences in how adult survival, density dependence and environmental variations are modelled. More importantly, it is not clear what strategy would be the most optimal if all three types of bet-hedging described above were considered simultaneously—should a female invest in (i) a constant, large egg size (conservative bet-hedging), (ii) egg sizes drawn from a fixed distribution (diversified bet-hedging), (iii) different egg sizes chosen randomly from year to year (adaptive coin flipping), or (iv) any combination of the above?
To investigate this question, we use an individual-based model incorporating explicit population dynamics, density-dependent recruitment and a direct trade-off between the size and number of offspring. The many sources of variation—environmental fluctuations and population dynamics as well as within- and between-year phenotypic variation—make an explicit calculation of fitness complicated and, to the best of our knowledge, lacking a firm theoretical underpinning. With our approach, we can simulate evolution without relying on a specific fitness measure (like mean intrinsic growth rate or mean lifetime reproductive success) to evaluate which strategy is the optimal bet-hedging strategy. We show that a female should produce propagules that are relatively large, but at the same time vary the mean propagule size of a clutch between years and the sizes of the propagules within a clutch. In other words, the optimal strategy is a combination of all the three strategies mentioned earlier: adaptive coin flipping, conservative and diversified bet-hedging. The exact location and evolutionary stability of this optimum depends, among other things, on the distribution of propagule size within the clutch. Adult survival, which is not allowed to evolve, affects how prone adults should be to take risks, but does not change our conclusions in any qualitative way.
2. Description of the model
(a). A general overview of the model
We have constructed an individual-based model where each female uses a specific, genetically determined bet-hedging strategy. Each strategy is characterized by three parameters that govern how a female should distribute the mass of the different propagules in a clutch within and between years. Reproduction is clonal, but new strategies are formed due to small, independent mutations in the three parameters. The new strategies are able to spread in the population depending on their net reproductive success, which is stochastic due to the fluctuating environment. The quality of the environment is described by a critical propagule size that is needed for propagules to survive that particular year. In ‘good years’, plenty of resources will be available for the propagules, and hence smaller-sized propagules will survive and develop into juveniles, while the opposite is true for harsher years. The recruitment of juveniles into the population is also density dependent. Adult survival is incorporated in the model but is not allowed to evolve.
(b). A detailed description of the model
Each female in the population produces a clutch of propagules every year during her adult lifespan; exactly what that clutch will look like is described by her strategy or her ‘genome’. In a given year, t, each female, i, produces a clutch of propagules whose individual masses, mi,t,j, are normally distributed with mean weight 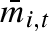 , and standard deviation σw,i. To investigate the possibility of adaptive coin flipping, we let the mean weight,
, and standard deviation σw,i. To investigate the possibility of adaptive coin flipping, we let the mean weight, 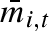 , be drawn from a uniform distribution with mean
, be drawn from a uniform distribution with mean 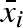 and standard deviation σb,i (see figure 1 for an illustration). In this way, a σb,i greater than zero gives an individual different mean propagule weight for different years, independent of other individuals. The parameters that describe the strategy of individual i are thus
and standard deviation σb,i (see figure 1 for an illustration). In this way, a σb,i greater than zero gives an individual different mean propagule weight for different years, independent of other individuals. The parameters that describe the strategy of individual i are thus 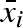 , σb,i (b for variation between years), and σw,i (w for variation within a year). In a stochastic environment, the particular type of propagule mass distribution may be of importance because it can potentially influence the optimal variation within a clutch (i.e. optimal bet-hedging strategy). We have therefore compared the result from the case with normally distributed propagule masses with the case of uniformly distributed propagule masses (
, σb,i (b for variation between years), and σw,i (w for variation within a year). In a stochastic environment, the particular type of propagule mass distribution may be of importance because it can potentially influence the optimal variation within a clutch (i.e. optimal bet-hedging strategy). We have therefore compared the result from the case with normally distributed propagule masses with the case of uniformly distributed propagule masses (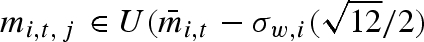 ,
, 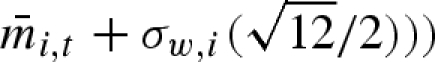 . The parameterization is chosen such that the standard deviation still is equal to σw,i. We will hereafter drop the individual index i for brevity.
. The parameterization is chosen such that the standard deviation still is equal to σw,i. We will hereafter drop the individual index i for brevity.
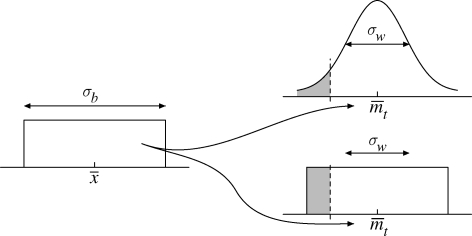
Figure 1.
An illustration of the parameters (‘genes’), σb, 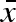 , and σw, in the females’ ‘genome’. σb is the standard deviation in a uniform distribution with mean
, and σw, in the females’ ‘genome’. σb is the standard deviation in a uniform distribution with mean 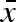 and corresponds to between year variation in the mean propagule size produced by each female. Every year, each female will ‘choose’ a value of her mean propagule size (
and corresponds to between year variation in the mean propagule size produced by each female. Every year, each female will ‘choose’ a value of her mean propagule size (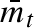 ), and the distribution within the clutch around that mean has a standard deviation of σw, independently of whether she produces a clutch with normally or uniformly distributed propagule sizes. The dashed line corresponds to an arbitrary mmin, which varies from one time step to another. All propagule sizes smaller than mmin (grey area) will not survive.
), and the distribution within the clutch around that mean has a standard deviation of σw, independently of whether she produces a clutch with normally or uniformly distributed propagule sizes. The dashed line corresponds to an arbitrary mmin, which varies from one time step to another. All propagule sizes smaller than mmin (grey area) will not survive.
In our parametrization, conservative bet-hedging corresponds to a large 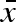 combined with zero within-clutch (σw) and between-year (σb) variability. Diversified bet-hedging, on the other hand, is characterized by a moderate
combined with zero within-clutch (σw) and between-year (σb) variability. Diversified bet-hedging, on the other hand, is characterized by a moderate 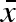 , large variation within a clutch (σw), but no variation between years (σb = 0). Finally, coin flipping corresponds to a moderate
, large variation within a clutch (σw), but no variation between years (σb = 0). Finally, coin flipping corresponds to a moderate 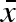 , a large σb and a zero σw.
, a large σb and a zero σw.
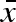 , σb and σw evolve independently of each other with a mutation probability equal to 0.001. The size of a mutation, δ, is drawn from a normal distribution with mean 0 and standard deviation 0.002 (δ ∈ N(0,0.0022)). Each clutch has a total mass of M (we use M = 100). In order to prevent females from producing propagules with negative weight, 0.01 was set as the smallest possible propagule size. All propagules smaller than this limit were given the weight 0.01 and then automatically labelled as non-viable. In the simulations, each individual produced propagules with weights drawn from the appropriate distribution until the total mass M was reached.
, σb and σw evolve independently of each other with a mutation probability equal to 0.001. The size of a mutation, δ, is drawn from a normal distribution with mean 0 and standard deviation 0.002 (δ ∈ N(0,0.0022)). Each clutch has a total mass of M (we use M = 100). In order to prevent females from producing propagules with negative weight, 0.01 was set as the smallest possible propagule size. All propagules smaller than this limit were given the weight 0.01 and then automatically labelled as non-viable. In the simulations, each individual produced propagules with weights drawn from the appropriate distribution until the total mass M was reached.
Each year, a smallest surviving propagule size, mmin,t, is drawn from a lognormal distribution with a mean value of 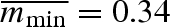 and a variance equal to 0.020, which gives a CV of 0.42. This corresponds to fluctuating weather conditions, where a small (large) mmin,t represents beneficial (poor) conditions (e.g. Einum & Fleming 2004). Propagule survival is a step-function, where all propagules in the clutch that are smaller than the threshold size mmin die and the other propagules develop into juveniles.
and a variance equal to 0.020, which gives a CV of 0.42. This corresponds to fluctuating weather conditions, where a small (large) mmin,t represents beneficial (poor) conditions (e.g. Einum & Fleming 2004). Propagule survival is a step-function, where all propagules in the clutch that are smaller than the threshold size mmin die and the other propagules develop into juveniles.
Juvenile survival is regulated according to the Beverton–Holt equation:
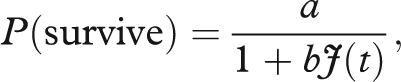 |
where 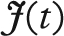 ) is the total number of juveniles produced in the population at time t, a is the maximum juvenile survival (a = 0.2) and b is the strength of density dependence (b = 5.3638 × 10−5 when adult survival is 0.01, b = 5.941 × 10−5 when adult survival is 0.1 and finally b = 9.1156 × 10−5 when adult survival is 0.4). The b value regulates the number of juveniles that are recruited into the population; therefore, it also regulates the total population size. The entire population grows (ignoring demographic stochasticity) according to:
) is the total number of juveniles produced in the population at time t, a is the maximum juvenile survival (a = 0.2) and b is the strength of density dependence (b = 5.3638 × 10−5 when adult survival is 0.01, b = 5.941 × 10−5 when adult survival is 0.1 and finally b = 9.1156 × 10−5 when adult survival is 0.4). The b value regulates the number of juveniles that are recruited into the population; therefore, it also regulates the total population size. The entire population grows (ignoring demographic stochasticity) according to:
where R(t) is the total number of recruits (surviving juveniles) and s is adult survival. The survival of juveniles and adults was implemented at the individual level, drawing one random number per individual and survival event. Since we compare three different adult survival rates, 0.01, 0.1 and 0.4, we use three different values of b in order to keep the population sizes at about the same size (about 3500 individuals).
3. Methods
Preparatory simulations of this model showed quick evolutionary convergence of the overall mean egg size (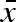 , figure 1), but a lot of genetic drift in the two variation parameters (σw and σb) due to weak selection (see below), which made identification of a possible evolutionarily stable strategy (ESS) difficult. To better evaluate the evolutionary dynamics, we calculated a ‘gradient landscape’ in the (σw, σb) trait space by averaging across a large number of short simulations. Starting with a population mean genotype at a given combination of σw and σb, we let the population dynamics, as described above, run for 10 time steps and measured the change in both parameters per time unit. After each 10 time steps, the whole distribution of genotypes was shifted to the same mean starting values and another 10 time steps were evaluated. Only the σw and σb values were back-shifted—the
, figure 1), but a lot of genetic drift in the two variation parameters (σw and σb) due to weak selection (see below), which made identification of a possible evolutionarily stable strategy (ESS) difficult. To better evaluate the evolutionary dynamics, we calculated a ‘gradient landscape’ in the (σw, σb) trait space by averaging across a large number of short simulations. Starting with a population mean genotype at a given combination of σw and σb, we let the population dynamics, as described above, run for 10 time steps and measured the change in both parameters per time unit. After each 10 time steps, the whole distribution of genotypes was shifted to the same mean starting values and another 10 time steps were evaluated. Only the σw and σb values were back-shifted—the 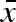 was left untouched. An initial time period of 20 000 time steps was allowed for transients and convergence of
was left untouched. An initial time period of 20 000 time steps was allowed for transients and convergence of 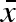 . Through this procedure, a stationary distribution of genotypes was developed at the fixed (σw, σb) point and repeated measurements (10 000 times) of the evolutionary rate gave a mean rate of change with sufficient accuracy. We thus got a measure of the evolutionary change per time unit of σw and σb, conditioned on the evolutionary convergence of
. Through this procedure, a stationary distribution of genotypes was developed at the fixed (σw, σb) point and repeated measurements (10 000 times) of the evolutionary rate gave a mean rate of change with sufficient accuracy. We thus got a measure of the evolutionary change per time unit of σw and σb, conditioned on the evolutionary convergence of 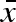 . The mean
. The mean 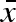 of all time steps was also calculated. This estimation procedure was repeated on a grid of equally spaced points in the (σw, σb) trait space (σw, σb = 0.01, 0.02, … ,0.15).
of all time steps was also calculated. This estimation procedure was repeated on a grid of equally spaced points in the (σw, σb) trait space (σw, σb = 0.01, 0.02, … ,0.15).
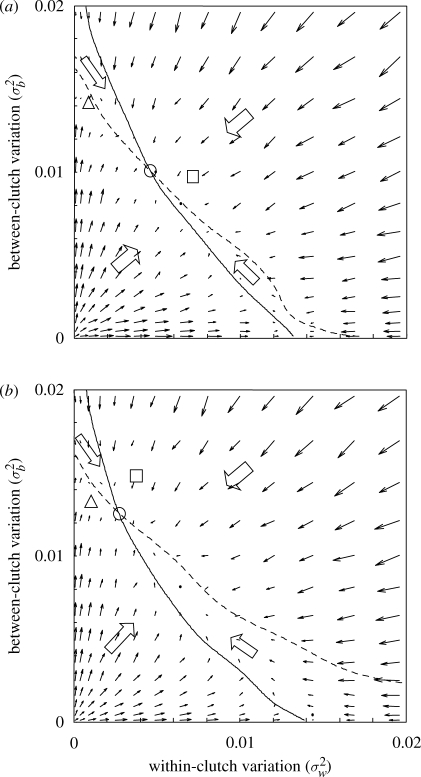
These evolutionary rate estimations resulted in a ‘gradient landscape’ (arrows, figure 2) in (σw, σb) trait space. We used these values to estimate the gradient nullclines, i.e. points in trait space where one parameter or the other has zero change. This was done through a weighted moving average of the grid values, with weights according to a two-dimensional Gaussian kernel with standard deviation equal to 0.01. The intersection of the two nullclines gives an estimated evolutionary equilibrium, and the surrounding gradient directions can be used to evaluate convergence stability.
Figure 2.
The rate of evolutionary change of the two variation parameters σw and σb, i.e. within- and between-clutch variation, respectively. The arrows indicate the direction of the mean rate of change from 10 000 simulations of 10 time steps each. A long (short) arrow corresponds to quick (slow) evolution. The solid and dashed lines are the nullclines of σw and σb evolutionary rates, respectively. The block arrows represent the principal directions of evolutionary change in each region of parameter space. The nullclines intersect at a convergent stable evolutionary equilibrium (solid circle). Only the case of intermediate adult survival is fully shown, but the convergent stable strategies of low survival (s = 0.01, square) and high survival (s = 0.4, triangle) are also depicted. (a) Normal within-clutch distribution. (b) Uniform within-clutch distribution. See text for further model and simulation details.
4. Results
In a constant environment, the strategy that produces the most surviving propagules will be selected, i.e. the ESS is a uniform clutch with minimal viable propagule mass: σw = σb = 0 and 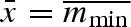 . This gives a clutch size of
. This gives a clutch size of 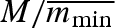 propagules with weight
propagules with weight 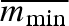 . However, in a variable, unpredictable environment, it is a high risk for females to produce only small propagules. Should the environment be too severe for her chosen propagule size, she will lose all her offspring that year. Therefore, a fixed propagule size is not necessarily selected for by natural selection.
. However, in a variable, unpredictable environment, it is a high risk for females to produce only small propagules. Should the environment be too severe for her chosen propagule size, she will lose all her offspring that year. Therefore, a fixed propagule size is not necessarily selected for by natural selection.
Figure 2 illustrates our main findings for the two cases of normal (figure 2a) and uniform (figure 2b) within-clutch distribution of propagule weights. The solid arrows indicate mean rates of evolutionary change of within-clutch variation (σw) and between-clutch variation (σb). The solid and dashed lines are the estimated nullclines of σb and σw selection, respectively. The two nullclines divide the parameter space into four regions of different general directions of evolutionary change (block arrows), from which we conclude that the nullcline intersection (solid circle) is a convergent stable evolutionary equilibrium. The square and triangle give the positions of similar evolutionary equilibria for low (s = 0.01) and high (s = 0.4) adult survival, respectively.
The overall mean propagule weight (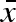 ) evolved freely in our simulations, but always converged relatively quickly and remained in close proximity to a value that was remarkably constant throughout (σw, σb) parameter space (not shown). The mean
) evolved freely in our simulations, but always converged relatively quickly and remained in close proximity to a value that was remarkably constant throughout (σw, σb) parameter space (not shown). The mean 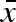 varied between 0.57 and 0.68 for all parameter combinations represented in figure 2, with the highest values close to the origin, where overall variation in propagule weight is the smallest. These values can be compared with the average minimal propagule weight
varied between 0.57 and 0.68 for all parameter combinations represented in figure 2, with the highest values close to the origin, where overall variation in propagule weight is the smallest. These values can be compared with the average minimal propagule weight 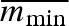 , which was always 0.34.
, which was always 0.34.
It can be concluded from our results that short-lived organisms (s = 0.01, square in figure 2) evolve to a strategy of relatively large propagule sizes, high total variance (σb2 + σw2) and a combination of both within- and between-clutch variation, i.e. a combination of conservative and diversified bet-hedging, as well as adaptive coin flipping. In contrast, more long-lived organisms with higher adult survival (s = 0.4, triangle in figure 2) converge to a strategy with lower total variance and very little within clutch variation, i.e. an almost pure coin-flipping strategy.
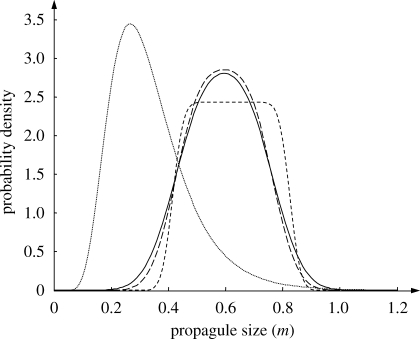
To illustrate the evolved strategies in more detail, figure 3 shows the total (between + within-clutch variation) probability distributions of the three ESSs for the case of a normal within-clutch distribution (figure 2a). The three distributions are rather similar in their position and variance, although the high adult survival case (short dashed line) has a more rectangular shape. This is because it is dominated by between-year variation, which has a uniform distribution (figure 1). For comparison, the distribution of mmin, i.e. the minimal viable propagule size, is also depicted (figure 3, dotted line). All three ESS distributions are clearly above the bulk of the mmin distribution, i.e. they are conservative bet-hedging strategies, but there is also substantial variation of propagule size. We conclude that variation in propagule weight is an important part of the evolved strategies.
Figure 3.
The distributions of propagule sizes corresponding to the convergent stable strategies marked in figure 2a. Each depicted distribution is a combination (convolution) of between- and within-clutch variation of propagule size, for the cases of low survival (s = 0.01, solid line), intermediate survival (s = 0.1, long-dashed line) and high survival (s = 0.4, short dashed line). For comparison, the distribution of the minimal viable propagule size, mmin, is also shown (dotted line).
Our preparatory simulations showed a lot of drift and the gradient landscapes (figure 2) offer an explanation. Especially in the case of normally distributed propagules (figure 2a), the two nullclines run close to each other and almost parallel through a large section of trait space. In the region between the two nullclines, any parameter combination is close to both nullclines and selection is weak in both parameters, which is verified by the very short arrows in this region. This region of weak selection coincides very well with the part of parameters space where our original simulations drifted back and forth. All simulations and calculations were performed on a linear (σw, σb) scale, but the results are depicted on the squared scale (σw2, σb2) to highlight that the ‘drift region’ is close to a straight line representing (almost) constant total variance of propagule size (σb2 + σw2).
We interpret the convergent stable points as ESS strategies, i.e. fitness maxima, once they are dominating the population, although we cannot directly measure the local curvature of the adaptive landscape. Our model has only density-dependent selection—there is no explicit frequency dependence. In a constant environment, density-dependent selection will maximize the population size (Charlesworth 1971), and any convergent stable strategy will be ESS. However, environmental fluctuations not only give a fluctuating selection pressure but also a variable population size, which makes possible frequency dependence. The dominant (or mean) strategy of the population dictates the population dynamics, and corresponding variable selection, in which new strategies are selected. This frequency dependence makes it difficult to characterize the ‘optimal’ strategy that will be selected—it is, for instance, no longer certain that population size will be maximized (Turelli & Petry 1980). ESS solutions are, however, still possible, as in any frequency-dependent game, and we here simply assume that the convergent stable strategies we find are ESS. We found no indications of evolutionary branching (sensu Geritz et al. 1998) and can think of no mechanistic reasons why the evolutionary equilibria should not be fitness maxima, i.e. uninvadable by alternative strategies.
To test the robustness of our results, we conducted some less complete, simulations with alternative model assumptions. First, we varied the average minimal propagule size (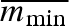 ). A doubling of
). A doubling of 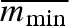 , keeping CV(mmin) the same, leads to a corresponding doubling of the evolved mean propagule size (
, keeping CV(mmin) the same, leads to a corresponding doubling of the evolved mean propagule size (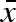 ), but the same coefficient of variation. Second, we changed the distribution of mmin values from lognormal to a (truncated) normal, all else being equal, which selected for much less variation in propagule size. This result is discussed below. Third, our standard model assumes propagule survival is a step function of the current environmental state (mmin). This was done for simplicity and to speed up simulations. However, a more gradual survival function is perhaps more realistic. We therefore tested a sigmoid survival:
), but the same coefficient of variation. Second, we changed the distribution of mmin values from lognormal to a (truncated) normal, all else being equal, which selected for much less variation in propagule size. This result is discussed below. Third, our standard model assumes propagule survival is a step function of the current environmental state (mmin). This was done for simplicity and to speed up simulations. However, a more gradual survival function is perhaps more realistic. We therefore tested a sigmoid survival: 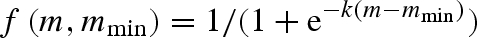 , where the parameter k determines the steepness of the survival function. A shallow slope (k = 10), selected for strategies without variation in propagule weight, i.e. no bet-hedging. This is not surprising since even the smallest propagules (m close to 0) have more than 3% chance of survival at the average mmin value (mmin = 0.34). A steeper slope (k = 30) gave results on par with our standard model (
, where the parameter k determines the steepness of the survival function. A shallow slope (k = 10), selected for strategies without variation in propagule weight, i.e. no bet-hedging. This is not surprising since even the smallest propagules (m close to 0) have more than 3% chance of survival at the average mmin value (mmin = 0.34). A steeper slope (k = 30) gave results on par with our standard model (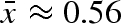 , CV(propagule weight) ≈ 0.14). We conclude that our choice of a step function is not critical to our results, but which strategy is selected does depend on the steepness of the survival function.
, CV(propagule weight) ≈ 0.14). We conclude that our choice of a step function is not critical to our results, but which strategy is selected does depend on the steepness of the survival function.
5. Discussion
We found that the convergent stable ESS, at our chosen level of environmental variability, is to have a mean propagule (egg or seed) size (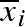 ) that is almost twice the average minimum viable mass, some year-to-year variation (σb > 0) and some variation within a clutch (σw > 0). This corresponds to a combination of conservative and diversified bet-hedging as well as ‘adaptive coin flipping’ (Cooper & Kaplan 1982; Kaplan & Cooper 1984), where the coin determines the mean propagule size any given year, drawn from a distribution determined by the individual's heritable strategy.
) that is almost twice the average minimum viable mass, some year-to-year variation (σb > 0) and some variation within a clutch (σw > 0). This corresponds to a combination of conservative and diversified bet-hedging as well as ‘adaptive coin flipping’ (Cooper & Kaplan 1982; Kaplan & Cooper 1984), where the coin determines the mean propagule size any given year, drawn from a distribution determined by the individual's heritable strategy.
There is some variation in ESS depending on the longevity of adults. Low adult survival, i.e. short lifespans, tends to select for higher variability in propagule size, which is a more cautious strategy. This makes sense, since short-lived organisms only have one or very few chances to reproduce and cannot afford a wasted season. A long-lived organism, on the other hand, may reproduce several times and can take higher risks. However, the density dependence in our model means that higher adult survival implies lower juvenile survival, which also might have an effect on the selected strategy. Either way, a high adult survival means reproduction comprises a relatively small portion of total fitness (survival + reproduction), and the organism is fairly insensitive to a variable reproductive success.
Our results differ from earlier theoretical studies (e.g. McGinley et al. 1987; Forbes 1991; Einum & Fleming 2004, 2007), which predict that the optimal strategy should be to form a clutch with equally sized propagules, at a size safely above the mean minimal viable size. This corresponds to pure conservative bet-hedging, or an ‘always play it safe’ strategy. Einum & Fleming (2004) found only weak selection for a slightly diversified strategy at a high level of environmental stochasticity, comparable to the level used here. In contrast, we find natural selection to favour fairly high amounts of variation in propagule size. In this context, we think that it is important to note that Einum & Fleming (2004) used normally distributed environmental fluctuations, whereas we used a lognormal distribution that has a thicker tail at high values. The probability of very high levels of minimal viable propagule size, mmin, is thus higher in our model, which makes it more worthwhile to produce a few very large propagules. By producing many small and just a few large propagules, a female will have a larger variation in propagule sizes than if she produced only large propagules. However, by increasing variation, she reduces her fitness costs compared with the costs of producing only several large propagules. We conclude that the exact distribution of environmental variability, especially the likelihood of extreme events, can have dramatic effects on the optimal bet-hedging strategy (this conclusion is supported by test simulations, see above).
Selection on the variation parameters (σw and σb, figure 2) is very weak around the ESSs and in stretched out regions corresponding to more or less constant total variance (σb2 + σw2 constant). Our model is free of genetic constraints or other trade-offs between the evolving parameters, which allowed for a lot of genetic drift along this region of weak selection. This means that several combinations of within-clutch variation (σw) and between-clutch variation (σb) are possible evolutionary outcomes in any real system. Should there be constraints of any kind, the set of possible evolutionary endpoints would probably be much smaller. We find small, yet notable differences between the two tested statistical distributions. In the case of normally distributed propagule sizes within a clutch, the two parameters σb and σw are more or less interchangeable (figure 2a), whereas a uniform distribution gives selection more towards between-clutch variation, i.e. a coin-flipping strategy (figure 2b). It is beyond the scope of this study to make a more thorough investigation of the impact of the actual shape of the distributions involved. We nevertheless conclude that distribution shape, as well as even weak genetic constraints, can be of importance for what kind of bet-hedging strategy will evolve.
Also of importance is the slope of the survival function, where a steep slope promotes more variable offspring sizes. This is not surprising since a very shallow slope makes the difference in survival between different propagule sizes negligible and variation in size of little value. The exact shape of the survival function in natural systems can be very hard to measure (but see Einum & Fleming 2000), but our results point at its potential importance for which type of strategy evolves.
Although some organisms, for example different species of salmon (Einum & Fleming 2004), have been found to have clutches that are relatively uniform and therefore conform with earlier theoretical results, this is not true for all organisms. In arthropods, as reviewed by, for example, Fox & Czesak (2000), egg sizes have been found to vary within and between clutches. Variation in propagule (egg or seed) masses have also been found in, tree frogs (Crump 1981), trematodes (Poulin & Hamilton 2000) and in plants (e.g. Janzen 1977). Fox & Czesak (2000) conclude that much of the variation seen within and between clutches in arthropods is non-adaptive variation due to morphological or physiological constraints in females. Our model suggests an alternative explanation; this variation might be an adaptive strategy, whereby genetically identical individuals produce clutches with weight differences within a clutch or between years. The question is then: how can we ever distinguish adaptive variation from non-adaptive variation due to developmental uncertainty? According to Philippi & Seger (1989), with reference to McGinley et al. (1987), it is, in theory, easier to distinguish adaptive variation among clutches from environmental noise than to distinguish adaptive within clutch variation from non-adaptive variation.
This model in particular, and bet-hedging theory in general, deals with the strategic choices of individuals in an unpredictable and stochastic environment. However, some organisms live in an environment that varies in an autocorrelated fashion, such that current environmental conditions are a good predictor of the future environment. In such cases, an individual can use cues from the current environment to predict the future success of alternative strategies, for example, to determine the appropriate propagule size (studied theoretically by Koops et al. 2003). Thus, a variable environment does not always select for bet-hedging strategies. Conversely, not all instances of a variable offspring size should necessarily be regarded as bet-hedging strategies. For example, some female insects shield their eggs with their bodies for some time after each clutch is produced, increasing the survival probabilities of the eggs. Eggs deposited in the middle of the clutch have a higher probability of surviving since they are shielded the most effectively. Those eggs are also the largest in each clutch (Kudo 2006). This is not, to our minds, bet-hedging—the female is simply investing the most in the safest place. Nevertheless, it would be interesting to investigate how behaviours such as parental care, plasticity and the use of environmental cues evolve in concert with bet-hedging strategies.
Our model specifically deals with the question of how a female should distribute resources to her eggs or seeds, but this is just one way to exemplify the general problem of evolution of bet-hedging strategies. Bet-hedging strategies are always facing the problem of lowering variance in fitness in order to maximize the geometric mean. Similar to Cooper and Kaplan's coin-flipping strategy, we show how increased variation at an individual level can be the most adaptive strategy to use. The individual females using this strategy will experience a lot of variation in reproductive output (and hence also in fitness) both within and between years. However, if the gene for this behaviour is present in many individuals, a great diversity of propagule sizes will be produced and at least some of them will survive to reproduce. From the gene's perspective, variation in total yearly fitness is thus minimized and long-term fitness maximized.
The model presented in this paper is, to our knowledge, the first to demonstrate that an optimal female strategy may be to produce propagules with size variation within a clutch as well as between clutches. There exists empirical support for such variation (e.g. Janzen 1977; Crump 1981; McLain & Mallard 1991; Fox & Czesak 2000; Poulin & Hamilton 2000) and we hope that, given our results, more empirical studies will look for this kind of variation in propagule sizes. At a more general level, we have shown the possibility for a combination of diversified bet-hedging and adaptive coin-flipping strategies, which calls for further theoretical work on more complex behaviours as adaptations to an unpredictable environment.
Acknowledgements
We thank Per Lundberg, Maria Servedio, Amanda Chunco, Sumit Dhole, Sarah Diamond, Alicia Frame, Jonathan Rowell and an anonymous reviewer, whose comments significantly improved the manuscript. N.J. and J.R. were financially supported by the Swedish Research Council.
References
- Brown J. S., Venable D. L.1986Evolutionary ecology of seedbank annuals in temporally varying environments. Am. Nat. 127, 31–47 (doi:10.1086/284465) [Google Scholar]
- Charlesworth B.1971Selection in density-regulated populations. Ecology 52, 469–474 (doi:10.2307/1937629) [Google Scholar]
- Cohen D.1966Optimizing reproduction in a randomly varying environment. J. Theor. Biol. 12, 119–129 (doi:10.1016/0022-5193(66)90188-3) [DOI] [PubMed] [Google Scholar]
- Cooper W. S., Kaplan R. H.1982Adaptive ‘coin-flipping’: a decision-theoretical examination of natural selection for a random individual variation. J. Theor. Biol. 94, 135–151 (doi:10.1016/0022-5193(82)90336-8) [DOI] [PubMed] [Google Scholar]
- Crump M. L.1981Variation in propagule size as a function of environmental uncertainty for tree frogs. Am. Nat. 117, 724–737 (doi:10.1086/283755) [Google Scholar]
- Einum S., Fleming I. A.2000Highly fecund mothers sacrifice offspring survival to maximise fitness. Nature 405, 565–567 (doi:10.1038/35014600) [DOI] [PubMed] [Google Scholar]
- Einum S., Fleming I. A.2004Environmental unpredictability and offspring size: conservative versus diversified bet-hedging. Evol. Ecol. Res. 6, 443–455 [Google Scholar]
- Einum S., Fleming I. A.2007Of chickens and eggs: diverging propagule size of iteroparous and semelparous organisms. Evolution 61, 232–238 (doi:10.1111/j.1558-5646.2007.00020.x) [DOI] [PubMed] [Google Scholar]
- Forbes L. S.1991Optimal size and number of offspring in a variable environment. J. Theor. Biol. 150, 299–304 (doi:10.1016/S0022-5193(05)80429-1) [Google Scholar]
- Fox C. W., Czesak M. E.2000Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 45, 341–369 (doi:10.1146/annurev.ento.45.1.341) [DOI] [PubMed] [Google Scholar]
- Geritz S. A. H., Kisdi É., Meszéna G., Metz J. A. J.1998Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol. Ecol. 12, 35–57 (doi:10.1023/A:1006554906681) [Google Scholar]
- Hopper K. R.1999Risk-spreading and bet-hedging in insect population biology. Annu. Rev. Entomol. 44, 535–560 (doi:10.1146/annurev.ento.44.1.535) [DOI] [PubMed] [Google Scholar]
- Ivarsson H., Ripa J., Lundberg P.2005Evolution of multi-stage dormancy in temporally autocorrelated environments. Evol. Ecol. Res. 7, 1–13 [Google Scholar]
- Janzen D. H.1977Variation in seed size within a crop of a Costa Rican Mucuna andreana (Leguminosae). Am. J. Bot. 64, 347–349 (doi:10.2307/2441978) [Google Scholar]
- Kaplan R. H., Cooper W. S.1984The evolution of developmental plasticity in reproductive characteristics: an application of the ‘adaptive coin-flipping’ principle. Am. Nat. 123, 393–410 (doi:10.1086/284211) [Google Scholar]
- Koops M. A., Hutchings J. A., Adams B. K.2003Environmental predictability and the cost of imperfect information: influences on offspring size variability. Evol. Ecol. Res. 5, 29–42 [Google Scholar]
- Kudo S.2006Within-clutch egg-size variation in a subsocial bug: the positional effect hypothesis. Can. J. Zool. 84, 1540–1544 (doi:10.1139/Z06-163) [Google Scholar]
- McGinley M. A., Temme D. H., Geber M. A.1987Parental investment in variable environments: theoretical and empirical considerations. Am. Nat. 130, 370–398 (doi:10.1086/284716) [Google Scholar]
- McLain D. K., Mallard S. D.1991Sources and adaptive consequences of egg size variation in Nezara viridula (Hemiptera: Pentatomidae). Psyche 98, 135–164 (doi:10.1155/1991/10123) [Google Scholar]
- Philippi T., Seger J.1989Hedging one's evolutionary bets, revisited. Trends Ecol. Evol. 4, 41–44 (doi:10.1016/0169-5347(89)90138-9) [DOI] [PubMed] [Google Scholar]
- Poulin R., Hamilton W. J.2000Egg size variation as a function of environmental variability in parasitic trematodes. Can. J. Zool. 78, 564–569 (doi:10.1139/cjz-78-4-564) [Google Scholar]
- Roff D. A.1992The evolution of life histories theory and analysis New York: Chapman and Hall [Google Scholar]
- Seger J., Brockmann H. J.1987What is bet-hedging? In Oxford surveys in evolutionary biology, vol. 4 (eds Harvey P. H., Partridge L.), pp. 182–211 Oxford, UK: Oxford University Press [Google Scholar]
- Slatkin M.1974Hedging one's evolutionary bets. Nature 250, 704–705 (doi:10.1038/250704b0) [Google Scholar]
- Turelli M., Petry D.1980Density-dependent selection in a random environment—an evolutionary process that can maintain stable-population dynamics. Proc. Natl Acad. Sci. 77, 7501–7505 (doi:10.1073/pnas.77.12.7501) [DOI] [PMC free article] [PubMed] [Google Scholar]