Abstract
Acoustic communication is fundamental in avian territory defence and mate attraction. In urban environments where sound transmissions are more likely to be masked by low-frequency anthropogenic noise, acoustic adaptations may be advantageous. However, minor modifications to a signal could affect its efficacy. While recent research has shown that there is divergence between songs from noisy and quiet areas, it is unknown whether these differences affect the response to the signal by its receivers. Here, we show that there is a difference in spectral aspects of rural and urban song in a common passerine, the great tit Parus major, at 20 sites across the UK. We also provide, to our knowledge, the first demonstration that such environmentally induced differences in song influence the response of male territory holders. Males from quiet territories exhibited a significantly stronger response when hearing song from another territory holder with low background noise than from those with high background noise. The opposite distinction in response intensity to homotypic versus heterotypic song was observed in males from noisy territories. This behavioural difference may intensify further signal divergence between urban and rural populations and raises important questions concerning signal evolution.
Keywords: song, great tit, urban noise, playback response, adaptation, signal divergence
1. Introduction
The urban environment provides unique selection pressures upon native flora and fauna, including air (Isaksson et al. 2005), noise (Brumm 2004) and light pollution (Fuller et al. 2007), as well as novel predators (Lepczyk et al. 2004). Ambient noise presents particular challenges for birds inhabiting cities owing to the integral part that acoustic communication plays in their life history (for reviews, see Brumm & Slabbekoorn 2005; Patricelli & Blickley 2006; Warren et al. 2006; Slabbekoorn & Ripmeester 2007).
In order to survive and breed in a city, birds may have to adapt their behaviour to transmit an acoustic signal effectively. Song is a sexually selected trait used to attract a mate (Baker et al. 1986) and to defend a territory (Krebs et al. 1978), so the masking of acoustic signals by anthropogenic noise could have a considerable effect on both inter- and intra-sexual selection. Even slight adjustments in song may lead to a change in the transmission efficacy or response (e.g. Nelson 1988, 1989). For example, in many species, low-frequency (pitch) signals typically depict hostility and are often used in threatening displays towards rivals (Morton 1977). Therefore, a male who reduces energy in low-frequency notes, or excludes them completely, may suffer a decrease in signal efficacy, less effective territorial defence and be perceived as less attractive by potential mates.
Variation in male song characteristics between individuals may encompass song rate, length, amplitude, repertoire size and frequency changes (Byers 2007), providing several possible targets for selection (Gil & Gahr 2002). Acoustic signals exchanged between males in defence of a territory may be associated with territory quality (e.g. Arvidsson & Neergaard 1991; Hoileitner et al. 1995) as well as male quality (Baker et al. 1986). Territory quality is an important aspect of mate choice where foraging is limited to the breeding territory when provisioning young in great tits (Baker et al. 1987) as well as sedge warblers Acrocephalus schoenobaenus (Buchanan & Catchpole 1997) and willow warblers Phylloscopus trochilus (Nyström 1997). Therefore, it is paramount that the receiver's perception of male song quality accurately reflects the signaller's true quality.
Many passerines engage in song matching, where the male replies to a stimulus using the most similar song in his repertoire (Falls et al. 1982). This behaviour, common in great tits, is thought to signify either an increase in aggression (Krebs et al. 1981; Vehrencamp 2001) or to whom the signal is directed (Peake et al. 2005). Modifications to song could therefore reduce the degree of similarity and the ability to song match.
Recently, Slabbekoorn & Peet (2003) showed that, within a city, male great tits from noisy territories sang at a higher minimum frequency than those holding quieter territories. Temporal changes have also been demonstrated between sites with different noise levels: urban birds sang shorter songs with shorter inter-song intervals and also had a shorter first note compared with rural birds (Slabbekoorn & den Boer-Visser 2006). Such specific adaptations to these acoustic environments could have implications for signal perception.
Great tits are socially monogamous territorial song birds (Cramp & Perrins 1993). They are present throughout Europe and inhabit a variety of environments including cities. Their song, sung only by males, consists of a basic element of between one and five notes, which is repeated several times to form a strophe. After a short pause, the strophe is repeated one or more times, creating a song. The frequency, timing and number of notes in a song vary within an individual's repertoire, each variety being a different ‘song type’. Generally, males have a repertoire consisting of between one and nine song types (Krebs et al. 1978; McGregor et al. 1981; McGregor & Krebs 1982; Slabbekoorn & Peet 2003). As great tits can disperse up to 3–5 km (Greenwood et al. 1979; Verhulst et al. 1997), the likelihood is that the natal territory, where the songs are learnt, will have a different level of background noise to the breeding territory. Therefore, an individual's repertoire may not contain the optimal songs for their post-dispersal breeding environment. Here, we investigate differences in song characteristics between territories with high and low levels of background noise. We also use playback of song to examine the effect of signal divergence on the response of both urban and rural males to simulated intruders from territories of low and high background noise.
2. Methods
(a). Song measurements
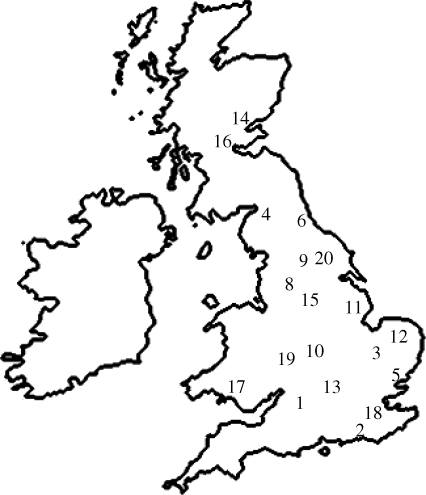
Great tit song was recorded in 20 different cities spanning mainland Great Britain between 25 February and 23 May 2008 (figure 1).
Figure 1.
A map of the UK showing the locations of the 20 cities used as sites in this study. 1, Bath; 2, Brighton; 3, Cambridge; 4, Carlisle; 5, Colchester; 6, Durham; 7, Exeter; 8, Halifax; 9, Harrogate; 10, Leamington Spa; 11, Lincoln; 12, Norwich; 13, Oxford; 14, Perth; 15, Rotherham; 16, Stirling; 17, Swansea; 18, Tunbridge Wells; 19, Worcester; 20, York.
The most southerly sites were visited first, and the study progressed north in an effort to move with the changing season and so minimize the effect of differing stages in breeding season. Two territorial males in each city were selected: one male with a territory within 1 km of the city centre and another with a territory approximately 4 km from the centre. The background noise levels of each territory were checked before any song was recorded to confirm that the site closer to the centre had a higher level of noise than the other. These territories were given the arbitrary labels ‘urban’ and ‘rural’, respectively (see table 1 for average noise measurements). Each pair of recording sites (a pair consisting of one rural and one urban site) was approximately 3 km apart (range 2.1–3.6 km; mean 3.0 km). Similarly, the playback sites were, on average, 3 km apart (range 1.7–3.8 km, mean 3.0 km). This reduces the likelihood of altered responses as a result of distance and local dialects (Baker et al. 1987), but is adequate to escape urban noise in a small city.
Table 1.
The range and mean noise measurements taken on rural and urban sites.
| site | minimum (dB) | maximum (dB) | mean (dB) |
|---|---|---|---|
| rural (recording) | 37 | 49 | 43 |
| urban (recording) | 48 | 68 | 57 |
| rural (playback) | 37 | 49 | 43 |
| urban (playback) | 46 | 68 | 57 |
Noise levels were averaged from three measurements, each taken over 15 s from 1 m above ground level at 07.00 and again at 08.00 using a CEM DT-805 sound meter (A-weighted; reference level 20 µPa) on the morning the recording took place. Each male's singing behaviour was observed to estimate territory boundaries. Noise-level measurements were then obtained from near the centre of the territory. The same sound meter was used throughout. Three readings were taken at each site from the same location, the noise meter pointing in three different directions, first at the greatest apparent source of noise and then at 120° clockwise and 120° anticlockwise from this point. The mean of these three values was taken to minimize the error (approx. 1.5 dB) of the meter.
Recordings were made at a quiet time of day, between sunrise and the start of the rush hour, to avoid high background noise. Songs were recorded from a distance of 5–10 m, using a Marantz (Longford, Middlesex, UK) CP430 tape recorder, with a Sennheiser (Wedemark, Lower Saxony, Germany) ME67 unidirectional microphone. Songs were analysed using Avisoft SASLAB Pro v.4.40 (Avisoft Bioacoustics, Berlin, Germany). We used a sampling rate of 22 050 Hz, and fast Fourier transform length was set at 256 with 50 per cent Frame in a Hamming Window, providing a resolution of 87 Hz and 11 ms (Brumm & Slater 2006).
Before analysis, background noise at a lower frequency than the song was removed from the spectrogram using a high-pass finite impulse response filter in AviSoft SASLAB Pro. Cut-off frequencies for each song were decided after visual inspection of the sonogram (settings as above) so as to not remove any song unintentionally. As recordings were carried out at a quiet time of day, there was no overlap between noise and the notes of the song. Six song parameters were measured: duration of note, duration of interval between each note, peak frequency (the frequency sung at the highest amplitude), bandwidth, minimum frequency and maximum frequency. This was done using the automatic parameter measurement tool, which was set to a −30 dB threshold, and measured the spectral characteristics at the beginning, middle and end of each note. The first four of these variables were averaged for each individual male. The minimum and maximum frequency data were examined for the most extreme value in each song. The mean of these values were calculated so that each bird had an average minimum and an average maximum frequency. All data were log transformed prior to analysis to ensure normality and to stabilize variances.
(b). Playback of rural versus urban song
Playback experiments were carried out on two new individuals, from different rural and urban territories (at least 250 m from recording territories), in the same cities from which the songs had been recorded. For each location (rural and urban), the lowest two-note song type was isolated from the recording, with low-frequency background noise removed using a high-pass filter (see above), and these two notes were repeated to make eight-note strophes. The average length of the silence between strophes was calculated from the original recording, and after this, the eight-note strophe was repeated. A total of 5 min of artificial urban or rural song was thereby created (e.g. Martin-Vivaldi et al. 2004; Bolton 2007; Osiejuk et al. 2007). Both artificial songs were played to both playback males using an SME-AFS (Saul Mineroff Electronics, Inc., Elmont, NY, USA) portable field speaker. A break of 20 min was included between the treatments, as our preliminary experiments showed that this provided sufficient time for the bird to return to its original behaviour and stop reacting to the playback/speaker. This is within the range used in other similar playback studies (e.g. 1 min (Bolton 2007), 10 min (Rios-Chelen & Garcia 2007), one to three days (Osiejuk et al. 2007)). The first song (rural or urban) to be played back was alternated at each consecutive site. The sound pressure level of playback songs was measured using the same sound meter and settings as in the noise measurements and was 68–69 dB at 10 m from the speaker (Blumenrath & Dabelsteen 2004). Figure 2 shows an example of the experimental set-up.
Figure 2.
A diagram showing the experimental set-up of the playback experiment.
Playback commenced when the focal male came within 25 m of the speaker. Behaviour was recorded every 5 s during the 5 min playback experiment, along with an estimate of the distance from the speaker. The experiments were all carried out by the same person to minimize subjectivity in distance estimations. The recorded behaviours were: flying towards the speaker, flying across the speaker, flying away from the speaker, alternating song with the playback song, overlapping song with the playback song and calling. From these data, five behavioural measures were extracted: (i) the latency to overlap the song on the tape (hereafter ‘LatOver’), (ii) the latency to fly towards the speaker (LatFly), (iii) the amount of time spent within a 5 m radius (Within5); on rare occasions when the bird was within 5 m but was not interacting with the tape (e.g. feeding), this time was not included, (iv) the closest approach the bird made towards the speaker (Approach), and (v) the amount of time spent not responding to the tape, here defined as the amount of time during the playback that the bird was not recorded participating in any of the recorded behaviours (Dormant). Time variables (i–iii and v) were measured in seconds, and the distance variable (iv) was measured in metres.
Each measure was tested for correlation with the distance from the recording site to control for neighbour–stranger discrimination. For each bird, the time or distance measurement recorded in response to the urban playback was subtracted from that recorded in response to the rural playback. This produced five variables describing the difference in response to the two playbacks. All difference variables were log transformed before analysis to acquire a distribution nearing normality. Variables that were significantly correlated with background noise of the territory were entered into a multivariate canonical variate analysis (CVA, also known as discriminant function analysis).
3. Results
(a). Noise and song measurements
Mean noise levels recorded were significantly different between urban and rural recording sites (paired t-test: t = −11.072, p < 0.001; table 1) and urban and rural playback sites (paired t-test: t = −8.962, p < 0.001; table 1).
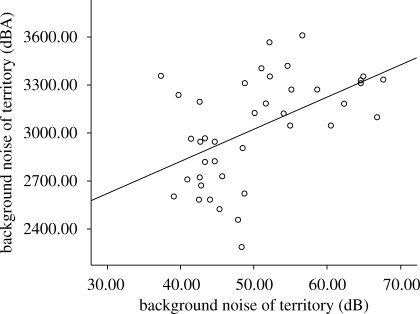
There was a significantly positive correlation between average minimum frequency and background noise (figure 3). Urban males also had a significantly higher average minimum frequency than rural males (mean difference: 478 Hz), and there was a non-significant trend for peak frequency to be significantly higher in urban males than in rural males (table 2). No other significant trends were identified among the spectral song characteristics. No temporal aspect significantly correlated with the level of background noise, although the average note duration length did show a non-significant negative trend with increasing noise (table 2).
Figure 3.
A scatterplot showing the relationship between the average minimum frequency of each male's song and the background noise in the centre of his territory.
Table 2.
Results of correlation analysis with background noise (n = 40) and a paired t-test between the two populations (for each group, n = 20) for each of the song variables. Significant values, after Bonferroni correction is applied, are indicated with asterisks.
| type of measure | variable | correlation with background noise |
paired t-test between rural and urban populations |
||
|---|---|---|---|---|---|
| r | adjusted p | t | adjusted p | ||
| temporal | average duration of note | −0.358 | 0.138 | 1.478 | 0.936 |
| average interval between notes | −0.283 | 0.462 | 1.231 | 1.000 | |
| spectral | average minimum frequency | 0.508 | 0.006* | −7.395 | <0.002* |
| average maximum frequency | 0.150 | 1.000 | −1.084 | 1.000 | |
| average peak frequency | 0.187 | 1.000 | −2.847 | 0.060 | |
| average bandwidth | 0.111 | 1.000 | 0.600 | 1.000 | |
(b). Playback of rural versus urban song
None of the original variables were shown to correlate with the distance between the playback and recording site after Bonferroni correction (all p > 0.2), and neither did any of the variables show any significant difference between cities. Therefore, for the purposes of this analysis, the birds were treated as independent from each other.
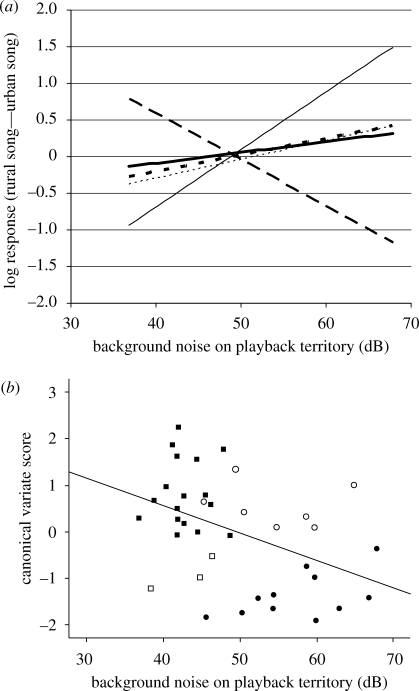
Background noise was significantly positively correlated with three response variables LatOver (r = 0.381, p = 0.018), Approach (r = 0.393, p = 0.015) and Dormant (r = 0.410, p = 0.011; figure 4a): male great tits holding quieter territories were significantly faster to sing over the playback song (LatOver) and approached the speaker more closely (Approach) when hearing a song taken from a quiet territory than from a noisy territory. Rural birds also spent more time overall interacting with the playback song when it was from a quiet territory (Dormant). Thus, birds from noisier territories were faster to sing over the playback song, approached the speaker more closely and spent more time interacting with playback song when it was from a noisy territory.
Figure 4.
(a) A graph showing the correlations of background noise with each of the five difference variables (Approach: thin solid line (r = 0.393, p = 0.015), the closest approach to the speaker; LatFly: dotted line (r = 0.275, p = 0.095), the latency to fly towards the speaker; LatOver: short dash line (r = 0.381, p = 0.018), the latency to overlap song with the tape; Within5: long dash line (r = −0.367, p = 0.023), the amount of time spent within 5 m of the speaker; Dormant: thick solid line (r = 0.410, p = 0.011), the amount of time spent not responding to the playback stimulus). The y-axis represents the difference in response to the rural and urban songs by an individual: a high value indicates a higher value in the rural response than the urban response. (b) A scattergraph showing the correlation between the canonical variate score and the background noise of the playback territory (r = −0.432, p = 0.007). Data points represent individual males; closed circles, urban birds; closed squares, rural birds. Empty shapes represent individuals that the model classified incorrectly (open circles, urban birds; open squares, rural birds).
The response variable Within5 was significantly negatively correlated with the background noise of the territory (r = −0.367, p = 0.023; figure 4a). Thus, males holding quieter territories spent more time close to the speaker when hearing song from a quiet territory than a noisy one and males from noisier territories spent more time close to the speaker when hearing song from a noisy territory than a quiet one.
There was a non-significant trend for great tits with quieter territories to fly towards the speaker more quickly when hearing song from a quiet territory, than when hearing song from a noisy territory, and vice versa for great tits from noisier territories (LatFly: r = 0.275, p = 0.095; figure 4a).
Significant differences in the behavioural variables were shown by CVA between males from quiet and noisy territories (Wilks' Lambda: 0.732, p = 0.032). The model correctly identified 73.7 per cent of cases. Of these, 85 per cent of the rural birds and 61.1 per cent of urban birds were classified correctly.
As there were two populations, one canonical variate was identified, accounting for 100 per cent of the variance. Of the variables comprising this canonical variate, the difference in time spent reacting within 5 m of the speaker had the highest loading (Within5: 0.865). Difference in latency to sing at the same time as the tape (LatOver: −0.282), difference in the closest approach to the speaker (Approach: 0.105) and difference in time spent not reacting (Dormant: −0.174) contributed in smaller amounts to the canonical variate. A correlation between the canonical variate and background noise showed a strong negative relationship (r = −0.432, p = 0.007; figure 4b), demonstrating that the differences in behavioural responses observed between the rural and urban populations are associated with background noise.
4. Discussion
We found consistent detectable differences between the songs of male great tits occupying territories of high and low background noise across the UK. This is consistent with other studies of the same species in continental Europe (Slabbekoorn & Peet 2003; Slabbekoorn & den Boer-Visser 2006), as well as in house finches Carpodacus mexicanus in California (Fernández-Juricic et al. 2005) and song sparrows Melospiza melodia in Oregon (Wood & Yezerinac 2006). However, we found this distinction present over just a few kilometres, between the city centre and the quieter outskirts, within the dispersal radius for young of this species (Greenwood et al. 1979; Verhulst et al. 1997). Furthermore, we found that territorial males responded at a significantly lower level when hearing song from a territory where background noise differed from their own.
The most prominent difference between rural and urban song was the minimum frequency. This was significantly lower in great tit song from quieter territories than noisy ones, and was significantly positively correlated with territory background noise.
Variation in noise levels at the spatial scales found here may pose problems for locally dispersing birds seeking to establish territories. Traffic noise in cities is generally low in frequency, and therefore creates direct competition in that acoustic space (an example of which can be seen in figure 5). In a multi-species study, Rheindt (2003) found a correlation between motorway noise and the dominant frequency of a species inhabiting the area around the road, suggesting that traffic noise causes declines in species that sing with a lower frequency song. Yearling great tits may avoid this situation by preferentially dispersing to areas with noise levels similar to their natal territory. However, this may not be possible: suitable habitat may be limited or already occupied and therefore, a dispersing male may have to occupy a territory where the noise is at a level different from their natal territory. The potential fitness detriment of decreased signal efficacy imposed upon males moving into noisier territories may be reduced if they were to avoid low frequencies.
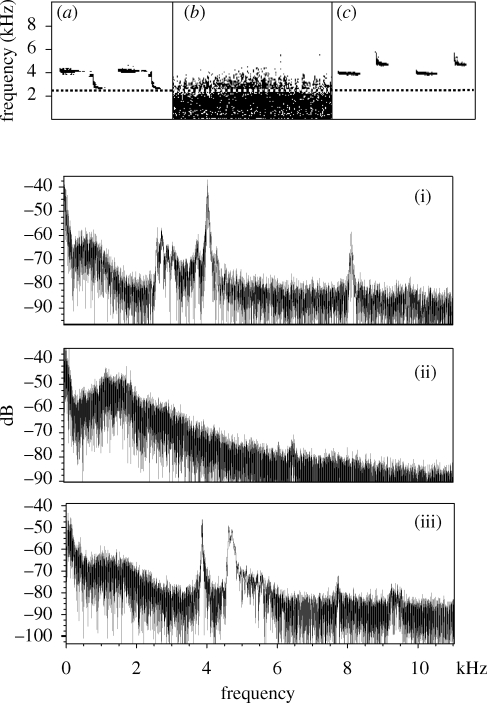
Figure 5.
(a–c) Sonograms of recordings taken in Cambridge and (i–iii) their associated power spectra. (a) Great tit at rural site (power spectrum (i)). (b) The background noise at the urban site during rush hour from exactly the same location as where (c) was recorded (power spectrum (ii)). (c) Great tit song at urban site at a quiet time of day (power spectrum (iii)). The dashed line represents the minimum frequency of the rural song. The background noise below 2 kHz has been removed from the sonograms to allow for clarity, but background noise is present in the power spectra.
Previously it has been found that urban great tits shorten the first note of their song (Slabbekoorn & den Boer-Visser 2006). However, neither this, nor any other temporal measure, was found to correlate with background noise, although we found a non-significant trend suggesting that great tits in noisy territories sang shorter notes overall.
Not only were there differences in signal characteristics in relation to background noise, but our analysis also clearly shows that male great tits respond significantly more strongly to songs from territories with noise levels similar to their own. This suggests that great tits dispersing to areas with different background noise levels from that which they have experienced previously will be at a competitive disadvantage in territorial disputes. A weaker response to songs may allow neighbouring males to encroach on a new arrival's territory. Alternatively, a reduced response to intruders may decrease the incidence of territorial disputes and permit larger, or overlapping, territories.
Birds from quieter territories show sufficient distinction in their behaviour towards the homo- and heterotypic songs, that the model correctly identified 85 per cent of them as rural individuals. Similarly, males from noisier territories showed a significantly lower response to songs from quieter territories. In both cases, great tits were slower to overlap their song with the playback song, did not approach the speaker as closely, spent less time within a 5 m radius of the playback song and spent less time reacting to the playback song throughout when that song was of a male with different level of background noise from their own.
However, the model was only able to identify 61 per cent of urban birds correctly from their behaviour during playback. This could be because of a difference in the behavioural plasticity of both rural and urban birds. A varying response to differing playback frequency has been shown in field sparrows Spizella pusilla (Nelson 1989). In controlled laboratory conditions, field sparrows were found to use a combination of five-song parameters in song recognition, although song frequency was given more weight than other temporal measures such as inter-note interval and phrase number (Nelson 1988). The great tits in our study may be using a similar weighting system, where frequency features strongly. Other song characteristics unidentified by us, possibly involving temporal measures, may be causing some ambiguity in the signal receiver's interpretation of the song, accounting for the shortfall of differentiation in the urban sample.
There are clear conservation implications of these adaptive variations in song characteristics, including population divergence (for a review, see Slabbekoorn & Ripmeester 2007). However, it is not yet known whether the difference in song between the two environments is because of a permanent change in the repertoire, or the individual's ability to detect masking of their signal and use this auditory feedback to select or alter their songs in order to increase detection (Patricelli & Blickley 2006). It is also possible that male great tits are seeking territories that optimize perception of their song. Until recently, great tits were thought to exhibit close-ended learning and so crystallize their repertoire at the end of a sensitive learning period in late autumn of their first year (McGregor & Krebs 1989). This would allow them to fit their repertoire to their environment and would select for songs that yearlings can hear from their neighbours (tutors). Presuming that young birds learn throughout the day, including quiet times, it is likely that the masking of songs is not sufficient to exclude learning of low-frequency notes completely, but instead produces a bias towards songs that are audible for the most amount of time. This would explain why, with a crystallized repertoire, birds with a higher minimum frequency still recognize lower frequency songs, albeit exhibiting a lower behavioural response. Nonetheless, recent work has suggested that adult great tits are able to assimilate new songs into their repertoire, suggesting lifelong plasticity controlled by social circumstances (Franco & Slabbekoorn 2009). A laboratory study on Bengalese finches has also shown that these song birds may adjust their song pitch on a short temporal scale (Tumer & Brainard 2007). Great tits may therefore be able to make small adaptations to their song post-dispersal. However, the extent to which they are able to adapt their songs in this manner, and thereby adapt to a new acoustic environment, remains unknown.
While background noise clearly has an effect on male song, we cannot rule out other factors completely. For example, air pollution poses a known threat to both a great tit's physiological condition (Isaksson et al. 2005) and song (Gorissen et al. 2005). The latter study found a significantly lower total amount of song during the dawn chorus from males close to the pollution sources than from the males 4 km away. However, there is no evidence that air pollution affects the frequency of the song. It is also unlikely that the difference in playback response to rural song is a result of reduced energy levels in urban birds, as their reaction to homotypic song was comparable to the rural bird's response to rural song.
In summary, our data show a clear difference in song characteristics between areas of high and low background noise. These variations in song elicit a significantly reduced behavioural response in territory-holding males when the level of background noise differs between the territories of the signaller and receiver. The adaptation of great tit song to overcome the increased noise levels associated with urban areas appears, therefore, to have occurred at a cost to their ability to respond appropriately to conspecifics from rural areas. Further cues as to the evolutionary significance of these behavioural alterations may be obtained from investigating how female great tits respond to these changes and the extent of cross-rural/urban great tit dispersal.
Acknowledgements
All work described in the paper has been carried out in accordance with ASAB/AB'S. Guideline for the Treatment of Animals in Research.
E.J.M. was supported by NERC studentship NE/F012756/1. Additional financial support was provided by a URF grant from Aberystwyth University to R.C.M. We are grateful to R. L. Wootton for guidance on the analysis and M. J. Wilkinson for helpful discussion. The manuscript was improved by the comments of two anonymous referees.
References
- Arvidsson B. L., Neergaard R.1991Mate choice in the willow warbler: a field experiment. Behav. Ecol. Sociobiol. 29, 225–229 (doi:10.1007/BF00166406) [Google Scholar]
- Baker M. C., Bjerke T. K., Lampe H., Espmark Y.1986Sexual-responses of female great tits to variation in size of males song repertoires. Am. Nat. 128, 491–498 (doi:10.1086/284582) [Google Scholar]
- Baker M. C., McGregor P. K., Krebs J. R.1987Sexual response of female great tits to local and distant songs. Ornis Scand. 18, 186–188 (doi:10.2307/3676765) [Google Scholar]
- Blumenrath S. H., Dabelsteen T.2004Degradation of great tit (Parus major) song before and after foliation: implications for vocal communication in a deciduous forest. Behaviour 141, 935–958 (doi:10.1163/1568539042360152) [Google Scholar]
- Bolton M.2007Playback experiments indicate absence of vocal recognition among temporally and geographically separated populations of Madeiran storm-petrels Oceanodroma castro. Ibis 149, 255–263 (doi:10.1111/j.1474-919X.2006.00624.x) [Google Scholar]
- Brumm H.2004The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73, 434–440 (doi:10.1111/j.0021-8790.2004.00814.x) [Google Scholar]
- Brumm H., Slabbekoorn H.2005Acoustic communication in noise. Adv. Study Behav. 35, 151–209 (doi:10.1016/S0065-3454(05)35004-2) [Google Scholar]
- Brumm H., Slater P. J. B.2006Ambient noise, motor fatigue, and serial redundancy in chaffinch song. Behav. Ecol. Sociobiol. 60, 475–481 (doi:10.1007/s00265-006-0188-y) [Google Scholar]
- Buchanan K. L., Catchpole C. K.1997Female choice in the sedge warbler, Acrocephalus schoenobaenus: multiple cues from song and territory quality. Proc. R. Soc. Lond. B 264, 521–526 (doi:10.1098/rspb.1997.0074) [Google Scholar]
- Byers B. E.2007Extrapair paternity in chestnut-sided warblers is correlated with consistent vocal performance. Behav. Ecol. 18, 130–136 (doi:10.1093/beheco/arl058) [Google Scholar]
- Cramp S., Perrins C. M.1993Handbook of the birds of Europe, the Middle East and North Africa: the birds of the Western Palearctic, vol. 7: flycatchers to shrikes, pp. 255–281 Oxford, UK: Oxford University Press [Google Scholar]
- Falls J. B., Krebs J. R., McGregor P. K.1982Song matching in the great tit (Parus major): the effect of similarity and familiarity. Anim. Behav. 30, 997–1009 (doi:10.1016/S0003-3472(82)80188-7) [Google Scholar]
- Fernández-Juricic E., Poston R., De Collibus K., Morgan T., Bastain B., Martin C., Jones K., Treminio R.2005Microhabitat selection and singing behavior patterns of male house finches (Carpodacus mexicanus) in urban parks in a heavily urbanized landscape in the Western US. Urban Habitats 3, 49–69 [Google Scholar]
- Franco P., Slabbekoorn H.2009Repertoire size and composition in great tits: a flexibility test using playbacks. Anim. Behav. 77, 261–269 (doi:10.1016/j.anbehav.2008.09.023) [Google Scholar]
- Fuller R. A., Warren P. H., Gaston K. J.2007Daytime noise predicts nocturnal singing in urban robins. Biol. Lett. 3, 368–370 (doi:10.1098/rsbl.2007.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D., Gahr M.2002The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 17, 133–141 (doi:10.1016/S0169-5347(02)02410-2) [Google Scholar]
- Gorissen L., Snoeijs T., Van Duyse E., Eens M.2005Heavy metal pollution affects dawn singing behaviour in a small passerine bird. Oecologia 145, 504–509 (doi:10.1007/s00442-005-0091-7) [DOI] [PubMed] [Google Scholar]
- Greenwood P. J., Harvey P. H., Perrins C. M.1979Role of dispersal in the great tit Parus major: causes, consequences and heritability of natal dispersal. J. Anim. Ecol. 48, 123–142 (doi:10.2307/4105) [Google Scholar]
- Hoileitner M., Nechtelberger H., Hoi H.1995Song rate as a signal for nest-site quality in blackcaps (Sylvia atricapilla). Behav. Ecol. Sociobiol. 37, 399–405 (doi:10.1007/BF001170587) [Google Scholar]
- Isaksson C., Örnborg J., Stephensen E., Andersson S.2005Plasma glutathione and carotenoid coloration as potential biomarkers of environmental stress in great tits. EcoHealth 2, 138–146 (doi:10.1007/s10393-005-3869-5) [Google Scholar]
- Krebs J. R., Ashcroft R., Webber M.1978Song repertoires and territory defence in the great tit. Nature 271, 539–542 (doi:10.1038/271539a0) [Google Scholar]
- Krebs J. R., Ashcroft R., Vanorsdol K.1981Song matching in the great tit Parus major. Anim. Behav. 29, 918–923 (doi:10.1016/S0003-3472(81)80029-2) [Google Scholar]
- Lepczyk C. A., Mertig A. G., Liu J. G.2004Landowners and cat predation across rural-to-urban landscapes. Biol. Conserv. 115, 191–201 (doi:10.1016/S0006-3207(03)00107-1) [Google Scholar]
- Martin-Vivaldi M., Palomino J. J., Soler M.2004Strophe length in spontaneous songs predicts male response to playback in the hoopoe Upupa epops. Ethology 110, 351–362 (doi:10.1111/j.1439-0310.2004.00971.x) [Google Scholar]
- McGregor P. K., Krebs J. R.1982Song types in a population of great tits Parus major: their distribution, abundance and acquisition by individuals. Behaviour 79, 126–152 (doi:10.1163/156853982X00210) [Google Scholar]
- McGregor P. K., Krebs J. R.1989Song learning in adult great tits (Parus major): effects of neighbours. Behaviour 108, 139–159 (doi:10.1163/156853989X00105) [Google Scholar]
- McGregor P. K., Krebs J. R., Perrins C. M.1981Song repertoires and lifetime reproductive success in the great tit (Parus major). Am. Nat. 118, 149–159 (doi:10.1086/283811) [Google Scholar]
- Morton E. S.1977Occurrence and significance of motivation structural rules in some bird and mammal sounds. Am. Nat. 111, 855–869 (doi:10.1086/283219) [Google Scholar]
- Nelson D. A.1988Feature weighting in species song recognition by the field sparrow (Spizella pusilla). Behaviour 106, 158–182 (doi:10.1163/156853988X00142) [Google Scholar]
- Nelson D. A.1989Song frequency as a cue for recognition of species and individuals in the field sparrow (Spizella pusilla). J. Comp. Psychol. 103, 171–176 (doi:10.1037/0735-7036.103.2.171) [DOI] [PubMed] [Google Scholar]
- Nyström K. G. K.1997Food density, song rate, and body condition in territory-establishing willow warblers Phylloscopus trochilus. Can. J. Zool. 75, 47–58 (doi:10.1139/z97-006) [Google Scholar]
- Osiejuk T. S., Ratynska K., Cygan J. P.2007Corn bunting (Miliaria calandra) males respond differently to alternating and overlapping playback of song. J. Ethol. 25, 159–168 (doi:10.1007/s10164-006-0010-3) [Google Scholar]
- Patricelli G. L., Blickley J. L.2006Avian communication in urban noise: causes and consequences of vocal adjustment. Auk 123, 639–649 (doi:10.1642/0004-8038(2006)123[639:ACIUNC]2.0.CO;2) [Google Scholar]
- Peake T. M., Matessi G., McGregor P. K., Dabelsteen T.2005Song type matching, song type switching and eavesdropping in male great tits. Anim. Behav. 69, 1063–1068 (doi:10.1016/j.anbehav.2004.08.009) [Google Scholar]
- Rheindt F. E.2003The impact of roads on birds: does song frequency play a role in determining susceptibility to noise pollution? J. Ornithol. 144, 295–306 [Google Scholar]
- Rios-Chelen A. A., Garcia C. M.2007Responses of a sub-oscine bird during playback: effects of different song variants and breeding period. Behav. Process. 74, 319–325 (doi:10.1016/j.beproc.2006.11.007) [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H., den Boer-Visser A.2006Cities change the songs of birds. Curr. Biol. 16, 2326–2331 (doi:10.1016/j.cub.2006.10.008) [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H., Peet M.2003Birds sing at a higher pitch in urban noise. Nature 424, 267–267 (doi:10.1038/424267a) [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H., Ripmeester E. A. R.2007Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83 (doi:10.1111/j.1365-294X.2007.03487.x) [DOI] [PubMed] [Google Scholar]
- Tumer E. C., Brainard M. S.2007Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature 450, 1240–1211 (doi:10.1038/nature06390) [DOI] [PubMed] [Google Scholar]
- Vehrencamp S. L.2001Is song-type matching a conventional signal of aggressive intentions? Proc. R. Soc. Lond. B 268, 1637–1642 (doi:10.1098/rspb.2001.1714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S., Perrins C. M., Riddington R.1997Natal dispersal of great tits in a patchy environment. Ecology 78, 864–872 [Google Scholar]
- Warren P. S., Katti M., Ermann M., Brazel A.2006Urban bioacoustics: it's not just noise. Anim. Behav. 71, 491–502 (doi:10.1016/j.anbehav.2005.07.014) [Google Scholar]
- Wood W. E., Yezerinac S. M.2006Song sparrow (Melospiza melodia) song varies with urban noise. Auk 123, 650–659 (doi:10.1642/0004-8038(2006)123[650:SSMMSV]2.0.CO;2) [Google Scholar]