Abstract
Bovine tuberculosis (Tb) caused by Mycobacterium bovis has proved refractory to eradication from domestic livestock in countries with wildlife disease reservoirs. Vaccination of wild hosts offers a way of controlling Tb in livestock without wildlife culling. This study was conducted in a Tb-endemic region of New Zealand, where the introduced Australian brushtail possum (Trichosurus vulpecula) is the main wildlife reservoir of Tb. Possums were trapped and vaccinated using a prototype oral-delivery system to deliver the Tb vaccine bacille Calmette–Guerin. Vaccinated and control possums were matched according to age, sex and location, re-trapped bimonthly and assessed for Tb status by palpation and lesion aspiration; the site was depopulated after 2 years and post-mortem examinations were conducted to further identify clinical Tb cases and subclinical infection. Significantly fewer culture-confirmed Tb cases were recorded in vaccinated possums (1/51) compared with control animals (12/71); the transition probability from susceptible to infected was significantly reduced in both males and females by vaccination. Vaccine efficacy was estimated at 95 per cent (87–100%) for females and 96 per cent (82–99%) for males. Hence, this trial demonstrates that orally delivered live bacterial vaccines can significantly protect wildlife against natural disease exposure, indicating that wildlife vaccination, along with existing control methods, could be used to eradicate Tb from domestic animals.
Keywords: bovine tuberculosis, oral vaccine, possum, wildlife, BCG, badger
1. Introduction
Mycobacterium bovis causes tuberculosis (Tb) in a wide variety of mammalian domestic and wildlife species, particularly among the Bovidae and Cervidae (Morris et al. 1994; Phillips et al. 2003; Wirth et al. 2008). Tb has a worldwide distribution and represents one of the most globally significant veterinary health problems (O'Reilly & Daborn 1995); it is also a significant zoonosis typically spread to humans by the inhalation of aerosols or the ingestion of unpasteurized cows' milk (Grange & Yates 1994; Etter et al. 2006). Human cases are still common in less-developed countries (Cosivi et al. 1998), and severe economic losses can occur from livestock deaths, chronic disease and trade restrictions (Etter et al. 2006). In some situations, this disease may also be a serious threat to endangered species such as lions, cheetahs and rhinoceros (Cleaveland et al. 2005; Renwick et al. 2007). In many developed countries, livestock-focused Tb-control programmes have reduced or eliminated Tb in cattle (Reviriego Gordejo & Vermeersch 2006; Ryan et al. 2006), and cases of human disease are rare (Sargeant 2008). However, where Tb also persists independently in wildlife reservoirs, complete eradication is much more difficult (Olmstead & Rhode 2002; Ryan et al. 2006; McDonald et al. 2008).
Wildlife reservoirs considered to constitute a major impediment to Tb control or eradication campaigns include the Eurasian badger in the UK and Ireland (Cheeseman et al. 1989; Delahay et al. 2002; Griffin et al. 2005), white-tailed deer in the USA (Schmitt et al. 1997), wild boar and red deer in Spain (Vicente et al. 2007) and the introduced Australian brushtail possum (Trichosurus vulpecula) in New Zealand (Montague 2000). Lethal control (culling) of wildlife reservoirs to reduce Tb incidence in cattle stocks has had mixed results to date. The utility of the approach has been demonstrated in New Zealand, where localized possum population control has been shown to cause subsequent reductions in the Tb reactor rate among adjacent cattle stocks (Caley et al. 1999). However, in the UK and Ireland, the impact of experimental badger culling on concurrent disease in domestic stock is more complex, with culling both increasing and decreasing cattle Tb reactor rates, depending on the circumstances (Griffin et al. 2005; Donnelly et al. 2006; Carter et al. 2007; McDonald et al. 2008; Woodroffe et al. 2009). Furthermore, social concerns in many countries limit the use of wildlife culling as a Tb control tool; the often heavy reliance on the use of poisons is controversial, and the wildlife in question may be valued for conservation or hunting purposes (Ryan et al. 2006; McDonald et al. 2008). This has driven a strong interest in non-lethal alternatives such as vaccination (Cross et al. 2007).
Both research theory and actual practice indicate that prophylactic vaccination can control or even eliminate some diseases in wildlife (Anderson & May 1992; Heesterbeek & Roberts 1995; Haydon et al. 2006). For example, sylvatic rabies has been successfully eradicated from foxes in several Western European countries by large-scale oral vaccination programmes (Rupprecht et al. 2004), and recent reports have indicated that it is feasible to interrupt borreliosis transmission in the wild by vaccinating wild white-footed mice (Peromyscus leucopus) against the Lyme disease spirochete (Borrelia burgdorferi; Tsao et al. 2004). Here we explore the potential use of vaccination for controlling Tb in wildlife reservoirs, through an experimental study of free-living possums in New Zealand. Possums are nocturnal 2–3 kg arboreal marsupials that occur in most parts of New Zealand, ranging in density from approximately 0.5 ha−1 in beech forests to approximately 10–12 ha−1 in podocarp-broadleaf forests (Cowan 2005). They are the principal wildlife reservoir of M. bovis infection in New Zealand (Morris & Pfeiffer 1995), with Tb believed to be established in possum populations over about 10 million ha (approx. 39% of New Zealand's area; Ryan et al. 2006).
In the current study, we investigated whether orally delivered bacille Calmette–Guerin (BCG), the Tb vaccine used in humans and derived from the attenuation of an M. bovis isolate (Behr 2002), can be used to protect possums from Tb. Although intranasal and intraconjunctival BCG vaccination has been shown to achieve some protection against Tb in wild possums (Corner et al. 2002a), oral baiting is generally considered the only feasible means of vaccine delivery for large-scale disease management in wildlife populations (World Health Organization 2004; Vial et al. 2006; Cross et al. 2007). Since BCG is a live-attenuated bacterium, it needs to be protected from degradation in the stomach of an animal if it is to be deployed as an oral vaccine. An edible lipid matrix has been developed that allows BCG bacilli to be maintained in a viable but static state that is suitable as an oral delivery vehicle for the vaccine (Aldwell et al. 2005). Studies in a range of animal species have shown that oral vaccination with this lipid-formulated BCG can induce a level of protection against experimental challenge with Tb that is comparable with that induced by injecting the vaccine (Aldwell et al. 2003a,b; Buddle et al. 2006; Nol et al. 2008). However, vaccination has generally not prevented animals from becoming infected as a result of experimental challenge; rather, it has slowed the progression of the disease relative to that in unvaccinated animals, as shown by a reduction in the severity of disease and bacterial counts in lungs and spleen (Aldwell et al. 1995, 2003a; Buddle et al. 2006).
As an ideal vaccine should either prevent the establishment of infection or reduce the incidence of clinical disease (Cross et al. 2007), here we address the key question of whether oral BCG vaccination can actually reduce Tb infection rates in free-living animals exposed to natural rather than experimental challenge, making it a useable tool for Tb control in wildlife reservoirs.
2. Methods
(a). Field study site and possum trapping
We studied a wild possum population inhabiting a 14 ha site in the Orongorongo Valley (lower North Island, New Zealand; 41°21′ S, 174°58′ E, elevation 100 m above sea level). The study site comprised a mixed broadleaf–conifer forest that supports about nine possums per hectare (Efford 2000). The trapping site consisted of a grid of 200 wire mesh cage traps at 30 m spacing. The surrounding area has been monitored intermittently over the last 25 years, and Tb has been reported in possums in this region since the 1980s (Brockie et al. 1987).
All traps were baited with apple coated with flour and aniseed and set over four consecutive nights on each trapping session, unless weather conditions dictated otherwise. During trapping sessions, all captured possums were anaesthetized with ketamine hydrochloride at 25 mg kg−1 body weight. Once sedated, possums were individually marked with either a metal ear tag and tattoo or two metal ear tags, had their Tb status ascertained by external examination and palpation of the major superficial lymph nodes, and were sexed, examined for tooth wear (an index of age; Winter 1980) and released. Captured possums were also initially assessed for prior exposure to M. bovis by blood testing; approximately 2 ml of blood was drawn from the tail or jugular vein of each animal, and a lymphocyte proliferation (LP) assay was conducted following the procedures described by Skinner et al. (2005). Previous work has shown palpation and LP assays to be robust approaches to disease assessment for Tb in wild possums (Corner et al. 2002a). For possums with suspected Tb lesions (i.e. open lesions or palpable subcutaneous lumps), either swabs (for open lesions) or aspirated fluid (for closed lesions) were collected for bacteriological culture confirmation of infection, following the procedures described by de Lisle & Havill (1985). Tb strain typing was conducted on selected positive cultures using restriction endonuclease analysis (REA; Collins et al. 1993).
(b). Vaccine formulation and delivery
BCG bacilli (strain Danish 1331) were grown to mid-log phase in Tween/albumin-supplemented Middlebrook 7H9 broth, and subsequently harvested, sedimented and formulated into the edible vaccine matrix Lipid-C as described elsewhere (Aldwell et al. 2006). For field vaccination, lipid vaccine samples containing 107 colony-forming units (cfu) of BCG per millilitre were maintained in 3 ml polypropylene syringes; captured and anaesthetized possums were immunized by applying 1 ml of the lipid formulation to the rear of the oral cavity and allowing the animals to ingest vaccine by reflex swallowing. Hence, each vaccine dose contained approximately 107 cfu of BCG.
(c). Experimental design and measures
The field trial was carried out between July 2004 and November 2006, with initial vaccinations applied in November–December 2004. Pairs of animals matched according to trap location within the grid, sex and age were identified during July–September 2004 for treatment application in November 2004, and during July–November 2004 for treatment application in early December 2004. Matching ensured no bias between treatment groups with respect to potential exposure to natural infection. Animals with suspected Tb infection, based on external examination and palpation, and/or positive LP assays from the initial surveys, were excluded from pairs. One animal of each pair was randomly chosen to be vaccinated, with the other sham handled as a control. The site was subsequently re-trapped every two months over the ensuing 2 year period, with clinical Tb cases being identified by palpation and bacteriological confirmation of lesion aspirates upon each capture. With the pre-clinical phase of infection resulting from experimental challenge estimated to last 6–16 weeks (Corner et al. 2002b) and a mean survival time of clinically tuberculous possums in the wild of 4.7 months (Ramsey & Cowan 2003), bimonthly monitoring was deemed sufficient for all cases of Tb infection to be documented.
At two months following initial vaccination, 2 ml blood samples were drawn from each vaccinated or control animal recaptured, and the induction of a Mycobacterium-specific immune response owing to the vaccine was assessed by the LP assay. Vaccine was re-applied to recaptured vaccine-group possums at six-month intervals, since captive trials have indicated that protection against artificial Tb challenge decreases after six months post-vaccination (Buddle et al. 2006). Although this is probably not the case for induced protection against natural infection in free-ranging animals (since artificial infection of possums with Tb can produce an un-naturally harsh disease challenge; Buddle et al. 1994; Ramsey et al. 2009), the re-vaccination protocol was carried out here to provide a ‘best’ test of the vaccine. New control and vaccinated animals were introduced to the study during May and November 2005 to maintain sample sizes (§3).
The study site was depopulated at the completion of the trial, in November 2006, through a prolonged trapping session (five nights), with two additional lines of leg-hold traps placed at 30 m intervals around the periphery of the trapping site and a further 20 leg-hold traps dispersed through the grid to catch any cage-shy animals. All recaptured possums were killed by a blow to the head and subjected to post-mortem examination to identify any visible tuberculous lesions. Reticuloendothelial tissues (spleen and liver) and lungs were closely examined, and several lymph node sites were located and excised (including retropharyngeal, axillary, inguinal, bronchial, hepatic and mesenteric nodes). Any suspect lesions were described and a sample taken for bacteriological culture confirmation of M. bovis infection (de Lisle & Havill 1985). Pooled lymph node samples were collected from all individuals for the identification of subclinical infection by bacteriological culture, with REA strain typing of selected positive samples (Collins et al. 1993).
(d). Statistical analyses
In the LP assays to investigate M. bovis reactivity and the immunological response to vaccination, data were expressed as a stimulation index (SI); that is, the proliferation of bovine-purified protein derivative stimulated blood lymphocytes divided by that of non-stimulated lymphocytes, as described in detail elsewhere (Skinner et al. 2005; Buddle et al. 2006). Log-transformed LP responses were compared between vaccinated and control possums using a one-sided t-test; the frequency of positive responses to the vaccine (SI > 4.5; Buddle et al. 2006) was compared by Fisher's exact test.
The capture histories of experimental group animals were analysed using multi-state mark-recapture analysis (Brownie et al. 1993), using the program MARK (White & Burnham 1999) to estimate survival and recapture probabilities, transition probabilities from ‘susceptible’ to ‘Tb-infected’ states, and the effects of sex and vaccine treatment on those probabilities (appendix A1, electronic supplementary material). Vaccine efficacy, as defined in appendix A1, was calculated from the Tb transition probabilities.
3. Results
(a). Oral vaccination
Initial vaccination treatments (in November and early December 2004) were applied to 32 possums, matched to a control group of 32 animals. All of the animals designated to the two groups, on the basis of having no evidence of Tb infection in the prior trapping sessions, were still clear of suspect Tb lesions at treatment application. Indeed, clinical signs of infection were not observed in either group until May 2005 (figure 1).
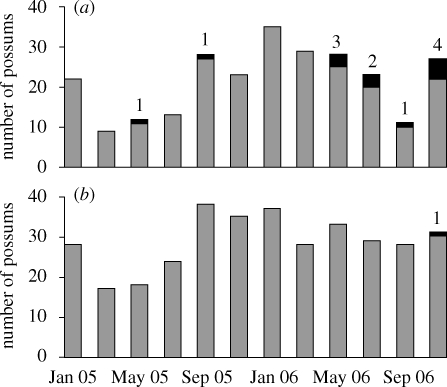
Figure 1.
Trapped possums with (black) and without (grey) culture-confirmed Tb lesions in (a) control and (b) vaccinated animals, and number identified as infected for the first time at each trapping session (numbers above columns).
At six to eight months into the vaccine trial (May–July 2005), 27 of the original 32 vaccinated animals were recaptured and vaccine was re-applied; in addition, 16 new animals were vaccinated for the first time and 20 new animals were designated as new controls. The inclusion of more animals as new controls was necessary owing to fewer controls being recaptured after an extreme rainfall event in March 2005 during which landslips covered parts of the study site, while flash floods covered others (figure 1).
At 12 months into the vaccine trial (November 2005), 34 animals were re-vaccinated, 3 new animals were vaccinated for the first time (bringing the number vaccinated at least once to 51) and 19 animals were designated as new controls (bringing the number designated as controls to 71). More animals were again introduced into the control group to ensure that sufficient control animals remained through to the end of the trial. At 18 months into the vaccine trial (May 2006), 33 animals were re-vaccinated. At the end of the vaccine trial (November 2006), 123 independent possums (i.e. not counting pouch young and attendant juveniles) were trapped and removed from the site, consisting of 31 vaccinated animals, 27 controls and 65 animals not belonging to either experimental group (generally animals recently trapped for the first time).
(b). Immunological responses to M. bovis infection and oral vaccination
In the July–September surveys prior to initial vaccination in November 2004, 5 of 75 possums examined were diagnosed with clinical signs of Tb; all were culture-positive for M. bovis and showed positive immune responses on the LP assay (SI range: 34–93). Of 46 possums without clinical signs also tested in the LP assay, an additional 5 animals showed positive immune responses (SI range: 11–305). All 10 animals with suspected Tb infection were subsequently excluded from the vaccinated and control groups, and acted as potential sources of M. bovis infection to the experimental animals.
LP assays conducted on blood samples drawn two months following initial vaccination showed no statistically significant response to vaccination; 35 per cent of 17 vaccinated animals showed positive immune responses compared with 17 per cent of 22 control animals (Fisher's exact p = 0.20). Median SI was 2.40 (SI range: 0.62–75.24) in vaccinates compared with 1.63 (SI range: 0.28–23.56) in controls (one-tailed t = 1.53, df = 37, p = 0.07).
(c). Tuberculosis incidence
The five possums diagnosed with clinical Tb in the July–September surveys prior to initial vaccination in November 2004 had swollen lymph nodes or discharging sinuses from these nodes and were all culture-positive for M. bovis. Three separate M. bovis isolates from these possums were strain typed, and all had the same REA strain type 21. This strain type is one of the most widespread in New Zealand and the same type identified in three earlier isolates from the same area (Collins et al. 1993).
Across all monitoring trips during the trial (excluding the final depopulation trap-out in November 2006), eight incident cases of culture-confirmed Tb infection were observed in experimental animals, all in the control group (figure 1; appendix B(A), electronic supplementary material). All infected animals had been designated controls at the site for at least six months prior to external lesions first being observed, indicating a high likelihood that all infections occurred after the animals were included in the trial. Hence, during the monitoring phase of the vaccine trial, significantly fewer vaccinated animals became naturally infected with Tb than control individuals exposed to the same force of infection (0 of 51 vaccinated animals infected versus 8 of 71 control animals; χ2 = 6.14, df = 1, p < 0.05).
Five further Tb cases were detected during the necropsies of experimental group possums at the end of the trial: four that had been designated as controls for at least 12 months and one that was vaccinated (figure 1; appendix B(B), electronic supplementary material). Combining the results from the trap-out with those from the monitoring trips, a highly significant protective effect of the vaccine was apparent—12 incident cases of infection in 71 control animals versus 1 in 51 vaccinated animals exposed to the same force of infection (χ2 = 6.94, df = 1, p < 0.01).
In the non-experimental group animals (i.e. those not designated as either controls or vaccinates) captured during the course of the trial, culture-confirmed Tb infection was only observed in three instances: 1 of 5 animals in November 2004, 1 of 18 animals in January 2006 and 1 of 25 animals in July 2006. In addition, one of the 65 non-experimental group possums necropsied at the end of the trial (larger sample owing to greater trapping effort and area during the site depopulation) also had culture-confirmed Tb infection.
(d). Multi-state capture–mark–recapture analysis
Model selection procedures undertaken initially for the recapture and survival probabilities established that the simplest, best-fitting models for these parameters had recapture probability varying only among treatments (vaccine and control) and the survival probability varying only among states (susceptible and infected; appendix C, electronic supplementary material). Recapture probability was lower for control versus vaccinated possums, and survival probability was much lower for Tb-infected versus susceptible possums (table 1).
Table 1.
Estimates of recapture probability (p) and survival probability (ϕ), for the best-fitting models for each parameter (p varying among vaccine treatment; ϕ varying among states).
| parameter | estimate | SE |
|---|---|---|
| p (control) | 0.59 | 0.028 |
| p (vaccinated) | 0.80 | 0.021 |
| ϕ (susceptible) | 0.96 | 0.009 |
| ϕ (infected) | 0.30 | 0.141 |
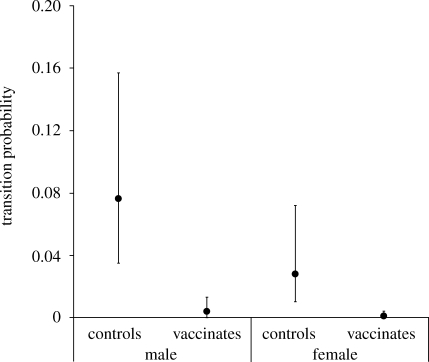
Using the best-fitting models for the recapture and survival probabilities, model selection for the transition probability (from susceptible to infected) indicated that models that included treatment were supported over models that did not (table 2). Among the best-supported models, transition probability varied with treatment both additively and interactively with sex (table 2). While the additive model had the most support, we calculated model-averaged estimates of transition probabilities to account for the uncertainty in model selection (Burnham & Anderson 2002). These indicated that the probability of becoming infected with Tb was almost three times higher for male versus female controls and at least an order of magnitude lower for vaccinated versus control possums (figure 2). Using these transition probabilities (i.e. controlling for differences in recapture and survival probabilities), vaccine efficacy (and 95% credible interval) was estimated as 0.96 (0.87–1.00) for females and 0.95 (0.82–0.99) for males.
Table 2.
Model selection results for the Tb transition probability (ψ). For each set of models, the covariate structure taken by the other parameters in the model (recapture probability, p; survival probability, ϕ) is indicated in other parameters. vacc, vaccine treatment (control/treatment); sex, sex (male/female); state, possum state (susceptible/infected); model, covariate structure of model; par, number of model parameters; QAICc, Akaike's information criterion corrected for small sample size and overdispersion; ΔQAICc, difference in QAICc values between this model and the model with the lowest QAICc; w, normalized model ‘weight’.
| other parameters | model | par | QAICc | ΔQAICc | w |
|---|---|---|---|---|---|
| p (vacc) ϕ (state) | ψ (sex+vacc) | 7 | 1287.359 | 0 | 0.58 |
| ψ (sex*vacc) | 8 | 1288.896 | 1.5369 | 0.27 | |
| ψ (vacc) | 6 | 1290.157 | 2.7976 | 0.14 | |
| ψ (sex) | 6 | 1302.01 | 14.651 | 0.00 | |
| ψ (.) | 5 | 1302.353 | 14.9936 | 0.00 |
Figure 2.
Model-averaged estimates of the bimonthly probabilities (with 95% confidence intervals) of the bimonthly probabilities of transition from susceptible to Tb-infected states for male and female possums in the control and BCG-vaccinated groups.
(e). Tuberculosis pathology
While all four of the infected control animals necropsied at the end of the trial had lesions in the lungs and/or lymph nodes typical of those seen in Tb-infected possums, the sole vaccinated animal infected had only small lesions in the liver (identified as the same M. bovis REA strain type as found on the study site immediately prior to vaccination); none of the infected control animals had similar liver lesions (appendix B, electronic supplementary material). In addition, the pooled lymph node sample collected from this vaccinated individual on necropsy proved culture-negative for M. bovis.
Apart from the pooled lymph node samples and tissue samples listed in appendix B(B) of the electronic supplementary material, none of the samples collected from other necropsied experimental group animals cultured positive for M. bovis and no cases of subclinical Tb were identified.
4. Discussion
Prophylactic vaccination of wildlife represents a means for controlling infectious diseases that have a transmission cycle in free-ranging hosts. To date, such vaccines have been successfully deployed on a wide-scale basis for the control of some endemic viral diseases in wildlife, including porcine pestivirus (hog cholera) among wild boar in Europe (Von Rüden et al. 2008) and lyssavirus (rabies) among carnivores in northern Europe and North America (Rupprecht et al. 2004). Additionally, prototypical vaccines against bacterial diseases in wildlife have been investigated, including vaccines against plague among prairie dogs (Cynomys ludovicianus), brucellosis among bison (Bison bison) and Lyme disease among white-footed mice in the USA (Olsen et al. 2002; Mencher et al. 2004; Tsao et al. 2004; Rocke et al. 2008), Tb among possums in New Zealand and badgers in the UK and Ireland (Corner et al. 2002a; Lesellier et al. 2006). However, while each of the bacterial disease studies employed a live agent as the test vaccine (either an attenuated bacterium or a recombinant, peptide-expressing poxvirus), none has described field protection against natural infection of the target species using an oral-delivery system (generally a necessary facet of a wildlife vaccine intended for wide-scale application; World Health Organization 2004; Vial et al. 2006; Cross et al. in press). In the present study, we delivered live BCG formulated into a protective lipid matrix orally to possums; the study design necessitated that vaccine was fed manually to restrained possums, although captive possums have been shown to readily and voluntarily consume the lipid-based vaccine if it is appropriately flavoured (Cross et al. 2009). In voluntary-uptake vaccination studies in captive possums, this vaccine has been shown to confer protection against artificial M. bovis infection, as demonstrated by lower lung and spleen pathogen burdens, reduced pulmonary tract pathology and a reduced occurrence of extra-pulmonary lesions (Aldwell et al. 2003a; Buddle et al. 2006; Collins et al. 2007). We are therefore confident that possums can be successfully orally vaccinated without having to capture them. Work is now under way to develop a vaccine package that can be aerially sown and to test the efficacy of aerially delivered vaccination versus poisoning in reducing the force of Tb infection from possum populations.
The experimental design employed here was successful in testing oral BCG vaccine efficacy against natural Tb infection in wild free-ranging possums. The recapture probability of animals varied between treatments during the trial, being lower in the control group, potentially because of both disease-induced morbidity in infected animals (survival of infected possums was one-third that of uninfected possums) and more transient individuals being introduced to this group than the vaccinated group later in the study. With culture-confirmed Tb infection only observed sporadically in non-experimental group animals, the inclusion of more animals as new controls ensured that sufficient animals were maintained in the experimental groups through the course of the study to allow a statistically valid test of the vaccine (figure 1). Interestingly, the transition probability from susceptible to infected was almost three times greater for males than for females (figure 2). This is in line with sex differences in Tb prevalence documented in previous studies that appear to be caused by behavioural factors (Coleman & Caley 2000): males forage more widely and juvenile males are more likely to disperse than juvenile females. Both activities presumably expose males to a greater risk of infection when fighting and maintaining larger activity areas (Jackson et al. 1995). Males may also have greater susceptibility to Tb infection than females (Ramsey et al. 2006).
Vaccine protection was expressed here as an order of magnitude reduction in the probability of wild possums becoming infected (figure 2), controlling for differences in recapture and survival probabilities, with an associated vaccine efficacy of 95–96 per cent against a natural force of Tb infection. Vaccine efficacy was similar in both sexes, even though the transition probability from susceptible to Tb infected was, as discussed earlier, greater for males than females. In addition, although not much weight can be placed on the observation that infection was less severe (in terms of number and size of lesions) in the single infected vaccinated animal compared with the infected controls (appendix B, electronic supplementary material), this is consistent with previous work showing that in cases where oral vaccination does not prevent Tb infection, it does slow and possibly prevent disease progression (Aldwell et al. 2003a; Buddle et al. 2006; Ramsey et al. 2009). This high level of vaccine efficacy compares very favourably with other wildlife vaccines. For example, recent trials of the protection conferred by an orally delivered recombinant poxvirus against artificial plague challenge in prairie dogs have demonstrated an efficacy of only 40–50 per cent (Mencher et al. 2004; Rocke et al. 2008), while commercial oral rabies vaccine baits have 70–100 per cent efficacy across different species (Cliquet et al. 2008). Furthermore, BCG vaccination of wild possums by intranasal and intraconjunctival routes only achieved 69 per cent efficacy of protection against natural Tb infection (Corner et al. 2002a). Unlike the current trial, the vaccinated animals in that trial that did contract Tb also displayed disease progression rates similar to controls (Corner et al. 2002a).
Protection against Tb was observed here even though the vaccinated possums had not mounted a statistically significant increase in the cellular immune response to BCG compared with the controls. This is in contrast with the previous trials with captive possums, where significant systemic cell-mediated immune responses to similar BCG dosages have been observed at 6+ weeks post-vaccination (Aldwell et al. 2003a), in addition to a strong relationship between the proportion of animals producing positive LP responses to M. bovis antigens and protection against artificial challenge with M. bovis (Buddle et al. 2006). The comparison of LP responses of vaccinated possums and controls here was probably confounded by actual Tb infection, since two of the control animals were positive responders to the assay (SI > 4.5). Unfortunately, these two animals were not observed again after the extreme rainfall event of March 2005, so infection could not be confirmed.
Another possible explanation for the differences in the LP responses for possums in the field versus those in captivity is that stress levels may be more variable in the wild situation; stress may be more controlled in captive possums where their body weight increases when food is supplied ad libitum. In addition, blood samples were only collected at two months post-vaccination in the current study, which may not have been the optimal time point for measuring increases in cellular responses. While reductions in LP responses by possums to oral BCG vaccination over time have been linked to waning protection against artificial challenge with M. bovis in captivity (Buddle et al. 2006), the high level of vaccine efficacy observed here without similarly high LP responsiveness indicates that the dynamics of conferred protection may be different against natural infection in the wild. The implications of this for the duration of protection conferred are the focus of an ongoing trial. In addition, although the vaccine was re-applied to recaptured vaccine group possums at six-month intervals in this study, the effectiveness of re-vaccinating possums with an oral bait BCG vaccine is not known. This is an important question that also needs to be addressed in future studies. However, captive work has shown that feeding multiple doses of BCG vaccine to possums and feeding dead BCG to possums three months prior to feeding live BCG do not interfere with the acquisition of protection against Tb (Buddle et al. 2006).
5. Conclusion
Mathematical modelling indicates that vaccination operations to eradicate Tb would need to maintain 40–52 per cent of a possum population in an immune state (Barlow 1991; Roberts 1996). Since techniques for the delivery of bait to possums are well advanced, with both aerial and bait-station dissemination able to reach over 90 per cent of the individuals in a population (Morgan & Hickling 2000; Tompkins & Ramsey 2007), the 95–96 per cent efficacy of orally delivered BCG vaccine demonstrated here should be more than sufficient for the purpose of Tb eradication from wild possum populations. This gives confidence that oral vaccination is a tenable solution for the control of Tb in wildlife.
In New Zealand, intensive culling of invasive possums is a justifiable and well-established Tb-control strategy, because possums not only carry Tb but are also major conservation pests (Montague 2000; Cowan 2005). The utility of vaccination will therefore depend largely on whether it can be implemented as cheaply as trapping, shooting or poisoning; its main use is likely to be in places where those culling methods are socially unacceptable or if it can be used in conjunction with culling to reduce the frequency of culling required.
Where wildlife hosts are valued species rather than pests, such as the Eurasian badger in the UK and Ireland, and the white-tailed deer in the USA, intensive culling is often not a politically desirable option, making vaccination a much more attractive alternative. However, demonstrations that oral vaccination is similarly efficacious against natural infection in free-living populations and that effective delivery at the required management scale can successfully be achieved are both still required for other host species.
Acknowledgements
The authors thank those who provided technical support for these studies: in the field, Kevin Drew, Peter Lei, Peter Berben, Paul Horton and Rachel Paterson (Landcare Research); in the laboratory, Matt Lambeth (University of Otago) for vaccine preparation and BCG bacteriology; Natalie Parlane, Allison McCarthy, Gary Yates, Yvette Ridley and Merie Joyce (AgResearch) for immunological assays and M. bovis bacteriology; and Des Collins for the REA of M. bovis strains. Special thanks to Graham Nugent, Robbie McDonald and two anonymous referees for comments on the manuscript. Funding was provided by contract research grants from the New Zealand Animal Health Board, the New Zealand Foundation for Research Science and Technology and the New Zealand Ministry of Agriculture and Forestry (Wellington, New Zealand). All animal manipulations were carried out under Landcare Research Animal Ethics Approval 04/01/01.
Footnotes
Present address: Department of Sustainability and Environment, Arthur Rylah Institute, PO Box 137, Heidelberg, Victoria 3084, Australia.
References
- Aldwell F. E., Pfeffer A., DeLisle G. W., Jowett G., Heslop J., Keen D., Thomson A., Buddle B. M.1995Effectiveness of BCG vaccination in protecting possums against bovine tuberculosis. Res. Vet. Sci. 58, 90–95 (doi:10.1016/0034-5288(95)90095-0) [DOI] [PubMed] [Google Scholar]
- Aldwell F. E., Keen D. L., Parlane N. A., Skinner M. A., de Lisle G. W., Buddle B. M.2003aOral vaccination with Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in brushtail possums. Vaccine 22, 70–76 (doi:10.1016/S0264-410X(03)00539-5) [DOI] [PubMed] [Google Scholar]
- Aldwell F. E., Tucker I. G., de Lisle G. W., Buddle B. M.2003bOral delivery of Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in mice. Infect. Immun. 71, 101–108 (doi:10.1128/IAI.71.1.101-108.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldwell F. E., Baird M. A., Fitzpatrick C. E., McLellan A. D., Cross M. L., Lambeth M. R., Buchan G. S.2005Oral vaccination of mice with lipid-encapsulated Mycobacterium bovis BCG: anatomical sites of bacterial replication and immune activity. Immunol. Cell Biol. 83, 549–553 (doi:10.1111/j.1440-1711.2005.01369.x) [DOI] [PubMed] [Google Scholar]
- Aldwell F. E., Cross M. L., Fitzpatrick C. E., Lambeth M. R., de Lisle G. W., Buddle B. M.2006Oral delivery of lipid-encapsulated Mycobacterium bovis BCG extends survival of the bacillus in vivo and induces a long-term protective immune response against tuberculosis. Vaccine 24, 2071–2078 (doi:10.1016/j.vaccine.2005.11.017) [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M.1992Infectious diseases of humans: dynamics and control Oxford, UK: Oxford University Press [Google Scholar]
- Barlow N. D.1991Control of endemic bovine Tb in New Zealand possum populations: results from a simple model. J. Appl. Ecol. 28, 794–809 [Google Scholar]
- Behr M. A.2002BCG—different strains, different vaccines? Lancet Infect. Dis. 2, 86–92 (doi:10.1016/S1473-3099(02)00182-2) [DOI] [PubMed] [Google Scholar]
- Brockie R. E., Hearfield M. E., White A. J., Waddington D. C., Hay J. R.1987Bovine tuberculosis in a possum from the Orongorongo Valley, Wellington. N. Z. Vet. J. 35, 201–203 [DOI] [PubMed] [Google Scholar]
- Brownie C., Hines J. E., Nichols J. D., Pollock K. H., Hestbeck J. B.1993Capture–recapture studies for multiple strata including non-Markovian transitions. Biometrics 49, 1173–1187 (doi:10.2307/2532259) [Google Scholar]
- Buddle B. M., Aldwell F. E., Pfeffer A., de Lisle G. W.1994Experimental Mycobacterium bovis infection in the brushtail possum (Trichosurus vulpecula): pathology, haematology and lymphocyte stimulation responses. Vet. Microbiol. 38, 241–254 (doi:10.1016/0378-1135(94)90005-1) [DOI] [PubMed] [Google Scholar]
- Buddle B. M., Aldwell F. E., Keen D. L., Parlane N. A., Hamel K. L., de Lisle G. W.2006Oral vaccination of brushtail possums with BCG: investigation into factors that may influence vaccine efficacy and determination of duration of protection. N. Z. Vet. J. 54, 224–230 [DOI] [PubMed] [Google Scholar]
- Burnham K. P., Anderson D. R.2002Model selection and multimodel inference: a practical information–theoretic approach New York, NY: Springer-Verlag [Google Scholar]
- Caley P., Hickling G. J., Cowan P. E., Pfeiffer D. U.1999Effects of sustained control of brushtail possums on levels of Mycobacterium bovis infection in cattle and brushtail possum populations from Hohotaka, New Zealand. N. Z. Vet. J. 47, 133–142 [DOI] [PubMed] [Google Scholar]
- Carter S. P., Delahay R. J., Smith G. C., Macdonald D. W., Riordan P., Etherington T. R., Pimley E. R., Walker M. J., Cheeseman C. L.2007Culling-induced social perturbation in Eurasian badgers Meles meles and the management of TB in cattle: an analysis of a critical problem in applied ecology. Proc. R. Soc. B 774, 2769–2777 (doi:10.1098/rspb.2007.0998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. L., Wilesmith J. W., Stuart F. A.1989Tuberculosis: the disease and its epidemiology in the badger, a review. Epidemiol. Infect. 103, 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaveland S., Mlengeya T., Kazwala R. R., Michel A., Kaare M. T., Jones S. L., Eblate E., Shirima G. M., Packer C.2005Tuberculosis in Tanzanian wildlife. J. Wildl. Dis. 41, 446–453 [DOI] [PubMed] [Google Scholar]
- Cliquet F., Barrat J., Guiot A. L., Cael N., Boutrand S., Maki J., Schumacher C. L.2008Efficacy and bait acceptance of vaccinia vectored rabies glycoprotein vaccine in captive foxes (Vulpes vulpes), raccoon dogs (Nyctereutes procyonoides) and dogs (Canis familiaris). Vaccine 26, 4627–4638 (doi:10.1016/j.vaccine.2008.06.089) [DOI] [PubMed] [Google Scholar]
- Coleman J., Caley P.2000Possums as a reservoir of bovine Tb. In The brushtail possum. Biology, impact and management of an introduced marsupial (ed. Montague T. L.), pp. 92–104 Lincoln, New Zealand: Manaaki Whenua Press [Google Scholar]
- Collins D. M., Erasmuson S. K., Stephens D. M., Yates G. F., de Lisle G. W.1993DNA fingerprinting of Mycobacterium bovis strains by restriction fragment analysis and hybridization with insertion elements IS1081 and IS6110. J. Clin. Microbiol. 31, 1143–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. M., de Lisle G. W., Aldwell F. E., Buddle B. M.2007A new attenuated Mycobacterium bovis vaccine protects brushtail possums (Trichosurus vulpecula) against experimental tuberculosis infection. Vaccine 25, 4659–4664 (doi:10.1016/j.vaccine.2007.04.014) [DOI] [PubMed] [Google Scholar]
- Corner L. A. L., Norton S., Buddle B. M., Morris R. S.2002aThe efficacy of bacille Calmette–Guerin vaccine in wild brushtail possums (Trichosurus vulpecula). Res. Vet. Sci. 73, 145–152 (doi:10.1016/S0034-5288(02)00038-3) [DOI] [PubMed] [Google Scholar]
- Corner L. A. L., Pfeiffer D. U., de Lisle G. W., Morris R. S.2002bNatural transmission of Mycobacterium bovis infection in captive brushtail possums (Trichosurus vulpecula). N. Z. Vet. J. 50, 154–162 [DOI] [PubMed] [Google Scholar]
- Cosivi O., et al. 1998Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4, 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan P.2005Brushtail possum. In The handbook of New Zealand mammals (ed. King C. M.), pp. 56–80 Oxford, UK: Oxford University Press [Google Scholar]
- Cross M. L., Buddle B. M., Aldwell F. E.2007The potential of oral vaccines for disease control in wildlife species. Vet. J. 174, 472–480 (doi:10.1016/j.tvjl.2006.10.005) [DOI] [PubMed] [Google Scholar]
- Cross M. L., Henderson R., Lambeth M. R., Buddle B. M., Aldwell F. E.In press Lipid-formulated BCG as an oral-bait vaccine for tuberculosis: vaccine stability, efficacy and palatability to New Zealand possums (Trichosurus vulpecula). J. Wildl. Dis. [DOI] [PubMed] [Google Scholar]
- de Lisle G. W., Havill P. F.1985Mycobacteria isolated from deer in New Zealand from 1970–1983. N. Z. Vet. J. 33, 138–140 [DOI] [PubMed] [Google Scholar]
- Delahay R. J., de Leeuw A. N., Barlow A. M., Clifton-Hadley R. S., Cheeseman C. L.2002The status of Mycobacterium bovis infection in UK wild mammals: a review. Vet. J. 164, 90–105 (doi:10.1053/tvjl.2001.0667) [DOI] [PubMed] [Google Scholar]
- Donnelly C. A., et al. 2006Positive and negative effects of widespread badger culling on cattle tuberculosis. Nature 439, 843–846 (doi:10.1038/nature04454) [DOI] [PubMed] [Google Scholar]
- Efford M.2000Possum density, population structure and dynamics. In The brushtail possum. Biology, impact and management of an introduced marsupial (ed. Montague T. L.), pp. 47–61 Lincoln, New Zealand: Manaaki Whenua Press [Google Scholar]
- Etter E., Donado P., Jori F., Caron A., Goutard F., Roger F.2006Risk analysis and bovine tuberculosis, a re-emerging zoonosis. Ann. N. Y. Acad. Sci. 1081, 61–73 (doi:10.1196/annals.1373.006) [DOI] [PubMed] [Google Scholar]
- Grange J. M., Yates M. D.1994Zoonotic aspects of Mycobacterium bovis infection. Vet. Microbiol. 40, 137–151 (doi:10.1016/0378-1135(94)90052-3) [DOI] [PubMed] [Google Scholar]
- Griffin J. M., Williams D. H., Kelly G. E., Clegg T. A., O'Boyle I., Collins J. D., More S. J.2005The impact of badger removal on the control of tuberculosis in cattle herds in Ireland. Prev. Vet. Med. 67, 237–266 (doi:10.1016/j.prevetmed.2004.10.009) [DOI] [PubMed] [Google Scholar]
- Haydon D. T., et al. 2006Low-coverage vaccination strategies for the conservation of endangered species. Nature 443, 692–695 (doi:10.1038/nature05177) [DOI] [PubMed] [Google Scholar]
- Heesterbeek J. A. P., Roberts M. G.1995Mathematical models for microparasites of wildlife. In Ecology of infectious diseases in natural populations (eds Grenfell B. T., Dobson A. P.), pp. 90–122 Cambridge, UK: Cambridge University Press [Google Scholar]
- Jackson R., Cooke M. M., Coleman J. D., Morris R. S., de Lisle G. W., Yates G. F.1995Naturally occurring tuberculosis caused by Mycobacterium bovis in brushtail possums (Trichosurus vulpecula): III. Routes of infection and excretion. N. Z. Vet. J. 34, 322–327 [DOI] [PubMed] [Google Scholar]
- Lesellier S., Corner L. A., Chambers M. A., Aldwell F. E., Costello E., Sleeman D. P., Hewinson R. G., Gormley E.2006. Oral vaccination of badgers (Meles meles) against tuberculosis: immune responses and protection following BCG delivery via the oral route and pulmonary challenge with Mycobacterium bovis. In Proc. Wildlife Disease Association 55th Annual Meeting, 2006 Wildlife Disease Association Online Publication; See http://www.wildlifedisease.org/Documents/Proceedings/Storrs_06.pdf [Google Scholar]
- McDonald R. A., Delahay R. J., Carter S. P., Smith G. C., Cheeseman C. L.2008Perturbing implications of wildlife ecology for disease control. Trends Ecol. Evol. 23, 53–56 (doi:10.1016/j.tree.2007.10.011) [DOI] [PubMed] [Google Scholar]
- Mencher J. S., Smith S. R., Powell T. D., Stinchcomb D. T., Osorio J. E., Rocke T. E.2004Protection of black-tailed prairie dogs (Cynomys ludovicianus) against plague after voluntary consumption of baits containing recombinant raccoon poxvirus vaccine. Infect. Immun. 72, 5502–5505 (doi:10.1128/IAI.72.9.5502-5505.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague T. L.2000The brushtail possum. Biology, impact and management of an introduced marsupial Lincoln, New Zealand: Manaaki Whenua Press [Google Scholar]
- Morgan D., Hickling G.2000Techniques used for poisoning possums. In The brushtail possum: biology, impact and management of an introduced marsupial (ed. Montague T. L.), pp. 143–153 Lincoln, New Zealand: Manaaki Whenua Press [Google Scholar]
- Morris R. S., Pfeiffer D. U.1995Directions and issues in bovine tuberculosis epidemiology and control in New Zealand. N. Z. Vet. J. 43, 256–265 [DOI] [PubMed] [Google Scholar]
- Morris R. S., Pfeiffer D. U., Jackson R.1994The epidemiology of Mycobacterium bovis infections. Vet. Microbiol. 40, 153–177 (doi:10.1016/0378-1135(94)90053-1) [DOI] [PubMed] [Google Scholar]
- Nol P., et al. 2008Efficacy of oral and parenteral routes of Mycobacterium bovis bacille Calmette–Guerin vaccination against experimental bovine tuberculosis in white-tailed deer (Odocoileus virginianus): a feasibility study. J. Wildl. Dis. 44, 247–259 [DOI] [PubMed] [Google Scholar]
- O'Reilly L. M., Daborn C. J.1995The epidemiology of Mycobacterium bovis infection in animals and man: a review. Tuberc. Lung Dis. 76(Suppl. 1), 1–46 [DOI] [PubMed] [Google Scholar]
- Olmstead A. L., Rhode P. W.2002An impossible undertaking: the eradication of bovine tuberculosis in the United States. Draft paper Chapel Hill: University of California, Davis and University of North Carolina; See http://aghistory.ucdavis.edu/TBDEC2.pdf [Google Scholar]
- Olsen S. C., Kreeger T. J., Schultz W.2002Immune responses of bison to ballistic or hand vaccination with Brucella abortus strain RB51. J. Wildl. Dis. 38, 738–745 [DOI] [PubMed] [Google Scholar]
- Phillips C. J., Foster C. R., Morris P. A., Teverson R.2003The transmission of Mycobacterium bovis infection to cattle. Res. Vet. Sci. B 74, 1–15 (doi:10.1016/S0034-5288(02)00145-5) [DOI] [PubMed] [Google Scholar]
- Ramsey D., Cowan P.2003Mortality rate and movements of brushtail possums with clinical tuberculosis (Mycobacterium bovis infection). N. Z. Vet. J. 51, 179–185 [DOI] [PubMed] [Google Scholar]
- Ramsey D., Coleman J., Coleman M., Horton P.2006The effect of fertility control on the transmission of bovine Tb in brushtail possums. N. Z. Vet. J. 54, 218–223 [DOI] [PubMed] [Google Scholar]
- Ramsey D. S., Aldwell F. E., Cross M. L., de Lisle G. W., Buddle B. M.2009Protection of free-living and captive possums against pulmonary challenge with Mycobacterium bovis following oral BCG vaccination. Tuberculosis 89, 163–168 (doi:10.1016/j.tube.2008.11.002) [DOI] [PubMed] [Google Scholar]
- Renwick A. R., White P. C. L., Bengis R. G.2007Bovine tuberculosis in southern African wildlife: a multi-species host–pathogen system. Epidemiol. Infect. 135, 529–540 (doi:10.1017/S0950268806007205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reviriego Gordejo F. J., Vermeersch J. P.2006Towards eradication of bovine tuberculosis in the European Union. Vet. Microbiol. 112, 101–109 (doi:10.1016/j.vetmic.2005.11.034) [DOI] [PubMed] [Google Scholar]
- Roberts M.1996The dynamics of bovine tuberculosis in possum populations, and its eradication or control by culling or vaccination. J. Anim. Ecol. 65, 451–464 (doi:10.2307/5780) [Google Scholar]
- Rocke T. E., Smith S. R., Stinchcomb D. T., Osorio J. E.2008Immunization of black-tailed prairie dogs against plague through consumption of vaccine-laden baits. J. Wildl. Dis. 44, 930–937 [DOI] [PubMed] [Google Scholar]
- Rupprecht C. E., Hanlon C. A., Slate D.2004Oral vaccination of wildlife against rabies: opportunities and challenges in prevention and control. Dev. Biol. 119, 173–184 [PubMed] [Google Scholar]
- Ryan T. J., Livingstone P. G., Ramsey D. S., de Lisle G. W., Nugent G., Collins D. M., Buddle B. M.2006Advances in understanding disease epidemiology and implications for control and eradication of tuberculosis in livestock: the experience from New Zealand. Vet. Microbiol. 112, 211–219 (doi:10.1016/j.vetmic.2005.11.025) [DOI] [PubMed] [Google Scholar]
- Sargeant J. M.2008The influence of veterinary epidemiology on public health: past, present and future. Prev. Vet. Med. 86, 250–259 (doi:10.1016/j.prevetmed.2008.02.011) [DOI] [PubMed] [Google Scholar]
- Schmitt S. M., et al. 1997Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33, 749–758 [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Keen D. L., Parlane N. A., Hamel K. L., Yates G. F., Buddle B. M.2005Improving protective efficacy of BCG vaccination for wildlife against bovine tuberculosis. Res. Vet. Sci. 78, 231–236 (doi:10.1016/j.rvsc.2004.07.007) [DOI] [PubMed] [Google Scholar]
- Tompkins D. M., Ramsey D.2007Optimising bait-station delivery of fertility control agents to brushtail possum populations. Wildl. Res. 34, 67–76 (doi:10.1071/WR05109) [Google Scholar]
- Tsao J. I., Wootton J. T., Bunikis J., Luna M. G., Fish D., Barbour A. G.2004An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl Acad. Sci. USA 101, 18 159–18 164 (doi:10.1073/pnas.0405763102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial F., Cleaveland S., Rasmussen G., Haydon D. T.2006Development of vaccination strategies for the management of rabies in African wild dogs. Biol. Cons. 131, 180–192 (doi:10.1016/j.biocon.2006.04.005) [Google Scholar]
- Vicente J., Höfle U., Garrido J. M., Fernández-de-mera I. G., Acevedo P., Juste R., Barral M., Gortazar C.2007Risk factors associated with the prevalence of tuberculosis-like lesions in fenced wild boar and red deer in south central Spain. Vet. Res. 38, 451–464 (doi:10.1051/vetres:2007002) [DOI] [PubMed] [Google Scholar]
- Von Rüden S., et al. 2008Retrospective analysis of the immunisation of wild boar populations against classical swine fever virus (CSFV) in region Eifel of Rhineland-Palatinate. Vet. Microbiol. 132, 29–38 (doi:10.1016/j.vetmic.2008.04.022) [DOI] [PubMed] [Google Scholar]
- White G. C., Burnham K. P.1999Program MARK: survival estimation from populations of marked animals. Bird Study 46, 120–139 [Google Scholar]
- Winter J. W.1980Tooth wear as an age index in a population of the brush-tailed possum, Trichosurus vulpecular (Kerr). Aust. Wildl. Res. 7, 359–363 (doi:10.1071/WR9800359) [Google Scholar]
- Wirth T., et al. 2008Origin, spread and demography of the Mycobacterium tuberculosis complex. Plos Pathog. 4, e1000160 (doi:10.1371/journal.ppat.1000160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroffe R., et al. 2009Bovine tuberculosis in cattle and badgers in localized culling areas. J. Wildl. Dis. 45, 128–143 [DOI] [PubMed] [Google Scholar]
- World Health Organization 2004WHO expert consultation on rabies. In WHO Technical Report Series, 931 Geneva, Switzerland: World Health Organization; [PubMed] [Google Scholar]