Abstract
Coral reefs are rich in biodiversity, in large part because their highly complex architecture provides shelter and resources for a wide range of organisms. Recent rapid declines in hard coral cover have occurred across the Caribbean region, but the concomitant consequences for reef architecture have not been quantified on a large scale to date. We provide, to our knowledge, the first region-wide analysis of changes in reef architectural complexity, using nearly 500 surveys across 200 reefs, between 1969 and 2008. The architectural complexity of Caribbean reefs has declined nonlinearly with the near disappearance of the most complex reefs over the last 40 years. The flattening of Caribbean reefs was apparent by the early 1980s, followed by a period of stasis between 1985 and 1998 and then a resumption of the decline in complexity to the present. Rates of loss are similar on shallow (<6 m), mid-water (6–20 m) and deep (>20 m) reefs and are consistent across all five subregions. The temporal pattern of declining architecture coincides with key events in recent Caribbean ecological history: the loss of structurally complex Acropora corals, the mass mortality of the grazing urchin Diadema antillarum and the 1998 El Nino Southern Oscillation-induced worldwide coral bleaching event. The consistently low estimates of current architectural complexity suggest regional-scale degradation and homogenization of reef structure. The widespread loss of architectural complexity is likely to have serious consequences for reef biodiversity, ecosystem functioning and associated environmental services.
Keywords: climate change, ecosystem degradation, ecosystem services, foundation species, habitat complexity, vulnerability
1. Introduction
The physical structure of a habitat profoundly influences its associated biodiversity and ecosystem functioning (MacArthur & MacArthur 1961), with more complex habitats facilitating species coexistence through niche partitioning and the provision of refuges from predators and environmental stressors (Bruno & Bertness 2001; Willis et al. 2005). In tropical shallow waters, the calcium carbonate skeletons of stony corals contribute to reef frameworks that sustain the most diverse ecosystem in our seas (Spalding et al. 2001). However, coral reefs have been heavily impacted worldwide by a combination of local and global stressors, including overfishing, climate change-induced coral bleaching, eutrophication and disease (Hughes et al. 2003). The marked declines in live hard coral cover documented over recent decades throughout the Caribbean and the Indo-Pacific regions (Gardner et al. 2003; Bruno & Selig 2007) exceed those reported for many other foundation species in terrestrial or marine ecosystems (Balmford et al. 2003). However, in contrast to other ecosystems where degradation usually indicates reductions in habitat area (e.g. deforestation), decreases in live coral cover on coral reefs do not immediately result in loss of available habitat because the reef framework can persist long after the death of corals.
In the Caribbean, declines in live coral cover began in the late 1970s, when substantial loss of the major reef-forming corals Acropora palmata and Acropora cervicornis occurred as a result of white-band disease (Aronson & Precht 2001). Coral mortality, in combination with the mass mortality of the black sea urchin (Diadema antillarum), which was a major remover of algae, and the long-term depletion of herbivorous fishes through overfishing, facilitated phase shifts to macro-algal dominance on many reefs (Carpenter 1988; Precht & Aronson 2006). In the Caribbean and elsewhere, reef-building corals now face new threats from climate change, particularly in the form of thermally induced coral bleaching and mortality, which are becoming increasingly frequent and extensive as thermal anomalies intensify and lengthen (Hughes et al. 2003; McWilliams et al. 2005).
A potential consequence of the widespread reduction in Caribbean coral cover is a reversal of the historic net accretion of calcium carbonate, resulting in a decrease in calcification and erosion of the reef framework. At local scales, hard coral mortality is associated with the loss of architectural complexity and ‘reef flattening’ after direct impacts such as hurricanes through the breakage of coral skeletons (e.g. Rogers et al. 1982). Reefs may also erode gradually owing to the natural activity of host organisms, such as herbivorous fishes and sea urchins, and by physical abrasion or geochemical shifts. However, widespread mortality of hard corals, for example, after severe bleaching events, moves the balance towards net reef erosion (Sheppard et al. 2002). These impacts could be exacerbated in the future by ocean acidification, which is expected to enhance calcium carbonate dissolution with negative consequences, initially for coral growth and eventually for the entire reef framework (Hoegh-Guldberg et al. 2007).
The ecological and socio-economic consequences of declining architectural complexity are likely to be substantial (Pratchett et al. 2008). For many reef organisms, risk of predation is influenced by access to refuges, and the densities of herbivores and grazing rates typically increase with architectural complexity (Beukers & Jones 1997; McClanahan 1999; Almany 2004; Lee 2006). Consequently, the species richness, abundance and biomass of coral reef fishes and invertebrates are all influenced by architectural complexity (e.g. Gratwicke & Speight 2005; Idjadi & Edmunds 2006; Wilson et al. 2007). The loss of architectural complexity may therefore drive declines in diversity, particularly of habitat specialists, and compromise fisheries productivity through elevated post-settlement mortality (Beukers & Jones 1997; Graham et al. 2007). Reef architectural complexity also plays a key role in providing important environmental services to humans, including enhancing coastal protection through the dissipation of wave energy transmitted over reefs (Lugo-Fernandez et al. 1998).
While recent regional-scale analyses have revealed declines in hard coral cover (Gardner et al. 2003; Bruno & Selig 2007), the consequences for reef habitat complexity on a similar large scale have not been quantified. The capacity of reefs to continue to perform key functions of refuge provision and coastal protection will depend on whether reef architecture persists for a substantial period of time following the loss of live coral. Here we collate published and unpublished estimates of reef complexity spanning four decades from reefs across the Caribbean, a region with clear evidence of recent declines in coral cover. We explore the rate and timing of changes in reef architecture in relation to region-wide events such as the demise of Acropora corals and grazing urchins. As the drivers of reef degradation are apparent throughout the Caribbean, we also examine whether the patterns are consistent throughout the entire region.
2. Material and methods
(a). Estimating architectural complexity
Habitat complexity on coral reefs has been measured using a variety of methods that differ in the attributes measured, the scale of measurement and the degree of subjectivity (with attendant variation in inter-observer comparability). The rugosity index is by far the most widely used method for measuring reef architectural complexity (see electronic supplementary material for further details) and is generally highly correlated with other methods (Wilson et al. 2007). Studies reporting the rugosity index were therefore chosen to quantify spatial and temporal variation in the architectural complexity of reefs across the Caribbean.
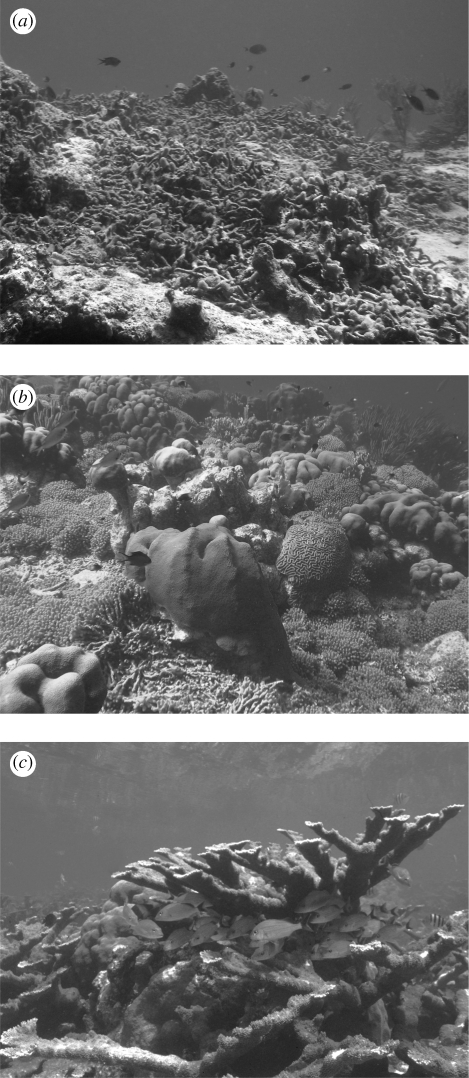
The rugosity index is expressed as the ratio between the total length of a chain and the length of the same chain when moulded to a reef surface. A perfectly flat surface would have a rugosity index of 1, with larger numbers indicating a greater degree of architectural complexity (figure 1). The index tends towards infinity with increasing architectural complexity; however, rugosity estimates greater than 3 are very rare.
Figure 1.
Examples of three different values of rugosity index of architectural complexity on Caribbean reefs. The values of the index are (a) 1.2, (b) 1.5 and (c) 2.5. Source for photos: L. Alvarez-Filip, M. Uyarra and W. Henry Marine Photobank.
(b). Data search
A database of quantitative surveys that measured reef rugosity within the wider Caribbean was compiled. We searched online ISI Web of Science, Google Scholar and other relevant databases (e.g. Reefbase) for peer-reviewed and grey literature using several search terms (see electronic supplementary material for examples). We also searched for papers that used the rugosity index in all issues of the journals Coral Reefs, Bulletin of Marine Science, Atoll Research Bulletin, Caribbean Journal of Science and in all Proceedings of the International Coral Reef Symposium. Additionally, we directly contacted coral reef scientists, site managers and those responsible for reef monitoring programmes throughout the Caribbean, asking for any available data pertaining to their study sites.
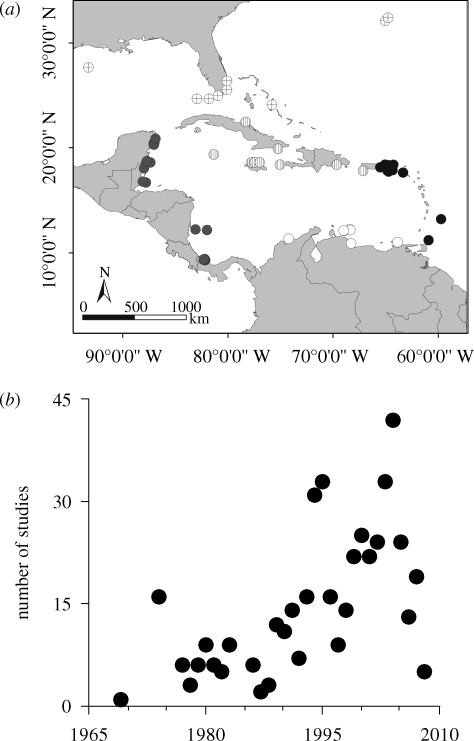
A total of 464 records from 200 reefs surveyed between 1969 and 2008 across the Caribbean were obtained (figure 2a,b). The database includes reefs that were surveyed only once (n = 214) and reefs where repeated measures of rugosity were collected over more than 1 year (n = 250). Both datasets provide highly consistent results (table S2, electronic supplementary material). We therefore present findings only from the whole dataset, because they offer a wider spatial and temporal representation.
Figure 2.
(a) Regional distribution of locations from which rugosity values were obtained. Grey circles, Central America; white circles, South America; black circles, lesser Antilles; circles with vertical lines, greater Antilles; circles with crosses, southwest North Atlantic. (b) Number of studies from which rugosity data were collated per year, from 1969 to 2008.
(c). Analyses
To assess the temporal pattern of change in region-wide architectural complexity, we calculated annual estimates of rugosity averaged across all available sites for each year from 1969 to 2008. We fitted a range of linear and nonlinear models to represent increasing degrees of complexity in the rate of change in rugosity over time and used the small-sample adjusted Akaike information criterion (AICc) to evaluate the models (Burnham & Anderson 2002). Linear models were fitted using both simple regressions and robust regression, to reduce the influence of outliers. We contrasted these linear models, which represent a hypothesis of constant change in rugosity over the whole time period, against segmented models that assumed piecewise linear relationships (i.e. two or more straight lines connected by breakpoints) and a general additive model (GAM) of an unspecified nonlinear (spline) function, which assumed that the rate of change in rugosity varied over time (Venables & Ripley 2002; Muggeo 2003). In addition, because the number of sites contributing to each annual rugosity estimate varied, with more sites available towards the end of the time period, we ran all models with annual estimates unweighted and weighted by sample size. Weighted models consistently provided a significantly better fit (lower AIC and higher variance explained) than unweighted models. All analyses were implemented in R (R 2008).
We used randomization techniques to evaluate whether the pattern and rate of change were sensitive to the inclusion of any particular site or year. For the best-supported model identified in the AICc analysis, we tested whether the rate of decline in rugosity was biased by the inclusion of any particular year, using a jackknife method to calculate the distribution of annual decline rates while sequentially removing each individual year. To evaluate any potential site selection bias, we used a bootstrap method to compare the annual decline rate with the range of possible decline rates for 10 000 random combinations of year, rugosity and weighting.
To explore whether the trends of changing architectural complexity varied with depth and within the region, we aggregated the data by decade to maximize the signal relative to interannual variation while retaining sufficient power. To evaluate the change in rugosity at different depths, we divided the data into three zones: (i) <6 m, which represents the optimal range of A. palmata (and therefore Acropora reefs); (ii) 6–20 m, to include the range of other reef-building scleractinian corals, including A. cervicornis; and (iii) >20 m, to reflect sites where hard corals are present but do not necessarily form complex three-dimensional structures. We also aggregated the data within five subregions to explore spatial variation in changes in rugosity within the Caribbean region (figure 2a).
A key question is whether the regional change in reef structure has produced more structurally homogeneous habitats throughout the Caribbean. We classified each reef into one of five rugosity index categories (1.0–1.49, 1.5–1.99, 2.0–2.49, 2.5–2.99 and greater than 3.0) to explore the change in the region-wide representation of complex (rugosity greater than 2.0) and flatter (rugosity less than or equal to 1.5) reefs for the four different decades.
3. Results
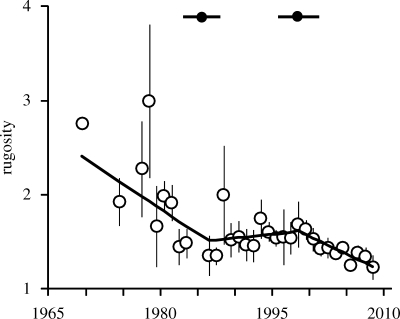
There has been a marked decline in the architectural complexity of Caribbean reefs over the past four decades (figure 3). The best-supported model of change in rugosity over time was a weighted segmented model (table 1), which suggests that the decline in rugosity has three distinct phases of change (figure 3). Architectural complexity declined steeply early in the time series (1969–1985), from reefs with indices of approximately 2.5 to much flatter reefs with indices of approximately 1.5. This period of decline ended in 1985 (±2.4 years s.e.), and architectural complexity throughout the region then remained relatively stable until the late 1990s. However, since 1998 (±2.8 years s.e.), the declining trend has resumed, with rugosity indices after the mid-2000s reaching the lowest levels recorded in the time series (approx. 1.2; see example in figure 1). The pattern of change is robust to the inclusion or exclusion of individual years (jackknife) and individual sites (bootstrap) (figure S1, electronic supplementary material).
Figure 3.
Changes in reef rugosity on reefs across the Caribbean from 1969 to 2008. Black line represents the best fitting model—a segmented regression weighted by the number of sites contributing to each annual rugosity estimate (mean ± 95% confidence intervals). Black dots at the top of the figure indicate the significant breakpoint in 1985 and 1998 (±1 s.e.) for the segmented regression. Model slopes: 1969–1984, −0.054; 1985–1997, 0.008; 1998–2008, −0.038.
Table 1.
Model structure and the temporal pattern of change in Caribbean architectural complexity. Summary of AICc analysis of linear and nonlinear models of change in yearly mean rugosity (derived from all 464 estimates), ordered by decreasing weight. (Models in which annual rugosity estimates have been weighted by sample size are indicated (wt). df, degrees of freedom of the model (for GAM, we use the estimated degrees of freedom). AICc is the Akaike information criterion corrected for small sample size; Δ is the difference in AICc between a given model and the best-supported model (indicated in bold); and W is the Akaike weight, which represents the probability that a given model is the best of those models considered. The asterisks indicate the average slope of the different model segments.)
| model | R2 | slope | df | AICc | ΔAICc | AICcW |
|---|---|---|---|---|---|---|
| segmented model (wt) | 0.64 | −0.028* | 26 | −25.8 | 0 | 0.8695 |
| linear model (wt) | 0.53 | −0.019 | 30 | −17.1 | 8.7 | 0.0112 |
| robust linear model (wt) | — | −0.018 | 30 | −16.9 | 8.8 | 0.0107 |
| segmented model | 0.65 | −0.038* | 26 | −2.9 | 22.9 | 0.0000 |
| generalized additive model (wt) | 0.99 | −0.033* | 3.6 | 0.1 | 25.8 | 0.0000 |
| linear model | 0.49 | −0.026 | 30 | 9.4 | 35.2 | 0.0000 |
| robust linear model | — | −0.021 | 30 | 11.2 | 37 | 0.0000 |
| generalized additive model | 0.59 | −0.044* | 3.3 | 22.8 | 48.6 | 0.0000 |
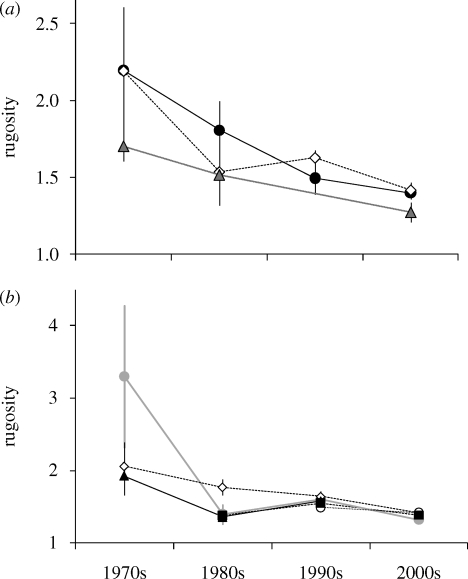
The decline in architectural complexity is widespread. The temporal pattern of change was consistent across all three depth intervals (figure 4a) and across the three subregions for which the available data spanned the whole time period, and the two regions with patchier data (Central America and southwest North Atlantic; figure 4b).
Figure 4.
Change in Caribbean reef rugosity in four different decades (a) at three depth intervals (filled circle, 0–6 m; open diamond, 6–20 m; filled triangle, 20 m) and (b) in five subregions (filled square, southwest North Atlantic; grey circle, greater Antilles; open diamond, lesser Antilles; filled triangle, South America; open circle, Central America) (mean index value ± 95% confidence intervals).
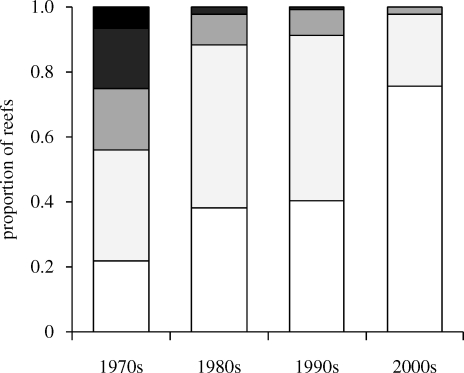
Caribbean reefs are becoming both flatter and more structurally homogeneous across the region. The proportion of complex reefs (rugosity greater than 2) has declined from approximately 45 per cent of sites to approximately 2 per cent in the past four decades (figure 5).
Figure 5.
Proportion of reefs in five rugosity index categories across the Caribbean between 1969 and 2008. Number of studies for each decade: 1970s: n = 32; 1980s: n = 52; 1990s: n = 136 and 2000s: n = 167. Black, >3; dark grey, 2.5–3; mid grey, 2–2.5; pale grey, 1.5–2; white, 1–1.5.
4. Discussion
The architectural complexity of coral reefs has declined drastically over the last 40 years throughout the Caribbean. Structurally complex reefs with a rugosity of greater than 2 have been virtually lost from the entire region. Today, the flattest reefs (rugosity less than 1.5) comprise approximately 75 per cent of the total compared with approximately 20 per cent in the 1970s, with most of the increase in the proportion of flattest reefs occurring in the 2000s. The high proportion of complex reefs in the 1960s and 1970s is unlikely to result from researchers tending to visit just the most pristine reefs at this time, because less architecturally-complex categories were also well represented during this period. The loss of architectural complexity is nonlinear and has occurred over three distinct phases that coincide closely with large-scale events that have affected Caribbean reef ecosystems. The rate of decline was steepest prior to 1985. The sample sizes are small and variance high during the 1960s and 1970s, hence it is unclear whether the decline began prior to the early 1980s, when widespread loss of acroporid corals began (Precht & Aronson 2006). After this period, average architectural complexity changed little until the late 1990s, when a new episode of decline began. The pattern of decline is consistent across depth zones and subregions. The widespread occurrence of flatter reefs could have serious implications for reef-associated biodiversity and reef-based environmental services.
The nonlinearity in the loss of architectural complexity suggests that different drivers operating at different times have influenced components of the reef community. Disturbances on reefs range in scale and intensity, from local tropical storms that can break and displace coral skeletons, to widespread events such as climate-induced bleaching and diseases that kill coral tissue without immediately compromising the reef structure (Pratchett et al. 2008). In the late 1970s, one key event is likely to have had a major role in the early, steep decline in Caribbean reef architecture. White-band disease killed approximately 90 per cent of the shallow-water, structurally dominant acroporid corals, exposing their fragile branching skeletons to erosion and hurricanes that probably led to their collapse in subsequent years (Aronson & Precht 2001). However, declines also occurred at depths greater than those at which acroporids were dominant, suggesting that the systematic loss of Caribbean reef corals was more widespread than previously thought during the 1970s and early 1980s.
After 1985, the main driver(s) of declining architectural complexity appear to cease; by this time, acroporids had disappeared almost entirely from the Caribbean, and the sea urchin D. antillarum had experienced a region-wide disease-induced mass mortality in 1983–1984 (Carpenter 1988). The loss of this important source of bioerosion may have slowed the decline following the first phase of reef flattening. This intermediate stable period of architectural complexity in the region persisted in the face of several disturbance events, including the first large-scale bleaching events and several major hurricanes (Gardner et al. 2005; McWilliams et al. 2005).
Around 1998, Caribbean reefs were tipped into a new phase of structural decline, following the most intense and widespread coral bleaching event to date (McWilliams et al. 2005). The coral mortality and reductions in growth rates that typically follow such bleaching events are likely to have precipitated the resumption of loss of architectural complexity. The low levels of coral cover, and presumably reef accretion, at this time (Gardner et al. 2003) may also have increased rates of erosion of underlying geological structures that were no longer shielded by actively growing hard corals. Since 1998, further mass bleaching events have occurred regularly (McWilliams et al. 2005), probably contributing to the continued decline in reef complexity.
All of the major events that are likely to have impacted reef complexity have occurred against a backdrop of changes not only in coral abundance but also in community composition. Following the disappearance of acroporids, massive species with slower growth rates, such as Montastrea spp., remained as the primary reef framework builders, and weedy corals, such as Porites spp. and Agaricia spp., that form rapidly growing, small colonies that are short-lived and quickly replaced, started to increase in abundance (Green et al. 2008). The shift from major reef-building species to weedy species that contribute less to maintenance of the reef framework, combined with increases in macro-algae (Côté et al. 2006) that compete for space with coral recruits (Mumby et al. 2007), probably reduced the rates of coral accretion on Caribbean reefs.
The loss of reef architecture is likely to have profound ecological, social and economic impacts. A growing body of evidence indicates severe repercussions for biodiversity of the loss of architectural complexity. On Indo-Pacific reefs, major changes in fish community composition have resulted from the long-term loss of structure following coral bleaching events (Pratchett et al. 2008 and references therein). The effects of bleaching are first manifest in obligate coral-dwelling species, followed by impacts on other small-bodied fishes (both small adults and juveniles of larger species) when the physical matrix of the reef collapses (Pratchett et al. 2008). In the Caribbean, the greatest impacts on biodiversity are expected to occur only with the breakdown of the reef matrix because no fish species feed exclusively on live coral, although many reef-associated species depend highly on rugose substrata to feed, recruit and hide (Gratwicke & Speight 2005). In this context, declining reef complexity may explain the onset of a decline in Caribbean reef fishes that has occurred since approximately 1996 (Paddack et al. 2009). Given that the loss of reef architecture began much earlier, our analysis supports the notion of a degradation debt for Caribbean reef fishes. Reduced recruitment resulting from a lack of settlement sites and refuges for species with commercial importance, such as lobsters and large fishes (Graham et al. 2007; Wynne & Côté 2007), may compromise the long-term sustainability of fisheries and fishing communities. Collapsing reef structures may also lead to the loss of important environmental services such as coastal protection. Simulation models predict that a reduction in reef surface roughness of approximately 50 per cent could produce a doubling of the wave energy reaching the shores behind those reefs (Sheppard et al. 2005). The vulnerability of coastal human communities in the Caribbean to projected increases in the intensity of Atlantic Ocean hurricanes and sea levels (Hopkinson et al. 2008) will therefore probably be compounded by the reduced wave dissipation function of architecturally simpler reefs.
Reversing declines in reef architecture will be a major challenge for scientists and policy-makers concerned with maintaining reef ecosystems and the security and wellbeing of Caribbean coastal communities. Although recent evidence suggests increases in coral cover on some Caribbean reefs (e.g. Cho & Woodley 2000; Idjadi et al. 2006), the effect of coral recovery on architectural complexity is unknown. If weedy corals dominate this recovery in the long term, future reef complexity is unlikely to mirror any improvement in coral condition. To regain the levels of architectural complexity that were prevalent prior to 1980, the recovery of large branching corals (i.e. Acropora spp.) and the maintenance of healthy populations of massive robust species (e.g. Montastrea spp.) are essential within the region. Not meeting these challenges will most probably result in a continued flattening of reefs throughout the region and seriously compromised biodiversity and environmental services.
Acknowledgements
We are grateful to Peter Edmunds, Michelle Paddack, Philip Molloy, Renata Goodridge and Hazel Oxenford (CARICOMP Barbados), Francisco Geraldes (Centro de Investigaciones de Biología Marina de la Universidad Autónoma de Santo Domingo and CARICOMP) and Simon Pittman and the NOAA Biogeography Branch for contributing unpublished data. L.A.-F. was supported by a scholarship from the CONACYT (171864) Mexico. I.M.C. and N.K.D. are supported by Discovery grants from the Natural Sciences and Engineering Research Council of Canada.
References
- Almany G. R.2004Differential effects of habitat complexity, predators and competitors on abundance of juvenile and adult coral reef fishes. Oecologia 141, 105–113 (doi:10.1007/s00442-004-1617-0) [DOI] [PubMed] [Google Scholar]
- Aronson R. B., Precht W. F.2001White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460, 25–38 (doi:10.1023/A:1013103928980) [Google Scholar]
- Balmford A., Green R. E., Jenkins M.2003Measuring the changing state of nature. Trends Ecol. Evol. 18, 326–330 (doi:10.1016/S0169-5347(03)00067-3) [Google Scholar]
- Beukers J. S., Jones G. P.1997Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114, 50–59 (doi:10.1007/s004420050419) [DOI] [PubMed] [Google Scholar]
- Bruno J. F., Bertness M. D.2001Habitat modification and facilitation in benthic marine communities. In Marine community ecology (eds Bertness M. D., Gaines S. D., Hay M. E.), pp. 201–218 Sunderland, MA: Sinauer [Google Scholar]
- Bruno J. F., Selig E. Z.2007Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2, e711 (doi:10.1371/journal.pone.0000711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham K. P., Anderson D. R.2002Model selection and multimodel inference: a practical information–theoretic approach. New York, NY: Springer-Verlag [Google Scholar]
- Carpenter R. C.1988Mass mortality of a Caribbean sea urchin: immediate effects on community metabolism and other herbivores. Proc. Natl Acad. Sci. USA 85, 511–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho L. L., Woodley J. D.2000Recovery of reefs at Discovery Bay, Jamaica and the role of Diadema antillarum. In 9th Int. Coral Reef Symp. vol. 1 (eds Moosa M. K., Soemodihardjo S., Soegiarto A., Romimohtarto K., Nontji A., Soekarno, Suharsono), pp. 331–338 Bali, Indonesia: Ministry of Environment, Indonesian Institute of Sciences and International Society for Reef Studies [Google Scholar]
- Côté I. M., Gardner T. A., Gill J. A., Hutchinso D. J., Watkinson A. R.2006New approaches to estimating recent ecological changes on coral reefs. In Coral reef conservation (eds Côté I. M., Reynolds D. J.), pp. 293–313 Cambridge, UK: Cambridge University Press [Google Scholar]
- Gardner T. A., Côté I. M., Gill J. A., Grant A., Watkinson A. R.2003Long-term region-wide declines in Caribbean corals. Science 301, 958–960 (doi:10.1126/science.1086050) [DOI] [PubMed] [Google Scholar]
- Gardner T. A., Côté I. M., Gill J. A., Grant A., Watkinson A. R.2005Hurricanes and Caribbean coral reefs: impacts, recovery patterns, and role in long-term decline. Ecology 85, 174–184 (doi:10.1890/04-0141) [Google Scholar]
- Graham N. A. J., Wilson S. K., Jennings S., Polunin N. V. C., Robinson J., Bijoux J. P., Daw T. M.2007Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 21, 1291–1300 (doi:10.1111/j.1523-1739.200700754.x) [DOI] [PubMed] [Google Scholar]
- Gratwicke B., Speight M. R.2005Effects of habitat complexity on Caribbean marine fish assemblages. Mar. Ecol. Prog. Ser. 292, 301–310 (doi:10.3354/meps292301) [Google Scholar]
- Green D. H., Edmunds P. J., Carpenter R. C.2008Increasing relative abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Mar. Ecol. Prog. Ser. 359, 1–10 (doi:10.3354/meps07454) [Google Scholar]
- Hoegh-Guldberg O., et al. 2007Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- Hopkinson C. S., Lugo A. E., Alber M., Covich A. P., Van Bloem S. J.2008Forecasting effects of sea-level rise and windstorms on coastal and inland ecosystems. Front. Ecol. Environ. 6, 255–263 (doi:10.1890/070153) [Google Scholar]
- Hughes T. P., et al. 2003Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (doi:10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- Idjadi J. A., Edmunds P. J.2006Scleractinian corals as facilitators for other invertebrates on a Caribbean reef. Mar. Ecol. Prog. Ser. 319, 117–127 (doi:10.3354/meps319117) [Google Scholar]
- Idjadi J. A., Lee S. C., Bruno J. F., Precht W. F., Allen-Requa L., Edmunds P. J.2006Rapid phase-shift reversal on a Jamaican coral reef. Coral Reefs 25, 209–211 (doi:10.1007/s00338-006-0088-7) [Google Scholar]
- Lee S. C.2006Habitat complexity and consumer-mediated positive feedbacks on a Caribbean coral reef. Oikos 112, 442–447 (doi:10.1111/j.0030-1299.2006.14247.x) [Google Scholar]
- Lugo-Fernandez A., Roberts H. H., Suhayda J. N.1998Wave transformations across a Caribbean fringing-barrier coral reef. Cont. Shelf Res. 18, 1099–1124 (doi:10.1016/S0278-4343(97)00020-4) [Google Scholar]
- MacArthur R. H., MacArthur J. W.1961On bird species diversity. Ecology 42, 594–598 [Google Scholar]
- McClanahan T. R.1999Predation and the control of the sea urchin Echinometra viridis and fleshy algae in the patch reefs of Glovers Reef, Belize. Ecosystems 2, 511–523 (doi:10.1007/s100219900099) [Google Scholar]
- McWilliams J. P., Côté I. M., Gill J. A., Sutherland W. J., Watkinson A. R.2005Accelerating impacts of temperature-induced coral bleaching in the Caribbean. Ecology 86, 2055–2060 (doi:10.1890/04-1657) [Google Scholar]
- Muggeo V. M. R.2003Estimating regression models with unknown break-points. Stat. Med. 22, 3055–3071 (doi:10.1002/sim.1545) [DOI] [PubMed] [Google Scholar]
- Mumby P. J., et al. 2007Trophic cascade facilitates coral recruitment in a marine reserve. Proc. Natl Acad. Sci. 104, 8362–8367 (doi:10.1073/pnas.0702602104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddack M. J., et al. 2009Recent region-wide declines in Caribbean reef fish abundance. Curr. Biol. 19, 590–595 (doi:10.1016/j.cub.2009.02.041) [DOI] [PubMed] [Google Scholar]
- Pratchett M. S., Munday P. L., Wilson S. K., Graham N. A. J., Cinner J. E., Bellwood D. R., Jones G. P., Polunin N., McClanahan T.2008Effects of climate-induced coral bleaching on coral-reef-fishes: ecological and economic consequences. Oceanogr. Mar. Biol. Annu. Rev. 46, 251–296 [Google Scholar]
- Precht W. F., Aronson R. B.2006Death and resurrection of Caribbean coral reefs: a paleoecological perspective. In Coral reef conservation (eds Côté I. M., Reynolds D. J.), pp. 40–77 Cambridge, UK: Cambridge University Press [Google Scholar]
- R. 2008 R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Rogers C. S., Suchanek T. H., Pecora F. A.1982Effects of hurricanes David and Frederic (1979) on shallow Acropora palmata reef communities: St-Croix, United States Virgin Islands. Bull. Mar. Sci. 32, 532–548 [Google Scholar]
- Sheppard C. R. C., Spalding M., Bradshaw C., Wilson S.2002Erosion vs. recovery of coral reefs after 1998 El nino: Chagos reefs, Indian Ocean. Ambio 31, 40–48 (doi:10.1579/0044-7447-31.1.40) [PubMed] [Google Scholar]
- Sheppard C., Dixon D. J., Gourlay M., Sheppard A., Payet R.2005Coral mortality increases wave energy reaching shores protected by reef flats: examples from the Seychelles. Estuar. Coast. Shelf Sci. 64, 223–234 (doi:10.1016/j.ecss.2005.02.016) [Google Scholar]
- Spalding M., Ravilious C., Green E. P.2001World atlas of coral reefs Berkeley, CA: University of California Press [Google Scholar]
- Venables W. N., Ripley B. D.2002Modern and applied statistics New York, NY: Springer-Verlag [Google Scholar]
- Willis S., Winemiller K., Lopez-Fernandez H.2005Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142, 284–295 (doi:10.1007/s00442-004-1723-z) [DOI] [PubMed] [Google Scholar]
- Wilson S. K., Graham N. A. J., Polunin N. V. C.2007Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 151, 1069–1076 (doi:10.1007/s00227-006-0538-3) [Google Scholar]
- Wynne S. P., Côté I. M.2007Effects of habitat quality and fishing on Caribbean spotted spiny lobster populations. J. Appl. Ecol. 44, 488–494 (doi:10.1111/j.1365-2664.2007.01312.x) [Google Scholar]