Abstract
Recent community-level studies have acknowledged that generalist species are more widespread than previously thought and highlighted their preponderant impact on community functioning and evolution. It is suggested that the type of interaction, trophic versus mutualistic, should affect species generalization level; however, no direct comparison has been made yet. Here, we performed such a comparison using 44 plant–insect networks describing either pollination or herbivory communities. Our analysis shows that the type of interaction does indeed have an impact on various aspects of species generalism, from the distribution of generalism in the community to the phylogenetic diversity of the plants with which a given insect species interacts. However, the amplitude of the observed differences depends on the aspect of species generalism studied. While the non-quantitative and quantitative measures of generalism suggest that pollinators interact with more plant species and more evenly than herbivores, phylogenetic measures clearly show that herbivores interact with plant species far more closely related to each other than pollinators. This comparative approach offers a promising perspective to better understand the functioning and evolution of multispecies assemblages by pointing out some fundamental singularities of communities depending on the type of interaction considered.
Keywords: generalism, herbivory, interaction web, mutualistic, pollination, trophic
1. Introduction
Interactions among species are one of the most important drivers of the ecology and evolution of species. Although historically studies in terrestrial plant–animal interactions have focused on direct pairwise interactions, it is now acknowledged that generalization in interactions among species is more widespread than previously thought. This relatively high prevalence of generalist species has been highlighted in both mutualistic (Waser et al. 1996) and trophic (Novotny & Basset 2005) interaction networks. Species generalism has some important consequences on the functioning and evolution of ecological systems, and the way we study them. Indeed, from an evolutionary perspective, the long-standing interest in coevolution between pairs of species is now challenged by the concept of diffuse coevolution, where selection pressures caused by one species change in the presence of other species (Janzen 1980; Fox 1988; Inouye & Stinchcombe 2001; Strauss & Irwin 2004). In the same way, from an ecological perspective, studies on the ecological dynamics of simple prey–predator systems are now replaced by multispecies systems and network approaches in which indirect effects among species via shared interacting partners can be as strong as the direct effects between interacting species (Lau & Strauss 2005; van Veen et al. 2006). Thus, generalism is clearly an important species property from both functional and evolutionary perspectives. But interestingly, the views on species generalization are different between the two research areas concerned, respectively, with mutualistic plant–pollinator systems and trophic plant–phytophagous insect systems.
In plant–pollinator studies, network approaches have been used to identify general patterns in community organization. It has been proposed that the distribution of species generalism follows power law family distributions (Jordano et al. 2003); but see Okuyama (2008). This implies that there is a higher proportion of specialist species and some higher generalist species than expected from a random distribution. These findings generate great interest in the internal structure of pollination webs with much emphasis on the importance of highly generalist species in the functioning and resistance to perturbations of pollination webs (Memmott et al. 2004; Fortuna & Bascompte 2006). Turning to plant–insect herbivore studies, the interest mainly focused on the proportion of extreme specialist species in a community. This is directly related to the controversy surrounding global estimates of arthropod species richness (Erwin 1982; Novotny et al. 2002) that range from 2 to 80 million species according to the percentage of specialist species (Thompson 1994). Whereas recent studies have shown that insect herbivores consume more species than previously thought (Novotny & Basset 2005), it has been proposed that these herbivores are often genus specialists, i.e. that the host plant range mainly lies within plant genera rather than within plant species or family (Novotny et al. 2002; Novotny & Basset 2005).
These different current views on insect generalism may simply arise for historical reasons. The scientific communities that focused on mutualism or herbivory were interested in different questions, resulting in apparent differences in insect generalization between these two types of communities. However, this might also reflect some real differences in insect generalization depending on whether insects establish mutualistic or trophic interactions with their plant partners. Indeed, it has been suggested that the type of interaction could have an effect on the way interactions are distributed among species within a community (Rezende et al. 2007; Bascompte et al. 2003). A few recent studies have indeed highlighted structural differences between network types (Lewinsohn et al. 2006; Guimaraes et al. 2007a; Thebault & Fontaine 2008; Van Veen et al. 2008). However, although having important evolutionary and ecological implications, the generalization of mutualistic and trophic interaction web has not been compared yet. In this study, we aim to carry out this comparison, asking the following questions: first, does the nature of the interaction affect the level of non-quantified species generalization (measured as the number of species with which a focal species interacts) within a community? Second, are patterns different when considering quantified generalization indexes that take into account the relative frequency of interaction? Third, is there a relationship between non-quantified generalism and phylogenetic generalism measured as the phylogenetic range of the species with which a given species interacts? And if this is the case, is it affected by the nature of the interaction considered? We performed the comparisons using community-level plant–insect interaction web datasets with resolution at the species level. We focused on insect generalization because plant–herbivore webs are mostly focused on one or a few insect groups, whereas pollination webs aim to consider all pollinators in the community. Thus, the estimation of plant vulnerability (i.e. total number of insects that feed on this plant) is not accessible.
2. Methods
(a). Datasets
We used 43 datasets from published community-wide studies of plant–pollinator (n = 24) and plant–phytophagous insect (n = 20) interactions (complete references are given in the electronic supplementary material, appendix 1). Each dataset had a species resolution level except for a few cases where a few species were lumped into groups owing to identification failure. Each dataset consisted of a list of insects associated with the plant species with which they interact.
(b). Measurement of non-quantified insect generalism
Distributions of non-quantified insect generalism within a community usually belong to the power family (Jordano et al. 2003). In order to detect differences among different parts of the distributions, such as the proportion of extreme specialist or generalist species, we partitioned them into octaves. Octaves are intervals that contain a constant increase with respect to the previous interval and for which divisions between the classes are equally spaced on a logarithmic scale (Williams 1964). We choose to use a log3 scale that partitions insect species into six classes of generalism: 1, 2–4, 5–13, 14–41, 42–123 and 124–367. The interest of this scale is that it allows an extreme specialist to be kept in a single octave. For each web, we calculated the proportion of insect species that belongs to the different octaves.
(c). Measurement of quantified insect generalism in quantified datasets
For 10 of the pollination webs and nine of the herbivory webs, data on the frequency of each interaction were available. For these datasets, we calculated the quantified generalism of each insect k using the metric 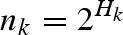 defined by Bersier et al. (2002), where
defined by Bersier et al. (2002), where 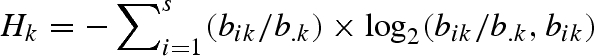 represents the number of recorded feeding events of the insect k on plant i and b.k represents the total number of feeding events recorded for the insect k.
represents the number of recorded feeding events of the insect k on plant i and b.k represents the total number of feeding events recorded for the insect k.
(d). Measurement of phylogenetical insect generalism
In order to quantify the degree of phylogenetic generalism (i.e. the trend for insects to interact with a broad phylogenetic range of plant species), we calculated the mean of the time to the nearest common ancestor of all possible pairs of plants with which a given insect interacts, using Phylocom software (Webb et al. 2008). A few plant species belonging to fern groups were excluded from the analysis since the available super tree only includes the plant family belonging to the Euphyllophyte clade. Similarly, the species name of a few plant species were not available in the original publication and were also removed from the analysis.
(e). Statistical analyses
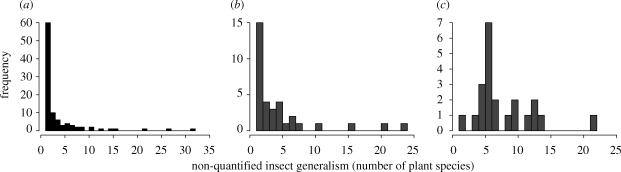
All statistical analyses were performed using R statistical software (v. 2.6). To test for different distributions of non-quantified insect generalism between pollination and herbivory webs, we performed a multivariate analysis of variance (MANOVA). The dependent variables were the percentage of insects belonging to the different octaves that were arcsin(square-root) transformed. The explanatory variables were the interaction type, the total number of plants per web and the interaction term. We incorporated this covariate in the analyses because estimates of species generalism should indeed be considered with regard to the number of potentially available alternative plants (Novotny & Basset 2005). To test which part of the distribution differed, we then analysed each octave separately using linear models (gls function of nlme package) with the same model structure. We used a variance function (varIdent of nlme library) that allows different variances for each level of a stratification variable (here, the type of interaction) in order to accommodate for heteroscedasticity when necessary. Analyses were not performed on the two largest octaves as insect never reached such a generalism level in the herbivory networks present in our dataset. Finally, since the three herbivory datasets that involved grasshoppers strongly differed from all other datasets in their insect generalism distribution (almost no extreme specialists; figure 1), we performed this analysis with and without these three networks.
Figure 1.
Distribution of insect generalism. Histograms representing the insect generalism distribution (in number of plant species) from the studies of (a) Arroyo (1982), (b) Prado (2004) and (c) Joern (1979). Pollination networks are in black and herbivory ones are in grey. Joern's dataset illustrates the singularity of grasshopper webs compared with other the datasets used in this analysis.
The quantified generalism of insects was analysed with a mixed linear model (lme function of nlme package) that included the type of interaction; the non-quantified insect generalism (number of plant species) and the interaction term as fixed effects. The random variable or grouping factor was the web and this affected the intercept estimates. We used a variance function (varPower of nlme library) that allows proportional variance to a covariate (here insect generalism) in order to accommodate for heteroscedasticity. To insure linearity, both the quantified and non-quantified insect generalism were log-transformed.
Phylogenetic generalism was analysed with a mixed linear model (lme function of nlme package) that included the type of interaction; the non-quantified insect generalism and the interaction term as fixed effects. The random variable or grouping factor was the web and this affected both the intercept and the slope estimates. Many pollinator species, mainly those belonging to Robertson's dataset, had a higher non-quantified generalism than the maximum of phytophagous insects. Therefore, we restricted the dataset to insect species that had a non-quantified generalism higher than 1 and lower than 40. The latter value roughly corresponds to the maximal species generalism observed in phytophagous webs (note that the results were similar to the complete dataset). Finally, we used a variance function (varPower of nlme library) that allows proportional variance to a covariate for each level of a stratification variable (here, the type of interaction), in order to accommodate for heteroscedasticity.
3. Results
(a). Non-quantified insect generalism
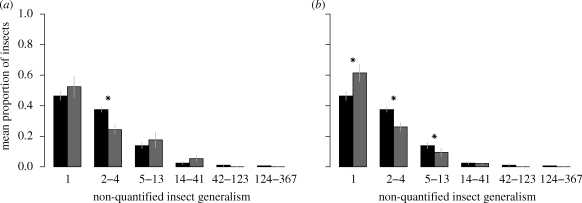
When analysing the complete dataset, the result of the MANOVA indicated that the distribution of non-quantified insect generalism significantly differed between pollination and herbivory networks (table 1). When analysing each octave separately, the model integrating unequal variance between network types always fitted the data better, indicating a higher variance in herbivory webs (2.5 times higher on average). We did not find any effect on the proportion of insect species belonging to the first octave (mean ± s.e. 0.49 ± 0.03; table 2 and figure 2). For the second octave, we found a significant effect of the type of interaction with a higher proportion of such insects in pollination webs (mean ± s.e. 0.38 ± 0.02 and 0.24 ± 0.03 for pollination and herbivory webs, respectively; table 2 and figure 2). Moreover, the interaction between the interaction type and the number of plants in the networks was significant, indicating that the proportion of such insects decreases with increasing plant number in pollination webs, whereas it remains constant in herbivory webs (slopes: −0.05, p = 0.002 and 0.03, p = 0.41, respectively, table 2). We did not find any significant effect on the proportion of insect species belonging to the third octave (mean ± s.e. 0.16 ± 0.03; table 2 and figure 2). For the fourth octave, we did not find any effect of the interaction type (mean ± s.e. 0.04 ± 0.01; table 2 and figure 2), but we found a significant positive effect of the number of plants present in the webs (slope estimate: 0.1). Finally, for the two largest octaves grouping insect species interacting with 42–123 and with 124–367 plant species, none of the hebivory webs contains such highly generalist insect species.
Table 1.
MANOVA of the distribution of insect generalism. Upper values correspond to the analysis on the complete dataset. Lower values correspond to the analysis without grasshopper webs.
| Effect | d.f. | app. F-value | p-value |
|---|---|---|---|
| interaction type | 6,35 | 2.91 | 0.0208 |
| 4.08 | 0.0038 | ||
| number of plant species | 6,35 | 11.90 | <0.0001 |
| 11.76 | <0.0001 | ||
| interaction type * number of plant species | 6,35 | 5.26 | 0.0005 |
| 5.05 | 0.0009 |
Table 2.
Analysis of the proportion of insect species belonging to different generalism octaves. Upper values correspond to the analysis of the complete dataset. Lower values correspond to the analysis without grasshopper webs.
| octave 1 (generalism range: 1) |
octave 2 (generalism range: 2–4) |
octave 3 (generalism range: 5–13) |
octave 4 (generalism range: 14–41) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| effects | d.f. | F-value | p-value | d.f. | F-value | p-value | d.f. | F-value | p-value | d.f. | F-value | p-value |
| interaction type | 1,40 | 1.20 | 0.280 | 1,40 | 10.64 | 0.002 | 1,40 | 0.86 | 0.359 | 1,40 | 0.05 | 0.945 |
| 1,37 | 5.25 | 0.027 | 1,37 | 12.22 | 0.001 | 1,37 | 4.68 | 0.037 | 1,37 | 2.66 | 0.111 | |
| number of plant species | 1,40 | 0.26 | 0.608 | 1,40 | 10.56 | 0.002 | 1,40 | 0.01 | 0.913 | 1,40 | 26.88 | <.0001 |
| 1,37 | 0.27 | 0.608 | 1,37 | 10.56 | 0.003 | 1,37 | 3.04 | 0.089 | 1,37 | 20.69 | 0.0001 | |
| interaction type * number of plant species | 1,40 | 1.04 | 0.31 | 1,40 | 4.30 | 0.045 | 1,40 | 0.78 | 0.380 | 1,40 | 0.004 | 0.825 |
| 1,37 | 2.55 | 0.119 | 1,37 | 6.26 | 0.017 | 1,37 | 1.89 | 0.177 | 1,37 | 2.83 | 0.101 | |
Figure 2.
Distribution of insect generalism transformed in to octaves. Bars represent the mean proportion of insects interacting with a number of partners included in the different octave ranges, in black for pollinators and grey for herbivores. (a) Histogram representing the complete dataset. (b) Histogram with grasshopper webs removed. Asterisk indicates significant differences between pollination and herbivory networks (see table 2).
When removing the three grasshopper datasets that exhibit really different distributions of insect generalism from the analysis (figure 1), the differences in generalism distribution between pollination and herbivory networks were more pronounced (table 1). When analysing each octave separately, higher variance in the herbivory web was still found for octaves 1 and 2 (1.7 times higher on average) but no longer for octaves 3 and 4. We found a significantly lower proportion of insects belonging to the first octave in pollination webs (mean ± s.e. 0.46 ± 0.004 and 0.61 ± 0.01 for pollination and herbivory webs, respectively; table 2 and figure 2), and a higher proportion of insects belonging to the second and third octaves in pollination webs (mean ± s.e. for pollination and herbivory webs, respectively, 0.38 ± 0.017 and 0.26 ± 0.028 for the second octave and 0.14 ± 0.002 and 0.09 ± 0.003 for the third octave; table 2 and figure 2).
(b). Quantified insect generalism
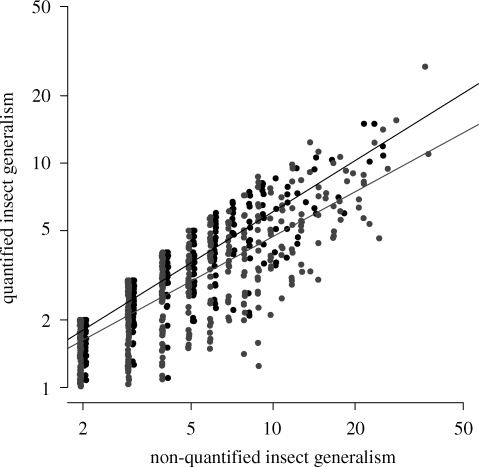
The model incorporating proportional variance to the non-quantified generalism fitted the data best, indicating an increase in quantified generalism variance with non-quantified generalism (figure 3). We found a significant interaction between the type of interaction and non-quantified generalism (table 2), indicating that quantified generalism increased with non-quantified generalism faster in pollination webs than in herbivory webs (slope estimates: 0.76 and 0.66, respectively).
Figure 3.
Relation between quantified and non-quantified insect generalism. Each point represents an insect species. Black points are pollinator species and grey points are phytophagous species. Lines represent significant regressions between non-quantified species generalism and associated quantified generalism.
(c). Phylogenetic insect generalism
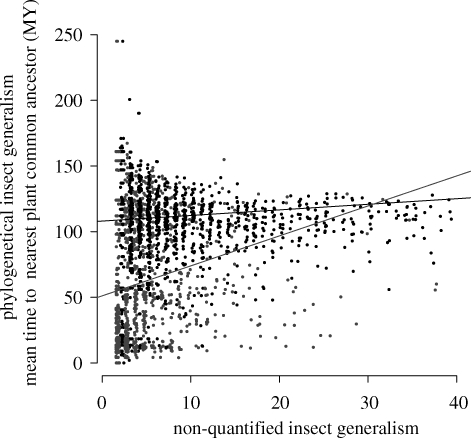
The models that integrated proportional variance to the non-quantified generalism for each type of interaction fitted the data best, indicating a higher decrease of variance with increasing non-quantified generalism in pollination data compared with trophic data. The mean time to the common ancestor of the plants with which an insect interacts was significantly higher in pollination than in phytophagous networks (table 3; mean estimates ± s.e. 108 MY ± 5.1 and 52 MY ± 5.6, respectively), indicating a much higher phylogenetic generalism for pollinators. Moreover, the significant interaction between non-quantified generalism and the type of interaction indicates that phylogenetic generalism was not related to non-quantified generalism in pollination networks, whereas there was a positive relationship in herbivory networks (slopes: 0.36, p = 0.31 and 2.17, p < 0.0001, respectively, for pollination and herbivory networks; table 4 and figure 4).
Table 3.
Analysis of the quantified insect generalism index.
| effect | d.f. | F-value | p-value |
|---|---|---|---|
| interaction type | 1,18 | 0.50 | 0.48 |
| non-quantified insect generalism | 1,995 | 915.50 | <0.0001 |
| interaction type * non-quantified generalism | 1,995 | 8.44 | 0.0038 |
Table 4.
Analysis of the phylogenetic generalism of insect species.
| effects | d.f. | F-value | p-value |
|---|---|---|---|
| interaction type | 1,42 | 56.79 | <0.0001 |
| non-quantified insect generalism | 1,2765 | 1.44 | 0.2291 |
| interaction type * non-quant. generalism | 1,2765 | 10.71 | 0.0011 |
Figure 4.
Relation between insect generalism (in number of plant species) and phylogenetic insect generalism. Each point represents an insect species: black points for pollinator species and grey points for phytophagous species. Lines represent significant regressions between species generalism and associated phylogenetic generalism index.
4. Discussion
This study integrates the growing body of recent literature aiming to understand how different interaction types influence community structure, dynamics and evolution (Bascompte et al. 2003; Lewinsohn et al. 2006; Vazquez et al. 2007; Thébault & Fontaine 2008). Our analysis suggests that the type of interaction, i.e. mutualistic versus trophic, has an impact on various aspects of species' generalism, from the distribution of non-quantified generalism in the community to the phylogenetic diversity of the plants with which a given species interacts. However, the amplitude of the observed differences depends on the aspect of species generalism studied. While the quantitative measure of generalism helped to support our results on non-quantified generalism, the strongest differences were obtained for the phylogenetic generalism. Hereafter, we will first discuss our results in the light of the current literature. Second, we will present different hypotheses involving either ecological mechanisms or evolutionary mechanisms, which could be made to explain the differences in generalization between pollination and herbivory communities.
When comparing species generalism measured as the number of interacting partners, the most striking result was the higher variance observed for herbivory webs. This could be owing to the higher diversity of tropic guilds or feeding groups (Simberloff & Dayan 1991) included in the herbivory networks (see electronic supplementary material, appendix 1). However, most of the variance observed was due to the three grasshopper networks that exhibit a singular distribution of generalism (figure 1) and was not related to classical feeding group partitioning since they belong to the leaf-chewing guild that was represented by five other webs in our dataset. When excluding these three networks from the analysis, extreme specialization appeared more common in herbivory networks, and in contrast, generalization was more widespread in pollination ones. The slightly higher prevalence of generalization in pollination communities was further confirmed by the results on the quantified generalism. Overall, despite some non-negligible variability and exception, our results are in accordance with the idea that mutualistic networks tend to be more generalist than antagonist ones. This idea is commonly held in the literature dealing with community-level interaction networks but has not been properly tested yet. Indeed, the nested structure of mutualistic networks (Bascompte et al. 2003; Guimaraes et al. 2007b; Ollerton et al. 2007) implies the presence of numerous generalist species, interacting with each other and forming a core to which specialized species bind. Although less studied, the structure of antagonistic bipartite networks tends to be described as compartmentalized (Prado & Lewinsohn 2004; Lewinsohn et al. 2006), i.e. characterized by cohesive groups of interacting species (compartments) with relatively few interactions among groups. Such a structure implies a lower prevalence of generalist species. In addition, herbivores appeared to interact with plant species far more closely related to each other than pollinators. These results strengthen previous findings showing that the host plant range of herbivores is often restricted within a genus (Novotny et al. 2002; Novotny & Basset 2005) and furthermore highlight the comparatively weaker restriction in the phylogenetic host range for pollinators (but see Rezende et al. 2007).
These differences in the various aspects of insect generalization suggest that the ecological and evolutionary processes generating these interaction webs might differ between pollination and herbivory communities. Several ecological factors might affect herbivore and pollinator generalism. In the plant–herbivore literature, it has been proposed that the predation pressure imposed by natural enemies could drive herbivores to specialize (Bernays & Graham 1988). As most predators of herbivores have searching patterns related to plant species identity, the specialization on a plant species not visited by predators will provide an enemy-free space (Jeffries & Lawton 1984). Such an ecological pressure towards specialization might be less strong for pollinators since the actual flower handling time by a pollinator is short compared with that of herbivores that live on a plant for part of their development (but see Reader et al. 2006). Although more work is needed to directly assess such difference in predation pressure intensity, this ecological process could be responsible for part of the difference observed.
Another potentially important ecological factor affecting species generalization is species density. Recent studies have linked it to species generalization through modification of the inter- and intra-specific competition strengths in both mutualistic and trophic systems (Bolnick 2001; Fontaine et al. 2008). Differences in relative abundances profiles between pollinator and herbivore communities could indeed influence their generalism level. However, good abundance data at the community level are very scarce, making this hypothesis difficult to test. Although ecological processes certainly play a role in determining species generalization, more studies are obviously needed to assess their impacts on mutualistic and trophic networks.
Evolutionary processes generating interaction web patterns might also be influenced by the type of interaction considered (Thompson 2005). Natural selection on mutualisms often specifically favours the development of multispecies networks through convergence and complementarity of traits in interacting species (Thompson 1994; Thompson 2005). Indeed, in pollination systems, the coevolution of flower and insect morphological traits exemplifies the convergence of disparate plant lineages into relatively few distinct floral types, reflecting their adaptation to different pollinator groups (Fenster et al. 2004). However, morphological adaptations (i.e. open versus tubular flowers and long versus short insect mouthparts) generate strongly asymmetrical constraints since pollinators with short mouthparts are restricted to open flower morphology, whereas insects with longer mouthparts have access to both floral morphologies (Fontaine et al. 2006). Such asymmetrical morphological constraints make the evolution toward generalism possible and have been shown to strongly structure pollination networks (Stang et al. 2006).
In contrast, for antagonistic interactions, the continuous coevolution of defences and counter defences may favour specialization (Thompson 2005). Indeed, the range of possible interactions in plant–phytophagous interactions is at least partially driven by the adequacy between the composition of toxic chemical compounds in plants and the detoxifying strategies adopted by phytophagous insects (Ehrlich & Raven 1964; Schultz 1988; Wittstock & Gershenzon 2002). Trade-off between the ability to detoxify numerous toxic compounds and the efficiency of the detoxifying processes might restrict the potential benefits of being generalist. It has been shown that generalist herbivores, on average, suffer more from a given toxic compound than specialist species (Cornell & Hawkins 2003).
Moreover, the specialization on a plant that herbivores can detoxify by sequestering the toxic compounds, providing them with protection against predators, has been documented in various insect taxa (Duffey 1980). The effect of such different evolutionary constraints on insect generalism could explain our strong results regarding the phylogenetic generalism. In pollination communities, convergence might lead to few phylogenetic constraints on pollinator generalism, whereas the tighter coevolution between plants and herbivores should lead to much stronger phylogenetic constraints on herbivore generalism.
Generalism is a species property that includes ecological and evolutionary components. By studying these various aspects in different types of communities, our study highlights the type of interaction as a determinant driver of community organization, functioning and evolution. Although more work is clearly needed to identify and assess the strength of the different processes involved, this comparative framework offers the promising perspective to better understand the functioning and evolution of multispecies assemblages.
Acknowledgements
We thank Mathilde Baude, Romain Gallet, Joaquin Hortal, Pedro Jordano, Gérard Lacroix, Nirmala Massin and Xavier Raynaud for their help and comments on the manuscript. We thank Owen T. Lewis, Jenella Loye, Teja Tscharntke, Lee A. Dyer and Dan H. Janzen for providing their datasets.
References
- Arroyo M. T. K., Primack R., Armesto J.1982Community studies in pollination ecology in the high temperate Andes of central Chile. Pollination mechanisms and altitudinal variation. Aus. J. Bot 69, 82–97 [Google Scholar]
- Bascompte J., Jordano P., Melian C. J., Olesen J. M.2003The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA 100, 9383–9387 (doi:10.1073/pnas.1633576100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernays E., Graham M.1988On the evolution of host specificity in phytophagous arthropods. Ecology 69, 886–892 (doi:10.2307/1941237) [Google Scholar]
- Bersier L. F., Banasek-Richter C., Cattin M. F.2002Quantitative descriptors of food-web matrices. Ecology 83, 2394–2407 [Google Scholar]
- Bolnick D. I.2001Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature 410, 463–466 (doi:10.1038/35068555) [DOI] [PubMed] [Google Scholar]
- Cornell H. V., Hawkins B. A.2003Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. Am. Nat. 161, 507–522 (doi:10.1086/368346) [DOI] [PubMed] [Google Scholar]
- Duffey S. S.1980Sequestration of plant natural-products by insects. Annu. Rev. Entomol. 25, 447–477 (doi:10.1146/annurev.en.25.010180.002311) [Google Scholar]
- Ehrlich P. R., Raven P. H.1964Butterflies and plants: a study in coevolution. Evolution 18, 586–608 (doi:10.2307/2406212) [Google Scholar]
- Erwin T. L.1982Tropical forests: their richness in Coleoptera and other arthropod species. Coleopt. Bull. 36, 74–75 [Google Scholar]
- Fenster C. B., Armbruster W. S., Wilson P., Dudash M. R., Thomson J. D.2004Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403 (doi:10.1146/annurev.ecolsys.34.011802.132347) [Google Scholar]
- Fontaine C., Dajoz I., Meriguet J., Loreau M.2006Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol. 4, 129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine C., Collin C. L., Dajoz I.2008Generalist foraging of pollinators: diet expansion at high density. J. Ecol. 96, 1002–1010 (doi:10.1111/j.1365-2745.2008.01405.x) [Google Scholar]
- Fortuna M. A., Bascompte J.2006Habitat loss and the structure of plant–animal mutualistic networks. Ecol. Lett. 9, 278–283 (doi:10.1111/j.1461-0248.2005.00868.x) [DOI] [PubMed] [Google Scholar]
- Fox L. R.1988Diffuse coevolution within complex communities. Ecology 69, 906–907 (doi:10.2307/1941243) [Google Scholar]
- Guimaraes P. R., Rico-Gray V., Oliveira P. S., Izzo T. J., dos Reis S. F., Thompson J. N.2007aInteraction intimacy affects structure and coevolutionary dynamics in mutualistic networks. Curr. Biol. 17, 1797–1803 (doi:10.1016/j.cub.2007.09.059) [DOI] [PubMed] [Google Scholar]
- Guimaraes P. R., Sazima C., dos Reis S. F., Sazima I.2007bThe nested structure of marine cleaning symbiosis: is it like flowers and bees? Biol. Lett. 3, 51–54 (doi:10.1098/rsbl.2006.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye B., Stinchcombe J. R.2001Relationships between ecological interaction modifications and diffuse coevolution: similarities, differences, and causal links. Oikos 95, 353–360 (doi:10.1034/j.1600-0706.2001.950218.x) [Google Scholar]
- Janzen D. H.1980When is it coevolution. Evolution 34, 611–612 (doi:10.2307/2408229) [DOI] [PubMed] [Google Scholar]
- Jeffries M. J., Lawton J. H.1984Enemy free space and the structure of ecological communities. 23, 269–286 [Google Scholar]
- Joern A.1979Feeding patterns in grasshoppers (Orthoptera: Acndidae)—factors influencing diet specialization. Oecologia 38, 325–347 (doi:10.1007/BF00345192) [DOI] [PubMed] [Google Scholar]
- Jordano P., Bascompte J., Olesen J. M.2003Invariant properties in coevolutionary networks of plant–animal interactions. Ecol. Lett. 6, 69–81 (doi:10.1046/j.1461-0248.2003.00403.x) [Google Scholar]
- Lau J. A., Strauss S. Y.2005Insect herbivores drive important indirect effects of exotic plants on native communities. Ecology 86, 2990–2997 (doi:10.1890/04-1779) [Google Scholar]
- Lewinsohn T. M., Prado P. I., Jordano P., Bascompte J., Olesen J. M.2006Structure in plant–animal interaction assemblages. Oikos 113, 174–184 (doi:10.1111/j.0030-1299.2006.14583.x) [Google Scholar]
- Memmott J., Waser N. M., Price M. V.2004Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B 271, 2605–2611 (doi:10.1098/rspb.2004.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny V., Basset Y.2005Review—host specificity of insect herbivores in tropical forests. Proc. R. Soc. B 272, 1083–1090 (doi:10.1098/rspb.2004.3023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny V., Basset Y., Miller S. E., Weiblen G. D., Bremer B., Cizek L., Drozd P.2002Low host specificity of herbivorous insects in a tropical forest. Nature 416, 841–844 (doi:10.1038/416841a) [DOI] [PubMed] [Google Scholar]
- Okuyama T.2008Do mutualistic networks follow pourer distributions? Ecol. Complexity 5, 59–65 (doi:10.1016/j.ecocom.2007.06.006) [Google Scholar]
- Ollerton J., McCollin D., Fautin D. G., Allen G. R.2007Finding NEMO: nestedness engendered by mutualistic organization in anemonefish and their hosts. Proc. R. Soc. B 274, 591–598 (doi:10.1098/rspb.2006.3758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado P. I., Lewinsohn T. M.2004Compartments in insect–plant associations and their consequences for community structure. J. Anim. Ecol. 73, 1168–1178 (doi:10.1111/j.0021-8790.2004.00891.x) [Google Scholar]
- Reader T., Higginson A. D., Barnard C. J., Gilbert F. S.2006The effects of predation risk from crab spiders on bee foraging behavior. Behav. Ecol. 17, 933–939 (doi:10.1093/beheco/arl027) [Google Scholar]
- Rezende E. L., Lavabre J. E., Guimaraes P. R., Jordano P., Bascompte J.2007Non-random coextinctions in phylogenetically structured mutualistic networks. Nature 448, 925–926 (doi:10.1038/nature05956) [DOI] [PubMed] [Google Scholar]
- Schultz J. C.1988Many factors influence the evolution of herbivore diets, but plant chemistry is central. Ecology 69, 896–897 (doi:10.2307/1941239) [Google Scholar]
- Simberloff D., Dayan T.1991The guild concept and the structure of ecological communities. Annu. Rev. Ecol. Syst. 22, 115–143 (doi:10.1146/annurev.es.22.110191.000555) [Google Scholar]
- Stang M., Klinkhamer P. G. L., van der Meijden E.2006Size constraints and flower abundance determine the number of interactions in a plant–flower visitor web. Oikos 112, 111–121 (doi:10.1111/j.0030-1299.2006.14199.x) [Google Scholar]
- Strauss S. Y., Irwin R. E.2004Ecological and evolutionary consequences of multispecies plant–animal interactions. Annu. Rev. Ecol. Evol. Syst. 35, 435–466 (doi:10.1146/annurev.ecolsys.35.112202.130215) [Google Scholar]
- Thébault E., Fontaine C.2008Does asymmetric specialization differ between mutualistic and trophic networks? Oikos 117, 555–563 (doi:10.1111/j.0030-1299.2008.16485.x) [Google Scholar]
- Thompson J. N.1994The coevolution process Chicago, IL: University of Chicago Press [Google Scholar]
- Thompson J. N.2005The geographic mosaic of coevolution Chicago, IL: University of Chicago Press [Google Scholar]
- van Veen F. J. F., Morris R. J., Godfray H. C. J.2006Apparent competition, quantitative food webs, and the structure of phytophagous insect communities. Ann. Rev. Entomol. 51, 187–208 (doi:10.1146/annurev.ento.51.110104.151120) [DOI] [PubMed] [Google Scholar]
- Van Veen F. J. F., Mueller C. B., Pell J. K., Godfray H. C. J.2008Food web structure of three guilds of natural enemies: predators, parasitoids and pathogens of aphids. J. Anim. Ecol. 77, 191–200 (doi:10.1111/j.1365-2656.2007.01325.x) [DOI] [PubMed] [Google Scholar]
- Vazquez D. P., Melian C. J., Williams N. M., Bluthgen N., Krasnov B. R., Poulin R.2007Species abundance and asymmetric interaction strength in ecological networks. Oikos 116, 1120–1127 (doi:10.1111/j.0030-1299.2007.15828.x) [Google Scholar]
- Waser N. M., Chittka L., Price M. V., Williams N. M., Ollerton J.1996Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060 (doi:10.2307/2265575) [Google Scholar]
- Webb C. O., Ackerly D. D., Kembel S. W.2008Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24, 2098–2100 [DOI] [PubMed] [Google Scholar]
- Williams C. B.1964Patterns in the balance of nature and related problems in quantitative ecology London, UK: Academic Press [Google Scholar]
- Wittstock U., Gershenzon J.2002Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 5, 300–307 (doi:10.1016/S1369-5266(02)00264-9) [DOI] [PubMed] [Google Scholar]