Abstract
Many animals use signals to assess the fighting ability of rivals and reduce the cost of aggressive competition. However, little is known about how an individual's own quality influences their signal assessment decisions. Polistes dominulus wasps have visual signals of fighting ability that provide a good model for testing the dynamics of rival choice. We found that rival assessment behaviour was influenced by the advertised quality of the individual, their rivals, and the interaction between individual and rival quality. Individuals of high advertised quality were more likely to challenge rivals and individuals of low advertised quality were more likely to be challenged. However, when choosing among two rivals with different advertised quality, individuals did not simply choose the lower quality rival. Instead, they only preferred the lower quality rival when there was a small difference between their own advertised quality and that of their rivals. Individuals were not choosy when both rivals advertised relatively high or relatively low quality. Therefore, although P. dominulus facial patterns function as conventional signals of fighting ability that provide valuable information about their bearer's behavioural strategy, there is substantial variation in signal responses based on the relative intensity of the senders' and receivers' signals.
Keywords: resource-holding potential, badge-of-status, self assessment, dominance, conventional signal, game theory
1. Introduction
Many animals reduce the costs of aggressive contests by using signals to assess the agonistic abilities of rivals. In some taxa, assessment is based on traits that are logically associated with fighting ability, such as body size or call frequency (Maynard Smith & Harper 2003; Searcy & Nowicki 2005). Other animals rely on conventional signals, traits that convey information about fighting ability although there is no logical, a priori connection between a signal and its bearer's fighting ability (Guilford & Dawkins 1995). There has been debate over the value of conventional signals, but a few studies have shown that animals use conventional signals during rival assessment (Senar & Camerino 1998; Tibbetts & Lindsay 2008). Typically, animals are less likely to challenge rivals that signal high quality than rivals that signal low quality.
Although there is increasing evidence that animals use signals to assess potential rivals, less is known about how individuals incorporate information about their own quality into rival assessment. Contest behaviour can be influenced by an individual's own abilities, its rival's abilities or a combination of the two. Research on the role of self versus rival assessment in animal contests has largely focused on behavioural dynamics during contests (Arnott & Elwood 2009), but we do not know how individuals use signals to make decisions before they engage in a contest. Do high-quality individuals make different signal assessment decisions from low-quality individuals?
Polistes dominulus paper wasps have conspicuous black facial patterns that provide a good model for testing how individual quality influences signal assessment decisions. Previous work has shown that the amount of disruption or ‘brokenness’ in a wasp's black facial patterns functions as a conventional signal of agonistic ability (Tibbetts & Dale 2004; Tibbetts & Lindsay 2008). The number of facial spots is a good proxy for brokenness, with ‘0’ facial spots indicating low quality and ‘2’ facial spots indicating high quality (see electronic supplemental material). Here, we allow wasps of varying signal intensities to choose among rivals. We test how the signal intensity of the focal wasp and rivals influences (i) whether or not the wasp challenges a rival and (ii) which rival she challenges.
2. Material and methods
Focal wasps were allowed to choose between two guarded patches of food. Guards and focal wasps were collected from wild single-foundress nests soon after nest foundation. Trials were performed in a triangle-shaped arena (7 cm wide × 6 cm long). At the narrow end of the arena there was a covered antechamber. At the opposite end of the arena there were two sugar cubes with a freshly freeze-killed ‘guard’ wasp positioned on top. Guards were similarly sized (within 5 mg) and had similar original facial patterns, but their facial patterns were experimentally altered with paint, so that one guard had more facial spots than the other guard. By choosing similar pairs of guards and randomly assigning the experimental treatment, we ensured that the only consistent difference between the guards was the number of facial spots. Two types of guard pairs were used in the experiment: 0 versus 1 spot and 1 versus 2 spots. There were 10 guard pairs: five 0 versus 1 spot pairs (185 trials) and five 1 versus 2 spot pairs (132 trials).
Every trial used a different focal wasp. The focal wasps were starved for 24 h before the trial and placed in the closed antechamber for 5 min before being released into the trial arena. A choice was made when the focal wasp opened her mandibles and licked a sugar cube. Wasps that did not eat after 20 min were scored as non-eaters that failed to challenge either guard (66/317 trials).
The advertised quality of each focal wasp was assessed by analysing ‘brokenness’ using a digital picture of their face. The central portion of the clypeus was converted into a 30 × 60 pixel bitmap and the standard deviation of the black pigment deposition from five to 55 pixels was calculated. Brokenness takes into account the number, size and shape of black spots on a wasp's clypeus and is the method commonly used to assess advertised quality in wasps (e.g. Tibbetts & Dale 2004).
Analyses were performed using generalized estimating equations (GEE) and unpaired t-tests. In the GEE, specific guard pair was included as a subject variable to control for potential similarity within specific guard pairs. Guard type (0 versus 1 spot; 1 versus 2 spots), focal wasp advertised quality, and their interaction were the dependent variables.
3. Results
The decision to challenge the guard with fewer facial spots, more facial spots, or neither guard was influenced by the focal wasps' own advertised quality (Wald's χ2 = 6.12, p = 0.013), the guards' advertised quality (Wald's χ2 = 12.9, p < 0.001), and their interaction (Wald's χ2 = 15.5, p < 0.001).
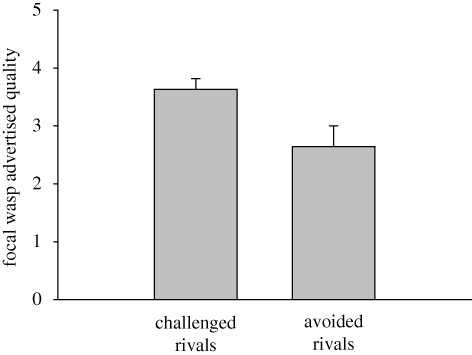
To provide a clearer picture of choices, the data were split to separately test the factors that influenced (i) whether or not the focal wasp challenged one of the guards and (ii) which guard she challenged. Whether or not the focal wasp challenged one of the guards was influenced by the focal wasps' own advertised quality (figure 1, Wald's χ2 = 4.3, p = 0.037), the guards' advertised quality (Wald's χ2 = 5.11, p = 0.024), but not their interaction (Wald's χ2 = 0.8, p = 0.37). Wasps were more likely to challenge one of the guards when the guard pair had lower advertised quality (0 and 1 spots) than when they had higher advertised quality (1 and 2 spots) (χ2 = 5.06, p = 0.024). Further, wasps that challenged one of the guards had higher advertised quality than wasps that challenged neither guard (t = 2.4, p = 0.018).
Figure 1.
Focal wasps that challenged a rival had facial patterns advertising a higher level of quality than focal wasps that did not challenge a rival. (mean + s.e.).
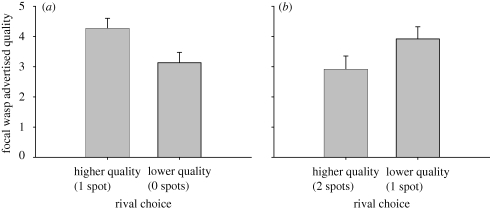
The particular guard challenged by the focal wasp was influenced by the guards' advertised quality (Wald's χ2 = 5.6, p = 0.017) and the interaction between the guards' and the focal wasp's advertised quality (Wald's χ2 = 11.24, p = 0.001). However, the focal wasps' own advertised quality had no independent effect on choice (Wald's χ2 = 0.014, p = 0.91). The significant interaction term occurs because wasps are most choosy when there is a small difference between their quality and the guard's advertised quality. High-quality wasps are not choosy in trials with 0 versus 1 spot guards, while low-quality wasps are not choosy in trials with 1 versus 2 spot guards. In trials with 0 versus 1 spot guards, individuals that chose the 0 spot guard had significantly lower signal intensity than individuals that chose the 1 spot guard (figure 2a; t = 2.3, p = 0.02). Within focal wasps given the choice between 1 and 2 spot guards, individuals that chose the 1 spot guard tended to have higher signal intensity than individuals that chose the 2 spot guard (figure 2b; t = 1.7, p = 0.10).
Figure 2.
Average (+s.e.) advertised quality of focal wasps that chose the higher versus lower quality guard when given a choice between (a) 0 versus 1 spot guards and (b) 1 versus 2 spot guards. (a) Individuals of high advertised quality were not choosy when faced with low-quality rivals, while (b) individuals of low advertised quality were not choosy when faced with high-quality rivals.
4. Discussion
Overall, an individual's rival choice decisions were influenced by the advertised quality of the individual, their rivals and the interaction between the two, consistent with a process of mutual assessment. Individuals were more likely to challenge one of the guards when the guards had few facial spots than when the guards had many facial spots. Further, focal wasps with facial patterns signalling high quality were more likely to challenge one of the guards than focal wasps with facial patterns signalling lower quality.
The particular guard that individuals challenged was also influenced by the signal intensity of the focal wasp and her rivals, but the relationship is more complex. Individuals did not use a simple decision rule like ‘always challenge the lower-quality rival.’ Instead, individuals chose the lower-quality rival when rivals were close to their own quality, but they were less choosy when both rivals were either higher or lower quality. For example, wasps with high-quality facial patterns were choosy when given a choice between relatively high-quality rivals (1 versus 2 spots), but they were not choosy when given a choice between relatively low-quality rivals (0 versus 1 spot). Similarly, wasps with low-quality facial patterns were choosy when given a choice between low-quality rivals (0 versus 1 spot), but they were not choosy when given a choice between high-quality rivals (1 versus 2 spots).
This study provides further evidence that conventional signals are important during rival assessment. Rivals were experimentally manipulated, so the only consistent difference between individuals was their facial pattern. Wasps used these facial patterns to quickly assess rival agonistic abilities without behavioural interactions, adding to mounting evidence that rival assessment may be particularly likely during the initial phases of a contest (e.g. Morrell et al. 2005). Therefore, conventional signals can reduce the costs of aggressive competition by allowing rapid rival assessment without overt aggression (Senar & Camerino 1998; Tibbetts & Lindsay 2008).
This study also demonstrates that an individual's advertised quality provides valuable information about its behavioural strategy. Wasps with high-quality facial patterns behaved differently than those with low-quality facial patterns. Previous work has shown that P. dominulus facial patterns are condition-dependent traits (Tibbetts & Curtis 2007), which provide information about their bearer's agonistic abilities (Tibbetts & Dale 2004; Tibbetts & Shorter 2009; Zanette & Field 2009). This study indicates that badges also provide reliable information about individual behaviour before contests. The effect of advertised quality on rival choices is particularly interesting because the relationship between advertised and ‘true’ quality is quite noisy in many systems (Szamado 2000). Nevertheless, conventional signals provide diverse, valuable information to receivers.
The strong effect of individual quality on rival choices underlines the importance of considering individual quality during communication experiments. The signal value of a trait may be underestimated or even completely obscured if the quality of the individual making the choice is not taken into account. For example, very high-quality individuals may largely ignore signals, and very low-quality individuals may occasionally take a desperado approach by challenging individuals regardless of their quality (Grafen 1987). Although more work is required to understand the factors that influence receiver responses across taxa, such experiments are essential to understanding how animals use signals. They may also clarify apparent inconsistencies in receiver responses across experiments.
The variation in receiver responses may be key to the evolutionary stability of conventional signals. Social costs are thought to maintain conventional signal accuracy (Rohwer 1977), but social costs will only provide an evolutionarily stable cost of ornamentation if receivers occasionally challenge rivals regardless of their signal. Individuals could get away with signalling an inaccurately high level of quality, if receivers always ‘trust’ signals, avoiding individuals who signal high quality and challenging individuals who signal low quality. Context-dependent receiver responses are one way to ensure that all individuals are occasionally challenged (Maynard Smith & Harper 1988; Tibbetts 2008). In wasps, receiver responses vary with the value of the resource being contested (Tibbetts 2008) and the relative quality of the sender and receiver (this study). Therefore, even though receivers pay attention to conventional signals, there is variation in receiver responses that may maintain signal accuracy.
Although little work has examined how individual and rival quality influence receiver responses prior to animal contests, there has been extensive theoretical and empirical research on the role of self, rival and mutual assessment during contests (Arnott & Elwood 2009). Researchers often have trouble differentiating among the types of assessment (Taylor & Elwood 2003), in part, because it is usually difficult to assess individual resource holding potential (RHP). Paper wasps provide a good system for studying assessment decisions, as they have an easily quantified and manipulated signal of agonistic ability. Although wasps use mutual assessment, they do not use a simple rule like ‘challenge lower-quality rival’. Considering previous work on mutual assessment, these experimental results are somewhat surprising (Briffa & Elwood 2009). Therefore, assuming that animals rely on simple decision rules may produce misleading predictions about behaviour during contests. Behavioural context, individual motivation and the value of the contested resource may be just as important as relative RHP in contest behaviour. Further, individuals of different quality may respond to these factors in different ways. Therefore, the dynamics of animal contests are surprisingly complex; it is important to consider how diverse factors influence assessment before and during contests.
Acknowledgements
Funding was provided by the University of Michigan. Thanks to J. Miyamoto for research assistance.
References
- Arnott G., Elwood R. W.2009Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004 (doi:10.1016/j.anbehav.2009.02.010) [Google Scholar]
- Briffa M., Elwood R. W.2009Difficulties remain in distinguishing between mutual and self-assessment in animal contests. Anim. Behav. 77, 759–762 (doi:10.1016/j.anbehav.2008.11.010) [Google Scholar]
- Grafen A.1987The logic of divisively asymmetric contests: respect for ownership and the desperado effect. Anim. Behav. 35, 462–467 (doi:10.1016/S0003-3472(87)80271-3) [Google Scholar]
- Guilford T., Dawkins M. S.1995What are conventional signals? Anim. Behav. 49, 1689–1695 (doi:10.1016/0003-3472(95)90090-X) [Google Scholar]
- Maynard Smith J., Harper D.2003Animal signals New York, NY: Oxford University Press [Google Scholar]
- Maynard Smith J., Harper D. G. C., Brookfield J. F. Y.1988The evolution of aggression: can selection generate variability? Phil. Trans. R. Soc. Lond. B 319, 557–570 (doi:10.1098/rstb.1988.0065) [DOI] [PubMed] [Google Scholar]
- Morrell L. J., Backwell P. R. Y., Metcalfe N. B.2005Fighting in fiddler crabs Uca mjoebergi: what determines duration? Anim. Behav. 70, 653–662 (doi:10.1016/j.anbehav.2004.11.014) [Google Scholar]
- Rohwer S.1977Status signaling in Harris sparrows: some experiments in deception. Behaviour 61, 106–112 (doi:10.1163/156853977X00504) [Google Scholar]
- Searcy W. A., Nowicki S.2005The evolution of animal communication Princeton, NJ: Princeton University Press [Google Scholar]
- Senar J. C., Camerino M.1998Status signalling and the ability to recognize dominants: an experiment with siskins (Carduelis spinus). Proc. R. Soc. Lond. B 265, 1515–1520 (doi:10.1098/rspb.1998.0466) [Google Scholar]
- Szamado S.2000Cheating as a mixed strategy in a simple model of aggressive communication. Anim. Behav. 59, 221–230 (doi:10.1006/anbe.1999.1293) [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Elwood R. W.2003The mismeasure of animal contests. Anim. Behav. 65, 1195–1202 (doi:10.1006/anbe.2003.2169) [Google Scholar]
- Tibbetts E. A.2008Resource value and the context dependence of receiver behaviour. Proc. R. Soc. B 275, 2201–2206 (doi:10.1098/rspb.2008.0477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts E. A., Curtis T. R.2007Rearing conditions influence quality signals but not individual identity signals in Polistes wasps. Behav. Ecol. 18, 602–607 (doi:10.1093/beheco/arm013) [Google Scholar]
- Tibbetts E. A., Dale J.2004A socially enforced signal of quality in a paper wasp. Nature 432, 218–222 (doi:10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- Tibbetts E. A., Lindsay R.2008Visual signals of status and rival assessment in Polistes dominulus paper wasps. Biol. Lett. 4, 237–239 (doi:10.1098/rsbl.2008.0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts E. A., Shorter J. R.2009How do fighting ability and nest value influence usurpation contests in Polistes wasps? Behav. Ecol. Sociobiol. 63, 1377–1385 (doi:10.1007/s00265-009-0764-z) [Google Scholar]
- Zanette L., Field J.2009Cues, concessions and inheritance: dominance hierarchies in the paper wasp Polistes dominulus. Behav. Ecol. 20, 773–780 (doi:10.1093/beheco/arp060) [Google Scholar]