Abstract
Killer whales (Orcinus orca) are large predators that occupy the top trophic position in the world's oceans and as such may have important roles in marine ecosystem dynamics. Although the possible top-down effects of killer whale predation on populations of their prey have received much recent attention, little is known of how the abundance of these predators may be limited by bottom-up processes. Here we show, using 25 years of demographic data from two populations of fish-eating killer whales in the northeastern Pacific Ocean, that population trends are driven largely by changes in survival, and that survival rates are strongly correlated with the availability of their principal prey species, Chinook salmon (Oncorhynchus tshawytscha). Our results suggest that, although these killer whales may consume a variety of fish species, they are highly specialized and dependent on this single salmonid species to an extent that it is a limiting factor in their population dynamics. Other ecologically specialized killer whale populations may be similarly constrained to a narrow range of prey species by culturally inherited foraging strategies, and thus are limited in their ability to adapt rapidly to changing prey availability.
Keywords: killer whale, Orcinus orca, predation
1. Introduction
The killer whale (Orcinus orca) is the oceans' apex non-human predator. As a species, it feeds on a diverse array of prey types, including many species of marine fishes, invertebrates, mammals, turtles and birds (Ford 2009). However, different populations of killer whales can have remarkably specialized foraging behaviours and diets, and these populations may coexist sympatrically. The coastal waters of the northeastern Pacific Ocean are home to three genetically distinct and socially isolated forms of killer whales, known as residents, transients and offshores (Ford et al. 2000). Resident killer whales feed on fishes, particularly salmon, but do not prey on marine mammals, while transient killer whales prey on marine mammals but do not feed on fishes (Ford et al. 1998). The poorly known offshore killer whales appear to be fish feeders, though stable isotope and fatty acid profiles from tissue samples suggest a diet distinct from that of residents (Herman et al. 2005). Similarly specialized mammal- and fish-feeding ecotypes of killer whales have been reported in Antarctic waters (LeDuc et al. 2008). Such ecological specialization has important implications for population regulation of these top predators through bottom-up processes, particularly if they have limited ability to switch to alternative prey types. In this study, we examined potential resource limitation in two populations of resident killer whales in relation to the fluctuating availability of their principal prey species over a 25-year period.
Resident killer whales that frequent coastal waters of British Columbia, Canada and Washington State, USA, have been the focus of annual field studies since the early 1970s, based on photo-identification of individuals from natural markings (Bigg et al. 1990; Ford et al. 2000). These whales live in stable societies comprised of matrilineal groups from which individuals rarely, if ever, disperse. Population demographics, including births and mortalities, can thus be assessed accurately each year. Distinct northern and southern populations of resident killer whales (219 and 84 whales, respectively in 2005) are found in the region. Despite overlapping ranges, the two populations do not mix. Resident killer whales are primarily salmonid predators that show strong selectivity for Chinook salmon (Oncorhynchus tshawytscha), probably because of this species' comparatively large size, high lipid content, and year-round availability in the whales' coastal habitat (Ford & Ellis 2006). Migrating chum salmon (Oncorhynchus keta), the second largest of the Pacific salmonids, are important prey during autumn although Chinook are targeted preferentially when available. The smaller but seasonally abundant sockeye (Oncorhynchus nerka) and pink salmon (Oncorhynchus gorbuscha), and some demersal fishes such as lingcod (Ophiodon elongatus) and Pacific halibut (Hippoglossus stenolepis), are consumed by resident killer whales but there is no evidence that these species comprise an important component of their diet.
When first censused in 1974, both resident killer whale populations were probably below carrying capacity (Olesiuk et al. 1990). From 1974 to the mid-1990s, the northern and southern populations grew by approximately 32 per cent and 74 per cent, respectively. Although there were some years of decline during this time, both populations showed extended periods of increase at nearly 2.6 per cent per year, the maximum intrinsic growth rate for this long-lived species (Olesiuk et al. 2005). However, abruptly in the mid-1990s, both populations entered a period of prolonged decline, with the southern population dropping 17 per cent and the northern population eight per cent by 2001. This decline ended in 2001, and by 2004 the southern and northern populations had increased by six per cent and nine per cent, respectively. In this study, we evaluated the hypothesis that the decline and subsequent increase of both resident killer whale populations was related to the abundance of their primary salmonid prey.
2. Material and methods
The population dynamics of resident killer whales were assessed from demographic data collected during annual field censuses in 1973–2005 using individual photo-identification (see Bigg et al. (1990) or Ford et al. (2000) for details). Temporal changes in survival and reproductive rates were examined by calculating an index derived from the ratio of the number of deaths and births actually observed to the number expected from a population model. The expected number of births and deaths each year was estimated by applying the sex- and age-specific mortality and fecundity schedules derived for a period of unrestrained growth during 1973–1996 (Olesiuk et al. 1990, 2005) to the observed sex- and age-structure of the population in each year. These indices explicitly take into account the demographic structure of the population and facilitate comparison among populations or population segments that differ in sex and age composition.
Annual indices of Chinook and chum salmon abundance were derived from Pacific Salmon Commission (2005a,b) estimates for coastal regions between Southeastern Alaska and Oregon, which cover most of the known range of the two resident killer whale populations. Indices were calculated by dividing the total abundance for each salmonid species in each year by its average abundance over the 1979–2004 period. Standard least-squares regression analysis was used to assess the strength and statistical significance of correlations between whale mortality and birth indices, and salmon abundance indices.
3. Results
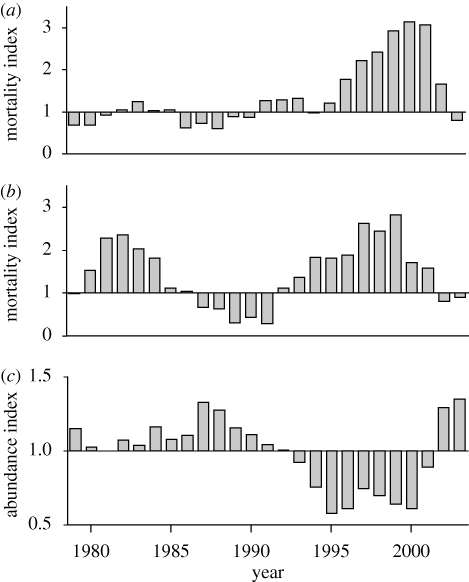
Annual mortality indices of the northern and southern resident populations were significantly correlated (F1,26 = 5.3, r2 = 0.345, p < 0.001). Both populations experienced a period of unusually high mortalities in the late 1990s (figure 1a,b), which were distributed widely among different social groupings and age/sex classes (see the electronic supplementary material). Birth rates in the two populations varied over a narrower range than did mortality rates. The southern residents experienced lower than expected birth rates during the two periods of high mortality, but this was not as apparent in the northern residents and there was no significant correlation between annual birth rate indices in the two populations (F1,29 = 0.52, r2 = 0.18, p = 0.48). Increased mortality was clearly the principal factor driving the synchronous declines in both populations.
Figure 1.
Annual indices of mortality of (a) northern and (b) southern resident killer whales and (c) abundance of Chinook salmon, 1979–2003. Deviations from an annual index value of 1 (a,b) indicate higher or lower than expected mortality rates. Annual abundance indices for Chinook salmon (c) reflect departures from the average abundance over the entire time series.
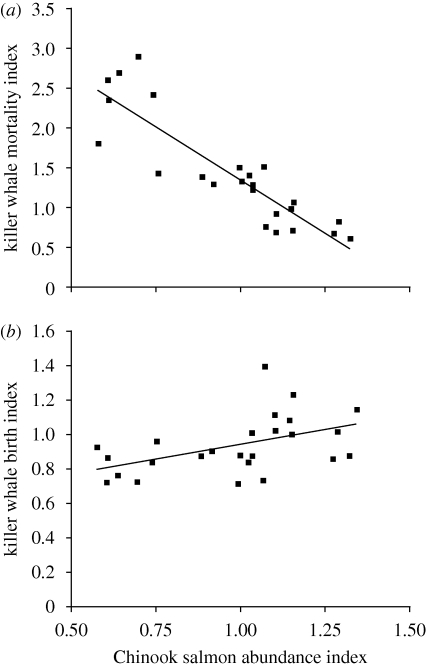
Range-wide abundance of Chinook salmon was at or above the time-series average until the mid 1990s, when abundance fell and remained well below average before recovering sharply in 2002–2003 (figure 1c). Causes of this sudden reduction in abundance are not clear, but probably involved poor ocean survival of juvenile Chinook salmon during several years of strong El Niño-like conditions in the early 1990s (Pacific Fisheries Resource Conservation Council 2001). This period of reduced Chinook abundance coincided distinctly with the period of unusually high mortalities in both resident killer whale populations. Mortality indices were most strongly correlated with changes in Chinook abundance after a lag of 1 year (figure 2a; F1,22 = 76.7, r2 = 0.777, p < 0.001; see the electronic supplementary material). Birth rate indices, also lagged by 1 year, showed a weaker but significant positive correlation with range-wide Chinook abundance indices (figure 2b; F1,23 = 6.77, r2 = 0.227, p < 0.05). Chum salmon abundance did not undergo a major coast-wide decline during the late 1990s, and no significant correlation was found with either killer whale mortality (F1,30 = 0.61, r2 = 0.020, p = 0.44) or birth rate indices (F1,30 = 1.92, r2 = 0.060, p = 0.18).
Figure 2.
(a) Mortality and (b) birth indices of northern and southern resident killer whales combined, as a function of coast-wide abundance indices for Chinook salmon over the period 1979–2003. Index values are expressed as 3-year running means and are lagged by 1 year after Chinook salmon abundance. (a) y = −2.6504x + 4.0066; r2 = 0.77707. (b) y = 0.3385x + 0.6012; r2 = 0.2273.
4. Discussion
The striking correspondence between changes in Chinook salmon abundance and mortality of northern and southern resident killer whales suggests that prey limitation was an important factor in recent population declines. As killer whales are apex predators, predation can be disregarded as a potential source of mortality, and anthropogenic threats to these populations that have been identified to date (Fisheries & Oceans Canada 2008) cannot explain the sudden and widespread increase in mortality rates that occurred concurrently in the two populations during the late 1990s. We hypothesize that these killer whale populations are dependent on Chinook salmon as their primary year-round food resource, and that alternative prey resources were insufficient to avert nutritional stress during years when availability of Chinook suddenly and sharply declined. This stress, potentially acting synergistically with immunosuppressive effects of the high levels of polychlorinated biphenyls and related pollutants found in these whales (Ross et al. 2000), resulted in increased susceptibility to disease and higher mortality rates.
Dependence on a single prey species in resident killer whales implies a high degree of foraging specialization, which is consistent with the propensity for specialized hunting techniques and diets observed in other killer whale populations (Baird 2000; Ford & Ellis in press). These specializations appear to represent fixed behavioural traditions that are passed across generations by mimicry and learning. Foraging traditions of killer whales, along with other cultural traits such as group-specific vocalizations, are probably important social isolating mechanisms that lead ultimately to the evolution of genetically and ecologically distinct populations that often coexist in sympatry (Hoelzel et al. 2007). Foraging specializations have enabled this versatile predator to exploit a diversity of marine environments and prey types, at the cost of dietary flexibility at the population level. Specialized hunting techniques enhance the efficiency and profitability of predation on particular prey types, but may also behaviourally constrain a predator's ability to successfully switch to alternative prey should the primary prey species become unavailable. Thus, although the killer whale occupies the top trophic position in the oceans, populations may be limited by a far narrower range of prey resources than the species is theoretically capable of consuming.
Acknowledgements
This field research was conducted under permits issued by federal authorities in Canadian and US jurisdictions.
Our long-term field studies have been supported by Fisheries and Oceans Canada, the Vancouver Aquarium, the BC Wild Killer Whale Adoption Program and the Earthwatch Institute. Helpful comments were provided by J. Durban, J. Watson, and an anonymous referee.
References
- Baird R. W.2000The killer whale: foraging specializations and group hunting. In Cetacean societies: field studies of dolphins and whales (eds Mann J., Connor R. C., Tyack P. L., Whitehead H.), pp. 127–153 Chicago, IL: University of Chicago Press [Google Scholar]
- Bigg M. A., Olesiuk P. F., Ellis G. M., Ford J. K. B., Balcomb K. C., III1990Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Rep. Int. Whal. Commn 12, 383–405 [Google Scholar]
- Fisheries & Oceans Canada. 2008. Recovery strategy for the northern and southern resident killer whales (Orcinus orca) in Canada. Species at Risk Act Recovery Strategy Series, Ottawa, Canada. See http://www.sararegistry.gc.ca/document/default_e.cfm?documentID=1341 .
- Ford J. K. B.2009Killer whale Orcinus orca. In Encyclopedia of marine mammals, 2nd edn (eds Perrin W. F., Wursig B., Thewissen J. G. M.), pp. 650–657 San Diego, CA: Academic Press [Google Scholar]
- Ford J. K. B., Ellis G. M.2006Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Mar. Ecol. Prog. Ser. 316, 185–199 (doi:10.3354/meps316185) [Google Scholar]
- Ford J. K. B., Ellis G. M. In press. You are what you eat: foraging specializations and their influence on the social organization and behaviour of killer whales. In Primates and Cetaceans: field studies and conservation of complex mammalian societies (eds Yamagiwa J., Karczmarski L.). Tokyo, Japan: Springer [Google Scholar]
- Ford J. K. B., Ellis G. M., Barrett-Lennard L. G., Morton A. B., Palm R. S., Balcomb K. C., III1998Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 76, 1456–1471 (doi:10.1139/cjz-76-8-1456) [Google Scholar]
- Ford J. K. B., Ellis G. M., Balcomb K. C.2000Killer whales: the natural history and genealogy of Orcinus orca in the waters of British Columbia and Washington. Vancouver, BC: and Seattle, WA: University of British Columbia Press and University of Washington Press [Google Scholar]
- Herman D. P., Burrows D. G., Wade P. R., Durban J. W., Matkin C. O., LeDuc R., Barrett-Lennard L. G., Krahn M. M.2005Feeding ecology of eastern North Pacific killer whales Orcinus orca from fatty acid, stable isotope, and organochlorine analyses of blubber biopsies. Mar. Ecol. Prog. Ser. 302, 275–291 (doi:10.3354/meps302275) [Google Scholar]
- Hoelzel A. R., Hey J., Dahlheim M. E., Nicholson C., Burkanov V., Black N.2007Evolution of population structure in a highly social top predator, the killer whale. Mol. Biol. Evol. 24, 1407–1415 (doi:10.1093/molbev/msm063) [DOI] [PubMed] [Google Scholar]
- LeDuc R. G., Roberston K. M., Pitman R. L.2008Mitochondrial sequence divergence among Antarctic killer whale ecotypes is consistent with multiple species. Biol. Lett. 4, 426–429 (doi:10.1098/rsbl.2008.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesiuk P. F., Bigg M. A., Ellis G. M.1990Life history and population dynamics of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Rep. Int. Whal. Comm 12, 209–242 [Google Scholar]
- Olesiuk P. F., Ellis G. M., Ford J. K. B.2005Life history and population dynamics of northern resident killer whales (Orcinus orca) in British Columbia. Research document 2005/45, Ottawa, Canada: Canadian Science Advisory Secretariat, Fisheries and Oceans Canada. See http://www.dfo-po.gc.ca/csas/Csas/Publications/ResDocs-DocRech/2005/2005_045_e.htm
- Pacific Fisheries Resource Conservation Council. 2001. Annual report. Vancouver, BC, Canada. See http://www.fish.bc.ca/annual-report-2000-2001 .
- Pacific Salmon Commission 2005aAnnual exploitation rate analysis and model calibration. Joint Chinook Technical Communication Report TCChinook (05)-3. See http://www.psc.org/pubs/TCCHINOOK05-3.pdf
- Pacific Salmon Commission. 2005b. Final 2002–2003 Post Season Summary Report. Joint Chum Technical Communication Report TCCHUM(05)-1. See http://www.psc.org/pubs/TCCHUM05-1.pdf .
- Ross P. S., Ellis G. M., Ikonomou M. G., Barrett-Lennard L. G., Addison R. F.2000High PCB concentrations in free-ranging Pacific killer whales, Orcinus orca: effects of age, sex and dietary preference. Mar. Pollut. Bull. 40, 504–515 (doi:10.1016/S0025-326X(99)00233-7) [Google Scholar]