Abstract
The adaptive significance of polyandry is an intensely debated subject in sexual selection. For species with male infanticidal behaviour, it has been hypothesized that polyandry evolved as female counterstrategy to offspring loss: by mating with multiple males, females may conceal paternity and so prevent males from killing putative offspring. Here we present, to our knowledge, the first empirical test of this hypothesis in a combined laboratory and field study, and show that multiple mating seems to reduce the risk of infanticide in female bank voles Myodes glareolus. Our findings thus indicate that females of species with non-resource based mating systems, in which males provide nothing but sperm, but commit infanticide, can gain non-genetic fitness benefits from polyandry.
Keywords: polyandry, infanticide, non-genetic benefit, bank vole, Myodes=Clethrionomys glareolus
1. Introduction
A number of potential benefits have been suggested to explain how the increased mating costs arising from polyandry are compensated and, thus, why it may pay females to mate multiply even though a single ejaculate typically assures fertilization (Jennions & Petrie 2000; Hosken & Stockley 2003). These benefits are often classified as either non-genetic or genetic and may increase female longevity (non-genetic), offspring numbers (non-genetic and genetic) and offspring quality (genetic; Hosken & Stockley 2003). While there is now increasing evidence that both types of benefits exist and contribute to the maintenance of polyandry (Arnqvist & Nilsson 2000; Jennions & Petrie 2000; Hosken & Stockley 2003; Simmons 2005), non-genetic benefits have been suggested to represent the most likely evolutionary origin of polyandry (Yasui 1998). In resource-based mating systems, non-genetic benefits seem obvious through e.g. nutritious courtship gifts or paternal care. However, few studies show that females can also gain non-genetic benefits in non-resource-based mating systems, where males provide nothing but sperm.
Avoidance of offspring loss caused by infanticide has been suggested as a subtle non-genetic benefit of polyandry. Males of numerous mammal species, but also of other vertebrate and of invertebrate taxa, have been observed to kill dependent young that they have not fathered (Van Schaik & Janson 2000; Wolff & Macdonald 2004). Infanticide is a potentially adaptive strategy, which may increase male fitness via nutritional gain, decreased competition or an increased access to mates when the loss of offspring shortens the time to the mother's next litter (reviewed in Ebensperger & Blumstein 2007). However, for females, it is a significant source of reproductive loss, and several physiological and behavioural mechanisms can be viewed as counteradaptations, including polyandry, which conceals paternity and so may deter infanticide (reviewed in Ebensperger & Blumstein 2007).
Previous studies have revealed indirect evidence for the infanticide avoidance hypothesis. First, in several rodent species, male infanticidal behaviour ceases after copulation and/or past association with the mother for the time that potential young are vulnerable (reviewed in Ebensperger & Blumstein 2007). Second, a comparative study showed that infanticide is common or likely in most polyandrous mammal species (Wolff & Macdonald 2004). However, that offspring of polyandrous females are less vulnerable to infanticide, and polyandrous females thus wean proportionally more offspring than monandrous females, has not been shown directly.
Here, we test this hypothesis using a common rodent species, the bank vole Myodes glareolus. Bank voles have a non-resource-based mating system, females are polyandrous (Klemme et al. 2007) and male infanticide is common (Ylönen et al. 1997). Because females mate and conceive in postpartum oestrus, i.e. within 48 h after parturition, they may spend considerable time away from the newborn litter at a period when the offspring are most vulnerable to infanticide. We conducted a combined laboratory and field study in which we compared juvenile recruitment of socially polyandrous and socially monandrous populations, while we controlled for potential genetic benefits of polyandry.
2. Material and methods
(a). Study species
The social organization of bank voles is characterized by exclusively defended female territories and large male home ranges that overlap with other male home ranges and several female territories (Bondrup-Nielsen & Karlsson 1985). Gestation is about 19 days, litter size is on average five and offspring are weaned approximately 20–22 days after birth.
(b). Experimental design
Animals used in this study were the second or third generation of wild-caught individuals, born and raised in the laboratory. At weaning, they were marked individually with small mammal eartags (Salt Lake Tags, USA). Until their release to the field, all individuals were housed singly in standard mouse cages (43 × 26 × 15 cm) with sawdust and hay as bedding under a 16 L∶8 D photoperiod. Food and water was available ad libitum. At the start of the experiment, all males were nine to 11 weeks old and mature, but sexually inexperienced. Females were 20–25 weeks old and primiparous.
Eight socially polyandrous and eight socially monandrous populations consisted of two females and three males each (see electronic supplementary material for further details). For socially polyandrous populations, both females mated once with each of the three males. For socially monandrous populations, both females mated once with one of the three males, both with the same male. The other two males in socially monandrous populations were not mated. However, to exclude an effect of the number of matings or mates on litter size or offspring survival, females of monandrous populations were additionally mated once with each of the two males that did not belong to any of the experimental populations. Thus, all populations were genetically polyandrous. Matings were conducted in the laboratory under continuous observation (see electronic supplementary material for further details).
Thirteen to 16 days after mating, the experimental populations were released to outdoor enclosures. This particular time delay was chosen to be able to confirm pregnancy with the help of palpation and body mass increase, and to avoid male-induced pregnancy disruption, the so-called Bruce effect (Bruce 1960), which is common in many rodent species when females are exposed to strange males during the first two-thirds of pregnancy (reviewed in Mahady & Wolff 2002).
The outdoor enclosures were 0.25 ha in size and fenced with 1.5 m high metal sheets. The habitat can be described as old field, the vegetation consisting mainly of tall grass, bushes and young trees. Each enclosure held a grid of 25 multiple-capture live traps (Ugglan, Sweden). About four weeks after the release of the animals, a 3-day live-trapping series was conducted to estimate adult survival and the recruitment rate, i.e. the number of offspring produced in each population.
A total of eight enclosures in two rounds, with four monandrous and four polyandrous populations in each, were used for the study. To exclude site effects, enclosures with polyandrous populations in the first round housed monandrous in the second round and vice versa. Because female size is positively correlated with litter size in bank voles (e.g. Klemme et al. 2007), both treatments were stocked with females of comparable mean body mass (mean ± s.d., monandrous: 17.8 ± 2.3 g, polyandrous: 17.7 ± 2.5 g; t30 = 0.135, p = 0.894). Also males in monandrous (19.3 ± 2.2 g) and polyandrous (19.4 ± 1.9 g) populations did not differ in weight (t46 = −0.200, p = 0.842).
(c). Ethical considerations
Infanticide is a natural behaviour in rodents and a controlled field study is essential to verify the significance of polyandrous mating as counterstrategy to infanticide. For ethical reasons, we followed the guidelines presented by Elwood (1991). We operated with the smallest population size controlling for variation in male infanticidal behaviour and in female reproductive success, and the minimum replicate number ensuring an appropriate statistical power. Because a mother detained in a trap cannot protect her offspring, trappings were only conducted at a time when young were no longer vulnerable to infanticide.
(d). Statistics
Statistical analyses were run in SAS v. 9.1. Unless stated otherwise, means are given with their ±s.d. We used generalized linear mixed models with a Poisson error distribution to study the effect of treatment on recruitment rate. The number of males and females survived in each population were entered as covariates. Round and enclosure were included as random factors to control for the non-independence of data for individuals tested in the same round or in the same enclosure. In one monandrous population, no female survived, it was therefore excluded from all statistical analyses.
3. Results
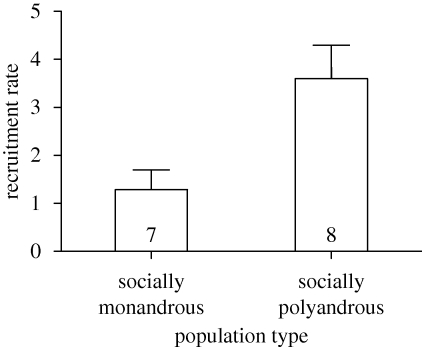
Socially polyandrous populations produced significantly more recruits than socially monandrous populations (F1,11 = 6.80, p = 0.024, figure 1). Adult survival was similar across populations. The number of surviving males was 1.9 ± 0.7 in monandrous populations and 2.2 ± 1.3 in polyandrous populations and did not affect the recruitment rate (F1,11 = 1.79, p = 0.208). Similarly, the number of surviving females (monandrous: 1.7 ± 0.5, polyandrous: 1.6 ± 0.5) had no effect on recruitment (F1,11 = 0.28, p = 0.607).
Figure 1.
Effect of population type on population offspring recruitment. Values are adjusted means (± s.e.) from the final model.
4. Discussion
Our findings indicate that female bank voles gain delayed non-genetic benefits from polyandry in the form of increased recruitment, probably via infanticide avoidance. As in other rodent species (see Ebensperger & Blumstein 2007 for review), copulation itself and/or sexual contact with a certain female may inhibit infanticidal behaviour in bank vole males. Because both the number of mates and matings were the same in the two treatments, genetic benefits as an explanation for our results can be excluded.
Despite the prominence of polyandry and its benefits in empirical and theoretical studies during the last decades, the generality of hypotheses for different taxa is still unclear. Wolff & Macdonald (2004) argued that the origin and benefits of polyandry in mammals might be different from other taxa, especially because infanticidal behaviour is extremely common. Thus, and because genetic benefits as the evolutionary origin of polyandry are questioned (Yasui 1998), our evidence for a non-genetic benefit may provide a general explanation for the evolution of this female reproductive behaviour in bank voles and other infanticidal mammal species.
Once established, however, polyandry leads invariably to the possibility of genetic benefits (Jennions & Petrie 2000), contributing to its maintenance. In fact, female bank voles may also gain long-term genetic benefits from polyandry, in the form of offspring with increased reproductive potential (Klemme et al. 2008). Additionally, other direct benefits are possible. For example, multiple mating in bank voles also increases the probability of pregnancy initiation owing to increased stimulation. However, because this stimulus can also be achieved by repeated matings with the same male, this benefit does not necessarily explain polyandry (Klemme et al. 2007).
Are there alternative explanations for our results? Maternal effects could also possibly explain decreased offspring numbers in monandrous populations (Simmons 2005). If females of an infanticidal species encounter strange males during pregnancy or lactation, they may perceive the potential for offspring loss and consequently decrease the resources they provide for the offspring. However, given the relatively large investment of females in this study until the first contact with strange males (at least two weeks of pregnancy), an increased effort in nest protection, which is effective in this species (Ylönen & Horne 2002), would seem a better cost-benefit strategy. Further, female infanticide, which is common in bank voles (Ylönen et al. 1997), as well as other mortality factors, may also have affected recruitment. However, if so, we would expect the magnitude of these effects to be similar among both treatments. Actually, female infanticide in addition to other mortality factors may explain the generally low recruitment rate in this study, despite the significant difference between the treatments (figure 1). Moreover, because some studies suggest that social interactions with the pregnant female are important for infanticide to be fully inhibited (e.g. Elwood 1985), and because males and females were housed separately until release to the field, some male infanticide may have occurred even in polyandrous populations. However, if so, the effect of infanticide avoidance via polyandry should be even stronger under natural conditions.
In conclusion, this study provides, to our knowledge, the first field evidence that polyandry is an efficient strategy for increasing offspring survival, probably via the avoidance of male infanticide. However, maternal effects cannot be entirely excluded. More studies are needed to test the general validity of the hypothesis and may help explain the ultimate origin of polyandry in bank voles and other infanticidal species.
Acknowledgements
The experiment was conducted under permission from the Board for Animal Experimentation of the University of Jyväskylä.
We are grateful to Dan Blumstein, Bob Elwood, Leigh Simmons, the late Jerry Wolff and one anonymous referee for comments on the manuscript. Lenka Trebatická and Marko Haapakoski helped with trappings and Konnevesi Research Station is thanked for financial and practical support.
References
- Arnqvist G., Nilsson T.2000The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 (doi:10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- Bondrup-Nielsen S., Karlsson F.1985Movements and spatial patterns in populations of Clethrionomys species: a review. Ann. Zool. Fenn. 22, 385–392 [Google Scholar]
- Bruce H. M.1960A block to pregnancy in the mouse caused by proximity of strange males. J. Reprod. Fertil. 1, 96–103(doi:10.1530/jrf.0.0010096) [DOI] [PubMed] [Google Scholar]
- Ebensperger L. A., Blumstein D. T.2007Functions of non-parental infanticide in rodents. In Rodent societies (eds Wolff J. O., Sherman P. W.), pp. 267–279 Chicago, IL: University of Chicago Press [Google Scholar]
- Elwood R. W.1985Inhibition of infanticide and onset of paternal care in male mice (Mus musculus). J. Comp. Psychol. 99, 457–467 (doi:10.1037/0735-7036.99.4.457) [Google Scholar]
- Elwood R. W.1991Ethical implications of studies on infanticide and maternal aggression in rodents. Anim. Behav. 42, 841–849 (doi:10.1016/S0003-3472(05)80128-9) [Google Scholar]
- Hosken D. J., Stockley P.2003Benefits of polyandry: a life history perspective. J. Evol. Biol. 33, 173–194 [Google Scholar]
- Jennions M. D., Petrie M.2000Why do females mate multiply? A review of the genetic benefits. Biol. Rev. Camb. Phil. Soc. 75, 21–64 (doi:10.1017/S0006323199005423) [DOI] [PubMed] [Google Scholar]
- Klemme I., Eccard J. A., Ylönen H.2007Why do female bank voles (Clethrionomys glareolus) mate multiply? Anim. Behav. 73, 623–628 (doi:10.1016/j.anbehav.2006.07.010) [Google Scholar]
- Klemme I., Ylönen H., Eccard J. A.2008Long-term fitness benefits of polyandry in a small mammal, the bank vole Clethrionomys glareolus. Proc. R. Soc. B 275, 1095–1100 (doi:10.1098/rspb.2008.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahady S. J., Wolff J. O.2002A field test of the Bruce effect in the monogamous prairie vole (Microtus ochrogaster). Behav. Ecol. Sociobiol. 52, 31–37 (doi:10.1007/s00265-002-0484-0) [Google Scholar]
- Simmons L. W.2005The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 36, 125–146 (doi:10.1146/annurev.ecolsys.36.102403.112501) [Google Scholar]
- Van Schaik C. P., Janson C. H.2000Infanticide by males and its implications Cambridge, UK: Cambridge University Press [Google Scholar]
- Wolff J. O., Macdonald D. W.2004Promiscuous females protect their offspring. Trends Ecol. Evol. 19, 127–134 (doi:10.1016/j.tree.2003.12.009) [DOI] [PubMed] [Google Scholar]
- Yasui Y.1998The ‘genetic benefits’ of female multiple mating reconsidered. Trends Ecol. Evol. 13, 246–250 (doi:10.1016/S0169-5347(98)01383-4) [DOI] [PubMed] [Google Scholar]
- Ylönen H., Horne T.2002Infanticide and effectiveness of pup protection in bank voles: does the mother recognise a killer? Acta Ethol. 4, 97–101 (doi:10.1007/s10211-001-0055-9) [Google Scholar]
- Ylönen H., Koskela E., Mappes T.1997Infanticide in the bank vole (Clethrionomys glareolus): occurrence and the effect of familiarity on female infanticide. Ann. Zool. Fenn. 34, 259–266 [Google Scholar]