Abstract
Gaze following is the ability to use the visual orientation of others as a trigger to look in the same direction. Thereby, animals may either align their head and eye orientation with others (gaze following into distant space) or may even reposition themselves to look behind barriers impairing their perception (geometrical gaze following). It has been proposed that these two different modes are functionally and cognitively distinct, but experimental evidence for this claim is lacking. We here, to our knowledge, demonstrate for the first time, that adult animals may be capable of following gaze into distant space, but not geometrically around barriers. We tested Northern bald ibises (Geronticus eremita) for their ability to follow a conspecific's gaze in two standard tasks. The birds readily looked up after seeing a model bird looking up; however, when seeing a model looking behind a barrier, they responded by looking at the barrier instead of walking around. These findings are in stark contrast to results obtained with great apes and corvids and provide the first experimental evidence, to our knowledge, for cognitive differences in gaze following tasks.
Keywords: Northern bald ibis, gaze following, geometrical gaze following, barrier
1. Introduction
Gaze following refers to the ability of looking in the same direction others are looking (Emery et al. 1997). In humans, this skill correlates with an important developmental step towards a theory of mind (Butterworth & Jarrett 1991). In non-human animals, gaze following was first reported for chimpanzees (Pan troglodytes; Povinelli & Eddy 1996) but recently also for other primates (e.g. Tomasello et al. 1998), ungulates (Kaminski et al. 2005) and corvids (Bugnyar et al. 2004). In those studies, animals have been tested with human or conspecific cue-givers, who looked either above, to the side or behind the test subject (gaze following into distant space) or, in a more complex setting, behind a visual barrier (geometrical gaze following). According to Povinelli & Eddy (1996), gaze following into distant space may simply reflect a socially facilitated response leading to an alignment of both individuals' head orientation (‘low-level’ model). When faced with an obstacle blocking their view, such a response would lead to looking at this obstacle instead of looking behind it. Individuals repositioning themselves to look behind the obstacle thus need to assess the difference in the visual perception between the cue-giver and themselves. This may either be achieved by mentally representing the looker's visual perspective (Povinelli & Eddy 1996) or by learning how visual barriers impair perceptions (Tomasello et al. 1999). Based on these different cognitive requirements, gaze following into distant space and geometrically around barriers have been regarded as two different modes (Gomez 2005). A similar dissociation has been found between following gaze and selecting the target of other's gaze in object choice situations (Call et al. 1998).
Only the great apes (Bräuer et al. 2005), ravens Corvus corax (Bugnyar et al. 2004) and rooks Corvus frugilegus (Schloegl et al. 2008a) have been tested for geometrical gaze following in a set-up inspired by developmental studies on human infants (Butterworth & Jarrett 1991). Interestingly, those species fail in traditional object choice tasks (Call et al. 1998; Schloegl et al. 2008b). However, they are renowned for using barriers in daily life, for instance to conceal sexual intercourse (Byrne & Whiten 1992) or caching of food (Bugnyar et al. 2007). In ravens, geometrical gaze following develops several months later during ontogeny than gaze following into distant space (Schloegl et al. 2007), and roughly coincides with the start of hiding behind barriers at caching (Bugnyar et al. 2007). Therefore, it has been suggested that gaze following into distant space may primarily serve to facilitate predator detection in those birds, whereas geometrical gaze following may aid the detection of food or conspecifics (Schloegl et al. 2007).
From both a cognitive and a functional point of view, gaze following into space is considered to be a general pattern in socially living animals, whereas geometrical gaze following may be restricted to species that possess advanced cognitive skills (Bräuer et al. 2005) and/or regularly conceal their actions behind obstacles (Schloegl et al. 2008a). To test this hypothesis, it would be crucial to demonstrate that some species may be skilled in following gaze into space but not around barriers. We investigated this idea using adult colony-breeding and free-flying Northern bald ibises (Geronticus eremita) of the Konrad Lorenz Research Station. Notably, ibises of this colony have ample experience with humans and wooden barriers and can be studied under similar experimental conditions as have been used for corvids. Furthermore, these ibises suffer from aerial predators but have not been described to hide behind barriers during daily life (Tintner & Kotrschal 2002). We thus expected them to follow others' gaze into distant space but not geometrically around a wall. Specifically, we predicted that they would co-orient with the models in both tasks, but that they would not search behind the barrier (see Povinelli & Eddy 1996).
2. Material and methods
(a). Subjects, housing and general procedure
We tested adult hand-raised ibises, which are free-flying during the day but return to a spacious aviary for roosting. The aviary consists of outdoor and indoor components, with the latter being divided by wooden walls into three rooms. Tests were conducted in one of the indoor rooms in the morning hours before the birds could leave the aviary (see electronic supplementary material). Experiment 1 was carried out on 12 birds in February 2006 and experiment 2 on 11 birds in February 2007 (see electronic supplementary material). In both years, experiments were conducted within a week. Test subjects received one session per day, consisting of either test or control trials.
(b). Experiment 1: following others' look-ups
We randomly chose one bird as a model and another bird as an observer (see electronic supplementary material). Both subjects were led into adjacent compartments (1.5 m2 each) within the testing room, separated by a wire-mesh partition with an opaque screen in 1 m height (figure 1a). In the test condition, we used a laser pointer to project a red dot at the opaque part of the partition above the model's head until the model had looked up (see electronic supplementary material, video S1). This dot was not visible from the observer's compartment. It was projected in four consecutive trials, with the inter-trial interval being set to a minimum of 30 s. For controls, we ran one trial in which we did not project the red dot (control 1) and one trial without model bird (control 2). For the test condition and control 2, we analysed whether the observer bird looked up within 10 s after cue presentation (i.e. after the model had looked up or after the projection of the red dot). For control 1, we randomly chose a 20 s sequence from a 1 min recording of the model and observer and screened the entire sequence for look-ups by the model bird. If no look-up was detected, we discarded the first 10 s (to avoid the fact that subjects may have responded to look-ups occurring prior to the onset of the sequence) and analysed the last 10 s. We used head orientation (beak lateral or horizontal) and head movement (e.g. switching from right to left eye) to assess the direction in which birds looked (Dawkins 2002).
Figure 1.
Set-up of (a) experiment 1, and (b) experiment 2. Arrows indicate the gaze direction of the model.
(c). Experiment 2: geometrical gaze following
The procedure was similar to experiment 1, with the following exceptions. The test compartments were parallel to each other. A wooden wall (1 m high) was placed inside the subject's compartment and we ensured that birds did not hesitate to move around the wall (see electronic supplementary material). The model bird could move up to the position of the barrier, allowing it to look, but not walk, behind it (figure 1b). To attract the model's attention, the experimenter pulled a piece of food attached to a thin nylon thread in and out of an opaque tube that was mounted vertically to the wall behind the barrier (opposite to the test subject's position). For testing, we waited until the test subject was on one side of the barrier and the model able to look towards the opposite side of the barrier; then, the experimenter released the stimulus from the tube until the model had oriented its head in its direction (i.e. looked at it; see electronic supplementary material, video S2). For controls, we ran one trial without stimulus presentation (control 1), and one trial with stimulus presentation but without the model present (control 2). We measured whether test subjects walked around the barrier or whether they looked at the barrier. To avoid habituation of the model birds to the stimulus, we conducted only one trial per condition.
(d). Statistical analysis
All trials were coded from videotapes. A second person coded 20 per cent of the trials and inter-observer reliability was excellent (Cohen's K = 0.87). Because of 1–0 coding, we used two-tailed McNemar tests with sequential Bonferroni correction.
3. Results
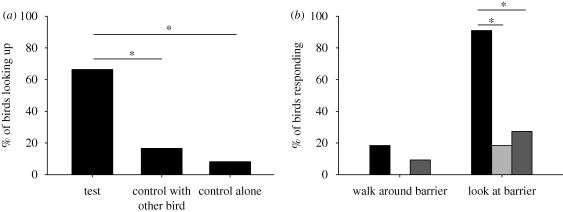
In experiment 1 (following look-ups), focal birds looked up significantly more often in the test than in the controls on the first trial (n = 12; test versus control 1: p = 0.031; test versus control 2: p = 0.016; figure 2a) and on the second trial (n = 12; test versus control 1: p = 0.008; test versus control 2: p = 0.004) but not on the two trials thereafter (all p > 0.05). In experiment 2 (geometrical gaze following), birds did not search behind the barrier more often in the test than in the control trials (n = 11; test versus control 1: p = 0.500; test versus control 2: p > 0.999). However, birds oriented towards the barrier significantly more often in the test than in the control trials (n = 11; test versus control 1: p = 0.016; test versus control 2: p = 0.008; figure 2b).
Figure 2.
(a) Percentage of birds (n = 12) responding to the model's look-up in the first trial of experiment 1 and (b) percentage of birds (n = 11) responding to the model's gaze by walking around the barrier and by looking at the barrier in experiment 2. Note that categories of experiment 2 are not exclusive. Black bar, test; light grey bar, control with other bird; dark grey bar, control alone. Asterisks denote p < 0.05.
4. Discussion
As predicted, Northern bald ibises followed other's gaze into distant space but not geometrically around a visual barrier. Intriguingly, birds also visually co-oriented with the model in the barrier task, indicating that they did respond to the gaze cue, but they did not reposition themselves to gain a view comparable to the model. These findings stand in contrast to those of corvids and apes, which succeeded in using the others' gaze direction in either paradigm (e.g. Tomasello et al. 1999; Bugnyar et al. 2004). Given the keeping conditions of the ibises and the age of the birds (6–9 years), a lack of experience with visual barriers can be ruled out. Furthermore, all ibises were fully habituated to the setup so that neophobia towards the barrier can hardly explain the results.
In combination with previous reports on corvids and apes (Tomasello et al. 1999; Bugnyar et al. 2004), our results strongly indicate cognitive differences between the two modes of gaze following. The gaze following skills of bald ibises may primarily be based on a low-level mechanism (Povinelli & Eddy 1996; Tomasello et al. 1999), as their responses in the barrier task suggest that they do not consider that visual barriers impair perception. However, their responses are compatible with the idea that individuals are co-orienting with the model and searching for something of interest at the first thing they see, i.e. in front of the barrier. The improvement of young ravens (Bugnyar et al. 2004), apes (Okamoto et al. 2004) and human children (Butterworth & Jarrett 1991; Moll & Tomasello 2004) over development led to the assumption that gaze following skills are modified by experience, eventually resulting in the ability to extrapolate lines of sight for other individuals. Our data reveal that this is not the case in ibises. These birds are clearly capable of tracking others' gaze into space but do not acquire geometrical gaze follow skills with age. The fact that ibises responded only in the very first trials may indicate that they are prone to rapid habituation, at least when co-orienting with others is not reinforced by the sight of external stimuli (Tomasello et al. 2001; Schloegl et al. 2007). Still, our interpretations have to be treated with caution, as they are partially based on negative findings and additional testing in different experimental contexts is required before drawing final conclusions.
Acknowledgments
The experiment described here complies with the laws of Austria, where it was carried out.
We thank the Herzog von Cumberland Stiftung and the ‘Verein der Förderer’. Funded by FWF projects R31-B03, P20538-B17 and Y366-B17. C.S. was supported by DAAD-Doktorandenstipendium.
References
- Bräuer J., Call J., Tomasello M.2005All great ape species follow gaze to distant locations and around barriers. J. Comp. Psychol. 119, 145–154 (doi:10.1037/0735-7036.119.2.145) [DOI] [PubMed] [Google Scholar]
- Bugnyar T., Stöwe M., Heinrich B.2004Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc. R. Soc. Lond. B 271, 1331–1336 (doi:10.1098/rspb.2004.2738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T., Stöwe M., Heinrich B.2007The ontogeny of caching in ravens, Corvus corax. Anim. Behav. 74, 757–767 (doi:10.1016/j.anbehav.2006.08.019) [Google Scholar]
- Butterworth G., Jarrett N.1991What minds have in common in space: spatial mechanism serving joint visual attention in infancy. Brit. J. Dev. Psychol. 9, 55–72 [Google Scholar]
- Byrne R. W., Whiten A.1992Cognitive evolution in primates: evidence from tactical deception. Man 27, 609–627 (doi:10.2307/2803931) [Google Scholar]
- Call J., Hare B., Tomasello M.1998Chimpanzee gaze following in an object-choice task. Anim. Cogn. 1, 89–99 (doi:10.1007/s100710050013) [DOI] [PubMed] [Google Scholar]
- Dawkins M. S.2002What are birds looking at? Head movements and eye use in chickens. Anim. Behav. 63, 991–998 (doi:10.1006/anbe.2002.1999) [Google Scholar]
- Emery N. J., Lorincz E. N., Perret D. I., Oram M. W., Baker C. I.1997Gaze following and joint attention in rhesus monkeys (Macaca mulatta). J. Comp. Psychol. 111, 286–293 (doi:10.1037/0735-7036.111.3.286) [DOI] [PubMed] [Google Scholar]
- Gomez C.2005Species comparative studies and cognitive development. Trends Cogn. Sci. 9, 118–125 (doi:10.1016/j.tics.2005.01.004) [DOI] [PubMed] [Google Scholar]
- Kaminski J., Riedel J., Call J., Tomasello M.2005Domestic goats, Capra hircus, follow gaze direction and use social cues in an object choice task. Anim. Behav. 69, 11–18 (doi:10.1016/j.anbehav.2004.05.008) [Google Scholar]
- Moll H., Tomasello M.200412- and 18-month-old infants follow gaze to spaces behind barriers. Develop. Sci. 7, F1–F9 (doi:10.1111/j.1467-7687.2004.00315.x) [DOI] [PubMed] [Google Scholar]
- Okamoto S., Tanaka M., Tomonaga M.2004Looking back: the ‘representational mechanism’ of joint attention in an infant chimpanzee (Pan troglodytes). Jpn. Psychol. Res. 46, 236–245 (doi:10.1111/j.1468-5584.2004.00255.x) [Google Scholar]
- Povinelli D. J., Eddy T. J.1996Chimpanzees: joint visual attention. Psychol. Sci. 7, 129–135 (doi:10.1111/j.1467-9280.1996.tb00345.x) [Google Scholar]
- Schloegl C., Kotrschal K., Bugnyar T.2007Gaze following in common ravens (Corvus corax): ontogeny and habituation. Anim. Behav. 74, 769–778 (doi:10.1016/j.anbehav.2006.08.017) [Google Scholar]
- Schloegl C., Schmidt J., Scheid C., Kotrschal K., Bugnyar T.2008aGaze following in non-human animals: the corvid example. In Animal behaviour: new research (ed. Columbus F.), pp. 73–92 New York, NY: Nova Science Publishers [Google Scholar]
- Schloegl C., Kotrschal K., Bugnyar T.2008bDo common ravens (Corvus corax) rely on human or conspecific gaze cues to detect hidden food? Anim. Cogn. 11, 231–241 (doi:10.1007/s10071-007-0105-4) [DOI] [PubMed] [Google Scholar]
- Tintner A., Kotrschal K.2002Waldrappe im Freiflug- Das Grünauer Projekt. Zool. Garten N.F. 71, 113–127 [Google Scholar]
- Tomasello M., Call J., Hare B.1998Five primate species follow the visual gaze of conspecifics. Anim. Behav. 55, 1063–1069 (doi:10.1006/anbe.1997.0636) [DOI] [PubMed] [Google Scholar]
- Tomasello M., Hare B., Agnetta B.1999Chimpanzees, Pan troglodytes, follow gaze direction geometrically. Anim. Behav. 58, 769–777 (doi:10.1006/anbe.1999.1192) [DOI] [PubMed] [Google Scholar]
- Tomasello M., Hare B., Fogleman T.2001The ontogeny of gaze following in chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta). Anim. Behav. 61, 335–343 (doi:10.1006/anbe.2000.1598) [Google Scholar]