Abstract
Allogrooming is probably one of the most common and most studied social behaviours in a variety of animals. Whereas the short-term benefits for the groomee have often been investigated, little is known about the effects for the groomer. Our study focused on the short-term effects of grooming another group member in seven adult female crested black macaques (Macaca nigra). We found reductions in self-directed behaviour, an indicator of anxiety, and aggressive tendencies soon after grooming, when compared to matched-control periods. These findings can be interpreted as evidence of distress prevention, possibly mediated by an increase in tolerance. Indeed, a former groomee was more likely to be the nearest neighbour of the former groomer in the 10 min after grooming ended. Thus, the role of grooming in short-term distress alleviation can be applicable to the groomer as well as the groomee. These short-term effects, together with the longer-term effects of large and/or strong grooming networks confirm that grooming, as well as receiving grooming, has great importance for social dynamics.
Keywords: aggression, grooming, scratching, stress, tolerance
1. Introduction
Allogrooming is probably the most common social behaviour among primates and, possibly, among other mammals and birds (Goosen 1987; Kutsukake & Clutton-Brock 2006; Radford & Du Plessis 2006). In addition to its hygienic function in terms of removal of ectoparasites (Zamma 2002), allogrooming is generally considered to maintain social bonds between group members (Dunbar 1988), being exchanged for further grooming or other services (Henzi & Barett 1999; Schino & Aureli 2008). This social function may be mediated by the release of brain opioids such as β-endorphins (Keverne et al. 1989).
The usually relaxed posture of the groomee and the massage-like actions of the groomer are probably what inspired Terry (1970) to propose a tension-reduction function for allogrooming. Recent studies have supported this view by showing a reduction in heart rate in individuals receiving grooming (Boccia et al. 1989; Aureli et al. 1999). This physiological evidence is further supported by a study using behavioural indicators of stress and anxiety. In primates, self-directed behaviours such as self-scratching and self-grooming are displayed in stressful situations and are sensitive to anxiolytic and anxiogenic drugs (Maestripieri et al. 1992; Schino et al. 1996). Long-tail macaques (Macaca fascicularis) were found to display less self-directed behaviour soon after they were groomed (Schino et al. 1988).
In addition to these short-term effects, Shutt et al. (2007) have recently shown that in Barbary macaques (Macaca sylvanus) the giving rather than the receiving of grooming is associated with lower stress levels in the longer term, as measured by faecal glucocorticoid concentrations. The authors deduced from their results that grooming creates a large and strong network of social support (see also Gust et al. 1993; Engh et al. 2006; Crockford et al. 2008).
Research on short-term effects of grooming has in general focused on the groomee, whereas studies on longer-term effects include the groomer as well. The present study focused on the groomer in an attempt to expand knowledge regarding the short-term effects of grooming another group member. Adult female crested black macaques (Macaca nigra) were observed after they groomed an adult group member in order to investigate whether they experienced a change in anxiety, aggressive tendency and social tolerance.
2. Material and methods
(a). Subjects and housing
The study subjects were adult females belonging to a large group of crested black macaques (M. nigra) housed at Chester Zoo, UK. The group consisted of three adult males, seven adult females, two subadults, five juveniles and several infants.
The macaques were housed in a large enclosure with access to indoor and outdoor sections. The 195 m2 (6 m high) indoor section had wood chips on the floor and a large number of enrichment devices, including logs, sacks, ropes and swings. The 2000 m2 outdoor section was landscaped with grassy areas, trees and bushes, as well as logs, platforms and ropes. The macaques were fed at varying times throughout the day and had access to water at all times.
(b). Data collection
Data were collected between 10.00 and 16.00 from November 2004 to January 2005, excluding periods in which the monkeys were fed. Allogrooming (hereafter grooming) was defined as the picking through and brushing aside of an adult partner's fur using one or both hands, or the teeth. A grooming bout that was interrupted for more than 30 s was considered terminated, and only bouts lasting over 60 s resulted in post-grooming observations (PG). Upon cessation of grooming, a 10 min focal animal observation of the groomer was carried out by continuously recording its behaviour. If the focal animal became re-involved in a grooming bout the PG observation was stopped. All aggressive patterns (i.e. threat, lunge, chase, physical contact, bite) displayed by the focal animal were recorded, as well as its self-scratching (a new bout was recorded after a break of 10 s). The duration of the focal animal's self-grooming was also recorded. Every minute the identity of the nearest neighbour of the focal animal was recorded using instantaneous sampling.
The behaviour collected during PGs was compared with that in corresponding matched-control observations (MC). This procedure followed the well-established methodology to study post-conflict behaviour (de Waal & Yoshihara 1983), which has already been used to study the effects of grooming on the groomee (Aureli et al. 1999). MCs were carried out on the next available day starting at about the same time as the corresponding PG, collecting the same type of data and following the same procedure as described for PGs. MCs were postponed if the focal animal was involved in a grooming bout in the 10 min before the planned starting time.
(c). Data analysis
Analyses were based on a total of 57 PG–MC pairs (7–9 per subject; mean ± s.e., 8.3 ± 0.4). In PGs and MCs, self-scratching and aggression rates were calculated by dividing their frequencies by the focal observation time for each adult female. The proportion of time spent self-grooming by each adult female was calculated as the total duration of self-grooming divided by the focal observation time. The proportion of instantaneous scans the groomee spent as the nearest neighbour of the groomer was also calculated for PGs and MCs.
Differences in behaviour between PGs and MCs were tested using Wilcoxon matched pairs tests. A Spearman test was used to correlate the mean proportion of PG instantaneous scans the groomee spent as nearest neighbour of the groomer and the minutes after the end of the grooming bout. All tests were two-tailed and the alpha value was set at 0.05.
3. Results
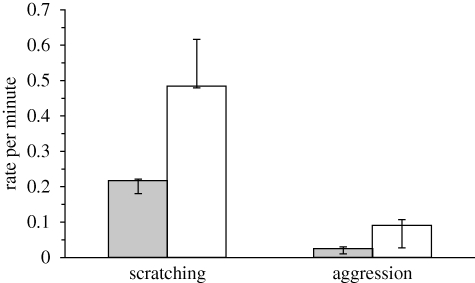
Self-scratching rates were lower after giving grooming than in control periods for all seven adult females (n = 7, T = 0, p = 0.016; figure 1). Similarly, the percentage of time spent in self-grooming was lower in PGs than in MCs (median (and interquartile range), 1.8 (1.3–2.2) versus 5.7 (5.6–6.7); n = 7, T = 0, p = 0.016).
Figure 1.
Median (and interquartile range) rates per minute of self-scratching and aggressive behaviour by the former groomer in post-grooming (PG) and matched-control (MC) observations. Filled bars, PG; open bars, MC.
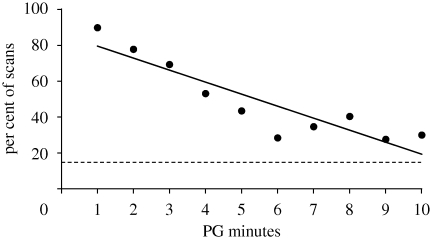
The former groomer initiated fewer aggressive interactions in the PGs than in the MCs (n = 7, T = 2, p = 0.047; figure 1). The former groomee was more likely to be the nearest neighbour of the former groomer in PGs than in MCs (n = 7, T = 0, p = 0.016; figure 2). The effect was stronger soon after the end of the grooming bout, as there was a negative correlation between the mean proportion of scans across the seven adult females in which the groomee was the nearest neighbour of the former groomer and the minutes after the end of the grooming bout (n = 10, rs = −0.891, p = 0.0005; figure 2).
Figure 2.
Correlation between the mean percentage of instantaneous scans per PG minute across the seven adult females in which the groomee was the nearest neighbour of the former groomer and the minutes after the end of the grooming bout is shown with the best fitting line. The dotted line represents the mean percentage of instantaneous scans for the whole MC period.
4. Discussion
Our study provides, to our knowledge, the first evidence for short-term distress reduction in the groomer. An early attempt on long-tailed macaques did not find such an effect for the groomer, whereas it found it for the groomee (although there was an increase in self-directed behaviour in the first 10 s after the end of the grooming bout; Schino et al. 1988; see also Manson & Perry 2000). These two studies used self-directed behaviour as an indicator of anxiety and distress. Studies using heart rate confirmed the calming effect of receiving grooming, but it is more difficult to use such a measure for the groomer because heart rate is strongly affected by physical activity (Boccia et al. 1989; Aureli et al. 1999).
Our findings can be interpreted as evidence of distress prevention. Close proximity with group members may be perceived as stressful given the potential for aggressive interactions or simply the unpredictability of their behaviour. Macaques, baboons, capuchin monkeys and chimpanzees display more self-directed behaviour and increased heart rate when in proximity with group members, depending on the relative dominance rank and degree of familiarity and affiliation (Troisi & Schino 1987; Schino et al. 1990; Aureli et al. 1999; Castles et al. 1999; Manson & Perry 2000; Kutsukake 2003). Grooming other group members, therefore, could be a means of preventing one's own distress while in their proximity.
Such an effect of grooming could be mediated by an increase in tolerance. Indeed, the former groomee was more likely to be the nearest neighbour of the former groomer in the 10 min after the end of grooming compared to control conditions, although the effect decreased over time. Similar results were found for two other macaque species (Troisi et al. 1989). The view that grooming increases tolerance is also supported by the reduction of aggressive tendency. Our study shows that the former groomer initiated fewer aggressive interactions after grooming than during control conditions. This complements findings in bonnet macaques (Macaca radiata) in which both the groomer and groomee were less likely to be attacked in the aftermath of grooming (Silk 1982).
The prevention of distress and the increase of tolerance provide evidence that grooming others is beneficial. Thus, our study lends support to the tension-reduction function of allogrooming (Terry 1970) and suggests that this function can be applicable to the groomer as well as the groomee. The groomer's distress alleviation may also be fitting the perspective that under certain conditions delivering benefits to others can be self-rewarding (de Waal et al. 2008). Following this perspective, the long-term benefits of large and/or strong social networks (Shutt et al. 2007; Crockford et al. 2008) may be subserved by a proximate mechanism based on the immediately rewarding consequences of grooming.
Acknowledgements
All research was approved by Chester Zoo.
We would like to thank the staff of Chester Zoo for their cooperation and C. Schaffner, G. Schino and two reviewers for their comments.
References
- Aureli F., Preston S. D., de Waal F. B. M.1999Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J. Comp. Psychol. 113, 59–65 (doi:10.1037/0735-7036.113.1.59) [DOI] [PubMed] [Google Scholar]
- Boccia M. L., Reite M., Laudenslager M.1989On the physiology of grooming in a pigtail macaque. Physiol. Behav. 45, 667–670 (doi:10.1016/0031-9384(89)90089-9) [DOI] [PubMed] [Google Scholar]
- Castles D. L., Whiten A., Aureli F.1999Social anxiety, relationships and self-directed behaviour among wild female olive baboons. Anim. Behav. 58, 1207–1215 (doi:10.1006/anbe.1999.1250) [DOI] [PubMed] [Google Scholar]
- Crockford C., Wittig R. M., Whitten P. L., Seyfarth R. M., Cheney D. L.2008Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus). Horm. Behav. 53, 254–265 (doi:10.1016/j.yhbeh.2007.10.007) [DOI] [PubMed] [Google Scholar]
- de Waal F. B. M., Leimgruber K., Greenberg A. R.2008Giving is self-rewarding for monkeys. Proc. Natl Acad. Sci. USA 105, 13 685–13 689 (doi:10.1073/pnas.0807060105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal F. B. M., Yoshihara D.1983Reconciliation and redirected affection in rhesus monkeys. Behaviour 85, 224–241 (doi:10.1163/156853983X00237) [Google Scholar]
- Dunbar R. I. M.1988Primate social systems New York, NY: Comstock Publishing [Google Scholar]
- Engh A. L., Beehner J. C., Bergman T. L., Whitten P. L., Hoffmeier R. R., Seyfarth R. M., Cheney D. L.2006Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc. R. Soc. B 273, 707–712 (doi:10.1098/rspb.2005.3378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosen C.1987Social grooming in primates. In Comparative primate biology, vol. 2B: behavior, cognition and motivation (eds Mitchell G., Erwin J.), pp. 107–131 New York, NY: Alan R. Liss [Google Scholar]
- Gust D. A., Gordon T. P., Hambright M. K., Wilson M. E.1993Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta). Horm. Behav. 27, 318–331 (doi:10.1006/hbeh.1993.1024) [DOI] [PubMed] [Google Scholar]
- Henzi S. P., Barrett L.1999The value of grooming to female primates. Primates 40, 47–59 (doi:10.1007/BF02557701) [DOI] [PubMed] [Google Scholar]
- Keverne E. B., Martensz N. D., Tuite B.1989Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 14, 155–161 (doi:10.1016/0306-4530(89)90065-6) [DOI] [PubMed] [Google Scholar]
- Kutsukake N.2003Assessing relationship quality and social anxiety among wild chimpanzees using self-directed behaviour. Behaviour 140, 1153–1171 (doi:10.1163/156853903322589687) [Google Scholar]
- Kutsukake N., Clutton-Brock T. H.2006Social function of allogrooming in cooperatively breeding meerkats. Anim. Behav. 72, 1059–1068 (doi:10.1016/j.anbehav.2006.02.016) [Google Scholar]
- Maestripieri D., Schino G., Aureli F., Troisi A.1992A modest proposal: displacement activities as an indicator of emotions in primates. Anim. Behav. 44, 967–979 (doi:10.1016/S0003-3472(05)80592-5) [Google Scholar]
- Manson J. H., Perry S.2000Correlates of self-directed behaviour in wild white-faced capuchins. Ethology 106, 301–317 (doi:10.1046/j.1439-0310.2000.00527.x) [Google Scholar]
- Radford A. N., Du Plessis M. A.2006Dual function of allopreening in the cooperatively breeding green woodhoopoe, Phoeniculus purpureus. Behav. Ecol. Sociobiol. 61, 221–230 (doi:10.1007/s00265-006-0253-6) [Google Scholar]
- Schino G., Aureli F.2008Grooming reciprocation among female primates: a meta-analysis. Biol. Lett. 4, 9–11 (doi:10.1098/rsbl.2007.0506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G., Maestripieri D., Scucchi S., Turilazzi P. G.1990Social tension in familiar and unfamiliar pairs of long-tail macaques. Behaviour 113, 264–272 (doi:10.1163/156853990X00518) [Google Scholar]
- Schino G., Scucchi S., Maestipieri D., Turillazzi P. G.1988Allogrooming as a tension-reduction mechanism: a behavioural approach. Am. J. Primatol. 16, 43–50 (doi:10.1002/ajp.1350160106) [DOI] [PubMed] [Google Scholar]
- Schino G., Perretta G., Taglioni A. M., Monaco V., Troisi A.1996Primate displacement activities as an ethopharmacological model of anxiety. Anxiety 2, 186–191 (doi:10.1002/(SICI)1522-7154(1996)2:4<186::AID-ANXI5>3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- Shutt K., MacLarnon A., Heistermann M., Semple S.2007Grooming in Barbary macaques: better to give than to receive? Biol. Lett. 3, 231–233 (doi:10.1098/rsbl.2007.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J. B.1982Altruism among female Macaca radiata: explanations and analysis of patterns of grooming and coalition formation. Behaviour 79, 162–188 (doi:10.1163/156853982X00238) [Google Scholar]
- Terry R.1970Primate grooming as a tension reduction mechanism. J. Psychol. 76, 129–136 [DOI] [PubMed] [Google Scholar]
- Troisi A., Schino G.1987Environmental and social influences on autogrooming behaviour in a captive group of Java monkeys. Behaviour 100, 292–303 (doi:10.1163/156853987X00161) [Google Scholar]
- Troisi A., Schino G., Aureli F.1989Allogrooming and interindividual proximity in two species of macaques (Macaca fascicularis and M. nemestrina). Behaviour 111, 196–206 (doi:10.1163/156853989X00655) [Google Scholar]
- Zamma K.2002Grooming site preferences determined by lice infection among Japanese macaques in Arashiyama. Primates 43, 41–49 (doi:10.1007/BF02629575) [DOI] [PubMed] [Google Scholar]