Abstract
Investigation of feathers from the famous Middle Eocene Messel Oil Shale near Darmstadt, Germany shows that they are preserved as arrays of fossilized melanosomes, the surrounding beta-keratin having degraded. The majority of feathers are preserved as aligned rod-shaped eumelanosomes. In some, however, the barbules of the open pennaceous, distal portion of the feather vane are preserved as a continuous external layer of closely packed melanosomes enclosing loosely aligned melanosomes. This arrangement is similar to the single thin-film nanostructure that generates an iridescent, structurally coloured sheen on the surface of black feathers in many lineages of living birds. This is, to our knowledge, the first evidence of preservation of a colour-producing nanostructure in a fossil feather and confirms the potential for determining colour differences in ancient birds and other dinosaurs.
Keywords: feather preservation, melanosome, feather colour, bird
1. Introduction
Melanin is an important pigment in animals for display, ultraviolet protection and predator avoidance (e.g. ink in cephalopods). Melanins are synthesized in a special class of pigment cells (melanocytes) and are packaged within organelles (melanosomes) that vary in morphology between tissues, colours and organisms; the chemically inert structure is not fully understood (Liu & Simon 2005). Wuttke (1983) interpreted the aligned oblate bodies that constitute the fossil feathers of the Messel Oil Shale as lithified bacteria. This interpretation was extrapolated to fossil feathers from other localities (Davis & Briggs 1995). Our recent investigation of a colour-banded feather from the Cretaceous Crato Formation of Brazil (Martill & Frey 1995) led us to interpret the oblate bodies as eumelanosomes, which contain black melanin (Vinther et al. 2008). They are preserved in the dark areas of this feather but not in the light areas, and their alignment matches the typical arrangement of eumelanosomes in feathers of living birds. We noted that different shapes and arrangements in the feathers of living birds result in different colours and predicted that such structure might be preserved in fossil examples, allowing their original colour to be diagnosed (Vinther et al. 2008). An obvious test of this hypothesis is provided by the Messel birds.
2. Material and methods
We surveyed birds from the Messel Shale held by the Senckenberg Museum, Frankfurt, Germany. All the birds have been transferred to resin and are too large to be accommodated easily in the chamber of an environmental scanning electron microscope (ESEM); they are typically covered by a thin layer of varnish. Isolated feathers, however, are retained in the original shale matrix and stored in glycerine. A representative selection of 12 isolated feathers was sampled for scanning electron microscopy (SEM). Specimens were photographed using a Nikon D90 DSLR camera with an AF Micro-NIKKOR 60 mm f/2.8D lens before and after sampling. Specimens were immersed in water to remove the glycerine. Several samples of each feather, just a few millimetres in dimension, were removed with a scalpel and mounted on SEM stubs and dried. The specimens were then replaced in glycerine.
The ultrastructure of the fossils was studied in a Philips XL 30 ESEM. Elemental composition was analysed using the energy dispersive X-ray analyser (EDX) at 10 kV; the distribution of the carbonaceous material was confirmed in the backscatter mode (Orr et al. 2002). Such elemental analysis was necessary to distinguish between colour differences on the fossils that are diagenetic/mineralogical in origin versus original differences. The samples were subsequently coated with gold to allow better resolution.
3. Results
The Messel feathers are typically well preserved, and it is possible to distinguish barbs from barbules (figure 1a). Many of the feathers appear to be brownish red or dark green (figure S2, electronic supplementary material). The distribution of these hues is diagenetic rather than original, and their origin remains problematic. We found no evidence that the feathers are mineralized in siderite (FeCO3); EDX and backscatter imaging showed that they are dominantly carbonaceous (contra Wuttke 1983). SEM revealed that melanosomes are ubiquitous over the surface of the feathers. Occasional pyrite framboids (FeS2) were detected, but not in quantities that could give rise to the red or green colours. These hues (figure S2, electronic supplementary material) may reflect absorption of metal ions into the melanosomes during diagenesis. Strong electronegativity of carboxyl groups is a property of melanin (Liu & Simon 2005) and may have attracted iron ions, which could generate the different observed colours in an oxidized or reduced state.
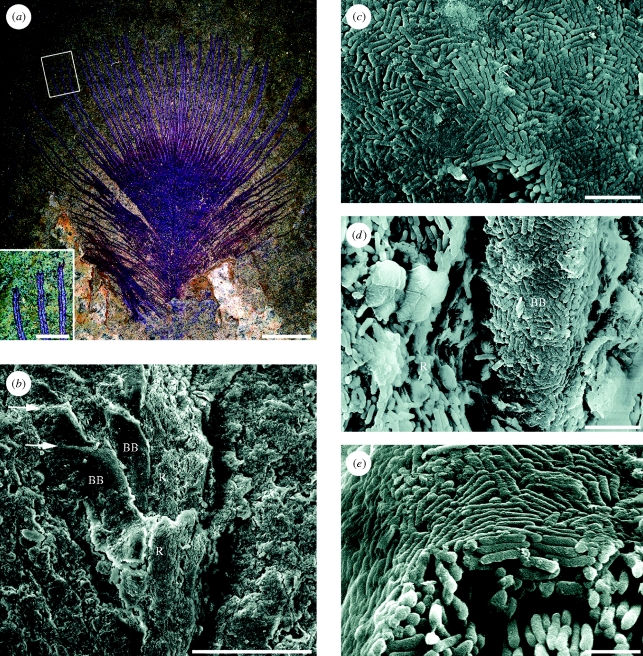
Figure 1.
Open pennaceous contour feather from the Eocene Messel Oil Shale, SMF ME 3850 showing evidence of original structural colour. (a) The part; the inset shows a detail of the distal barb rami and reflective barbules. (b–e) SEMs of samples from the counterpart (figure S1, electronic supplementary material). (b) Distal part of barb ramus. (c) Surface of the barbule melanosome layer. (d) Base of a barbule and its attachment to the barb ramus; note the lack of a uniform melanosome layer on the ramus. (e) Cross-section of barbule showing thin outer layer and aligned melanosomes within. R, ramus with loose aligned melanosomes; BB, associated barbules with surface layer of melanosomes; arrows show cell boundaries. Scale bars: (a) 5 mm, inset 1 mm; (b) 50 µm; (c,d) 2 µm; (e) 1 µm.
SEM investigation of representative isolated feathers from Messel showed that the keratin has degraded, exposing the melanosomes, which display two main types of organization. Most feathers revealed elongate oblate eumelanosomes roughly aligned along barbs and barbules, with little variation in their morphology or arrangement over the surface of the feather (as in the Crato example illustrated by Vinther et al. 2008). Three contour feathers, on the other hand, showed a striking contrast in the arrangement of melanosomes in the proximal and distal regions of the feather. These specimens range up to 3 cm in length. In the best-preserved specimen (SMF ME 3850, figure 1a), the basal 20 mm of the vane displays a closed pennaceous structure with interlocking barbules. The distal 8 mm of the vane has a conspicuously open pennaceous structure with prominent barbules that do not overlap or interlock. The barbules, which are 10–15 µm wide and oriented to the barb ramus at approximately 30°, are preserved as swaths of melanosomes (figure 1b). The margins of the barbules are defined by an abrupt transition from melanosomes to sediment. In places, barbule cells can be distinguished (figure 1b). In the proximal portion of the vane, both barbs and barbules are preserved as loosely packed aligned rod-shaped eumelanosomes (figure S1, electronic supplementary material). In the distal, open pennaceous portion, the eumelanosomes of the barb rami are similarly organized, but the melanosomes on the surface of the barbules form a solid, smooth and continuous external layer of closely packed rods (figure 1b–e). The melanosomes in this sheet are not oriented in a consistent direction, but neighbours are locally oriented in common directions (figure 1c–e). Underneath the superficial layer, the melanosomes become less organized both in packing and orientation with greater distance from the surface. Within the barbule, loose melanosomes are aligned along the axis (figure 1e).
The closed proximal portion of SMF ME 3850 (figure 1a) is preserved as a red material. On the part of the specimen, in the distal, open pennaceous portion of the vane, the barb rami appear red, but the barbules are silvery white (figure 1a). On the counterpart, the barbules appear silvery in a few places (figure S1, electronic supplementary material), presumably reflecting uneven splitting of the shale when the feather was revealed. We sampled and examined with SEM one of these silvery portions on the counterpart. The silvery sheen may be produced by reflection off the superficial sheet of melanosomes, exposed by decay of the surrounding beta-keratin.
4. Discussion
Structural details evident in the Messel feathers confirm our identification of melanosomes, which have a high preservation potential (Vinther et al. 2008). Bacteria cannot generate the organized layers (figure 1c–e) which are, in any case, characteristic of coloured feathers in living birds. Iridescent colours in many living birds are produced by constructive interference of light scattered by melanosomes in the barbules (Prum 2006). The size, shape and spatial distribution of melanosomes in these colour-producing nanostructures are highly variable (Durrer 1977; Prum 2006). The simplest nanostructure for the production of an iridescent colour is a single solid layer of melanosomes underneath a layer of superficial keratin. These nanostructures produce a structural colour by interference among light waves scattered by the keratin layer and the underlying layer of melanosomes (Prum 2006; Shawkey et al. 2006). This type of thin-film nanostructure has evolved numerous times in passeriform and non-passeriform birds (Prum 2006).
Feathers with this type of colour-producing nanostructure generally appear black with a glossy or oily iridescent sheen (figure 2). Depending on the thickness of the keratin layer (from approx. 100 to over 300 nm), the iridescent colour varies in reflectance from saturated ultraviolet (Ptilonorhynchus: Doucet et al. 2006) or blue (Quiscalus, Molothrus: Shawkey et al. 2006) to an oily appearance (e.g. Sturnus vulgaris: Prum 2006). The most distinctive feature of these nanostructures is the highly uniform superficial layer of closely packed melanosomes (figure 2e,f) (Durrer 1977; Prum 2006; Shawkey et al. 2006). The melanosomes may be rod shaped as in some passerines (e.g. Quiscalus: figure 2a,b,e), galliform birds (e.g. Tetrao) and the fossils described here (figures 1 and 2g) or flattened as seen in some ducks and swifts (e.g. Tachycineta and Hemiprocne) (figure 2d,f). Their orientation varies from uniformly aligned along the axis of the barbule to highly variable with some local uniformity among neighbouring melanosomes (e.g. Hemiprocne: figure 2d).
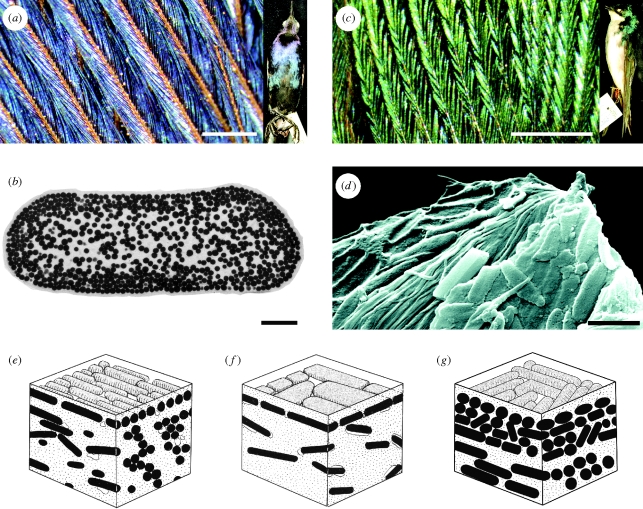
Figure 2.
Structural coloration in birds formed by a single thin film of beta-keratin above dense melanin sheets. (a) Belly contour feather of a male boat-tailed grackle (Quiscalus major: Icteridae; YPM 128134), showing blue iridescent colour in distal barbules. (b) Transmission electron microscopy of barbule from Q. major showing melanosomes in cross-section (by M. Shawkey). (c) Back contour feather of a tree swallow (Tachycineta bicolor: Hirundinidae; YPM 127371). (d) SEM image of a fractured barbule from the moustached treeswift (Hemiprocne mystacea: Apodiformes; YPM 74982), showing melanosomes randomly oriented as in the swallow and SMF ME 3850 (compare figure 1c). (e,f) Diagrammatic representation of melanosome organization illustrated in (b) and (d) (after Durrer 1977, Abb 21 and 27, with permission). (g) Reconstruction of organization in the fossil feather SMF ME 3850 illustrated in figure 1. Scale bars: (a,c) 0.5 mm; (b,d) 1 µm.
Although the Messel feathers show a specific combination of melanosome shape and orientation (i.e. rod shaped but unaligned: figures 1 and 2g) unknown in extant birds (reviewed in Prum 2006), the dense external layer of melanosomes indicates that they displayed some form of structural colour. The occurrence of this layer only in the barbules of the distal, open pennaceous portion of the feather suggests a display function.
In a phylogenetic study of this type of iridescence in a clade of blackbirds (Icteridae) and their close relatives with matte black plumage, Shawkey et al. (2006) demonstrated that the critical nanostructural feature is the uniformity of the superficial layer of melanosomes. Dense aggregations of melanosomes may occur in black feathers that lack iridescence (Shawkey et al. 2006), but the superficial layer of melanosomes is irregular with many gaps. Furthermore, as in the fossil, iridescence is usually restricted to the exposed barbules of the distal portion of the vane; the basal portion, which is overlapped by other feathers, is matt black. The distinct arrangement of melanosomes within the uniform layer in the fossil Messel feathers would have the same optical function.
Iridescence in the living bird is a result of constructive interference between light scattered at the surface of the feather and the interface between the surface keratin layer and the much higher refractive index of the immediately underlying melanosomes. The keratin in the fossil feather has degraded, eliminating this constructive interference, even though the preserved layer of melanosomes is apparently highly reflective. Like fossil feathers from the great majority of localities elsewhere (see table 1 in Davis & Briggs 1995), the Messel feathers are carbonaceous. Wuttke's (1983) original interpretation of these structures as siderite was based on X-ray diffraction, but the samples analysed may have been soft tissues other than feathers.
We cannot determine the original hue of the Messel feathers, given that there is no evidence of the thickness of the outer keratin, which has degraded. Comparisons with living birds with similar structures, however, suggest that these feathers were originally dark black in colour with a strongly lustrous iridescent blue, green or coppery sheen. Given that similar melanosome sheets and associated structural colours occur in an array of birds (e.g. many galliforms and swifts, stem members of which have been described from the Messel deposit: reviewed in Mayr 2009), referral of the feather to a specific taxon is not possible.
Acknowledgements
We are grateful to Stephan Schaal and Elvira Brahm, Forschungsinstitut Senckenberg and Kristof Zyskowski, Yale Peabody Museum, for access to fossil and living birds. Matthew Shawkey and Michael Wuttke offered valuable comments. Erica Champion helped produce the figures. The research was funded by NSF EAR-0720062, the National Geographic Society and the Yale University W.R. Coe Fund.
References
- Davis P. G., Briggs D. E. G.1995The fossilization of feathers. Geology 23, 783–786 (doi:10.1130/0091-7613(1995)023<0783:FOF>2.3.CO;2) [Google Scholar]
- Doucet S. M., Shawkey M. D., Hill G. E., Montgomerie R.2006Iridescent plumage in satin bowerbirds: structure, mechanisms and nanostructural predictors of individual variation in colour. J. Exp. Biol. 209, 380–390 (doi:10.1242/jeb.01988) [DOI] [PubMed] [Google Scholar]
- Durrer H.1977Schillerfarben der Vogelfeder als Evolutionsproblem: elektronenmikroskopische Untersuchung der Schillerstrukturen, ihrer Morphogenese und Analyse von Selektionsmechanismen (speziell dargelegt am Beispiel der Hühnervögel). Denkschriften der Schweizerischen Naturforschenden Gesellschaft 91, 127 [Google Scholar]
- Liu Y., Simon J. D.2005Metal–ion interactions and the structural organization of Sepia eumelanin. Pigment Cell Res. 18, 42–48 (doi:10.1111/j.1600-0749.2004.00197.x) [DOI] [PubMed] [Google Scholar]
- Martill D. M., Frey E.1995Colour patterning preserved in Lower Cretaceous birds and insects: the Crato Formation of N.E. Brazil. N. Jb Geol. Paläont. Mh 1995, 118–128 [Google Scholar]
- Mayr G.2009Paleogene fossil birds, p. xiii+262 Berlin, Germany: Springer [Google Scholar]
- Orr P. J., Kearns S. L., Briggs D. E. G.2002Backscattered electron imaging of fossils exceptionally preserved as organic compressions. Palaios 17, 110–117 (doi:10.1669/0883-1351(2002)017<0110:BEIOFE>2.0.CO;2) [Google Scholar]
- Prum R. O.2006Anatomy, physics, and evolution of avian structural colors. In Bird coloration, vol. 1 (eds Hill G. E., McGraw K. J.), pp. 295–353 Cambridge, MA: Harvard University Press [Google Scholar]
- Shawkey M. D., Hauber M. E., Estep L. K., Hill G. E.2006Evolutionary transitions and mechanisms of matte and iridescent plumage coloration in grackles and allies (Icteridae). J. R. Soc. Interface 3, 777–786 (doi:10.1098/rsif.2006.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinther J., Briggs D. E. G., Prum R. O., Saranathan V.2008The color of fossil feathers. Biol. Lett. 4, 522–525 (doi:10.1098/rsbl.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke M.1983‘Weichteil-Erhaltung’ durch lithifizierte Mikroorganismen bei mittel-eozänen Vertebraten aus den Ölschiefern der ‘Grube Messel’ bei Darmstadt. Senck. Leth. 64, 509–527 [Google Scholar]