Abstract
Regressive evolution of structures associated with vision in cave-dwelling organisms is the focus of intense research. Most work has focused on differences between extreme visual phenotypes: sighted, surface animals and their completely blind, cave-dwelling counterparts. We suggest that troglodytic systems, comprising multiple populations that vary along a gradient of visual function, may prove critical in understanding the mechanisms underlying initial regression in visual pathways. Gene expression assays of natural and laboratory-reared populations of the Atlantic molly (Poecilia mexicana) revealed reduced opsin expression in cave-dwelling populations compared with surface-dwelling conspecifics. Our results suggest that the reduction in opsin expression in cave-dwelling populations is not phenotypically plastic but reflects a hardwired system not rescued by exposure to light during retinal ontogeny. Changes in opsin gene expression may consequently represent a first evolutionary step in the regression of eyes in cave organisms.
Keywords: regressive evolution, vision, qPCR, Poecilia mexicana, Poeciliidae
1. Introduction
Regressive evolution—the loss or simplification of derived traits—is a widespread phenomenon, the understanding of which provides important insights into the consequences of natural selection and genetic drift (Porter & Crandall 2003; Wilkens 2007). Most of the research on regressive evolution has focused on embryonic eye and pigment development in the blind Mexican cave tetra, Astyanax mexicanus (Jeffery 2005). While this system provides a powerful model for elucidating the developmental mechanisms associated with regressive eye evolution (Jeffery 2005), we suggest that elucidating the initial mechanisms underlying regressive evolution may be facilitated by studying systems in which eye regression is less advanced and eyes are still functional.
This is the case in the Cueva del Azufre system in southern Mexico, where a small livebearing fish, the Atlantic molly (Poecilia mexicana), has colonized at least two caves (Tobler et al. 2008a). Cave-dwelling P. mexicana occur parapatrically with their epigean ancestors, but despite spatial proximity and the lack of major physical barriers, gene flow between surface and cave populations as well as between different cave chambers is extremely low (Tobler et al. 2008a). Cave populations are morphologically distinct and exhibit reductions in pigmentation and eye size (Parzefall 2001). Unlike in other cave organisms (Langecker 2000), the eyes of cave-dwelling P. mexicana are fully functional, and fish readily respond to visual stimuli when given an opportunity (Tobler et al. 2008b). In this study, we compare the expression of opsin genes, which are responsible for visual sensitivity (Yokoyama 2000), in surface-dwelling P. mexicana to cave-dwelling conspecifics from two photically distinct cave environments: front cave chambers where natural skylights provide dim light, and deep cave chambers with perpetual darkness. We also compare photoreceptor cell densities between the two cave populations.
Theory predicts that natural selection favours the evolution of sensory systems that are locally adapted to ecological conditions (Endler & Basolo 1998). Locally adapted visual systems can increase an individual's sensitivity to available wavelengths of light in two ways: (i) changes in the amino-acid sequence of opsin proteins as a result of mutations in the opsin genes can ‘tune’ spectral sensitivities of photoreceptors to the local photic environment (Yokoyama 2000) and (ii) opsin genes may be differentially expressed to maximize an individual's sensitivity to particular wavelengths of light (Fuller et al. 2004; Parry et al. 2005). Functional changes in amino-acid sequences are absent in Atlantic mollies from different habitats, as cave and surface-dwelling fish have identical spectral sensitivities of photoreceptors (Körner et al. 2006). Hence, we focus on the latter mechanism in three opsin genes: short-wavelength-sensitive (SWS1) opsin coding for an ultraviolet-sensitive opsin protein, medium-wavelength-sensitive (Rh2) opsin coding for a green-sensitive opsin protein, and long-wavelength-sensitive (LWS) opsin coding for a red-sensitive opsin protein (Yokoyama 2000). Upregulating particular opsin genes—and hence increasing translation of corresponding opsin proteins in the retina—may increase an individual's sensitivity to particular wavelengths of light. Likewise, downregulation of an opsin gene may decrease sensitivity to certain wavelengths. Since individuals that occupy perpetually dark cave habitats have no use for vision, we predicted that opsin gene expression would be reduced in these populations relative to populations exposed to natural light because of either the fixation of now neutral mutations by drift, or natural selection on opsin gene expression (Jeffery 2005).
2. Material and methods
All fish were captured near the village of Tapijulapa (Tabasco, Mexico). Cavefish were collected in the chambers V (in dim light) and X (in perpetual darkness) of the Cueva del Azufre. Surface fish were collected in a nearby creek (Arroyo Tres). Immediately after capture, individuals were sacrificed, measured (standard length and eye diameter) and eyes were extracted and preserved in RNAlater (Ambion, Inc.). Laboratory stocks of cave and surface fish were established in May 2007 and maintained as randomly outbred populations at the University of Oklahoma. All stocks were exposed to identical environmental conditions and a natural sunlight cycle. Laboratory-reared fish were sacrificed and eyes were preserved as described above. For further information about field collections and husbandry of laboratory stocks, see electronic supplementary material.
RNA was isolated from retinal tissue using a TRIzol reagent (Invitrogen) isolation. Superscript III First-Strand Synthesis System (Invitrogen) was used to create cDNA. qPCR was conducted using Sybr Green (Invitrogen) chemistry and primers developed from sequences of Xiphophorus opsin genes (see electronic supplementary material). We compared relative eye size among populations using ANOVA. ANCOVA was used to investigate population differences in opsin expression patterns. Since the size of an eye potentially could affect the amount of mRNA present, eye size was used as a covariate in all analyses.
We quantified photoreceptor cell density of laboratory fish from cave chamber V and chamber X using immunohistochemistry. Eyes were enucleated from adult fish, fixed in 4 per cent paraformaldehyde in phosphate buffered saline and equilibrated in 30 per cent sucrose prior to cryosectioning. We used the monoclonal antibodies ZPR1 and ZPR3 (Vihtelic et al. 1999) to label an unknown epitope on the surface of red/green double cones and rhodopsin, respectively. Slides were counterstained with DAPI (Invitrogen) to label DNA (see electronic supplementary material). We estimated cone density based on photographs of transverse retinal sections near the optic nerve. Densities were compared between populations using a t-test. All analyses met the assumption of normality.
3. Results
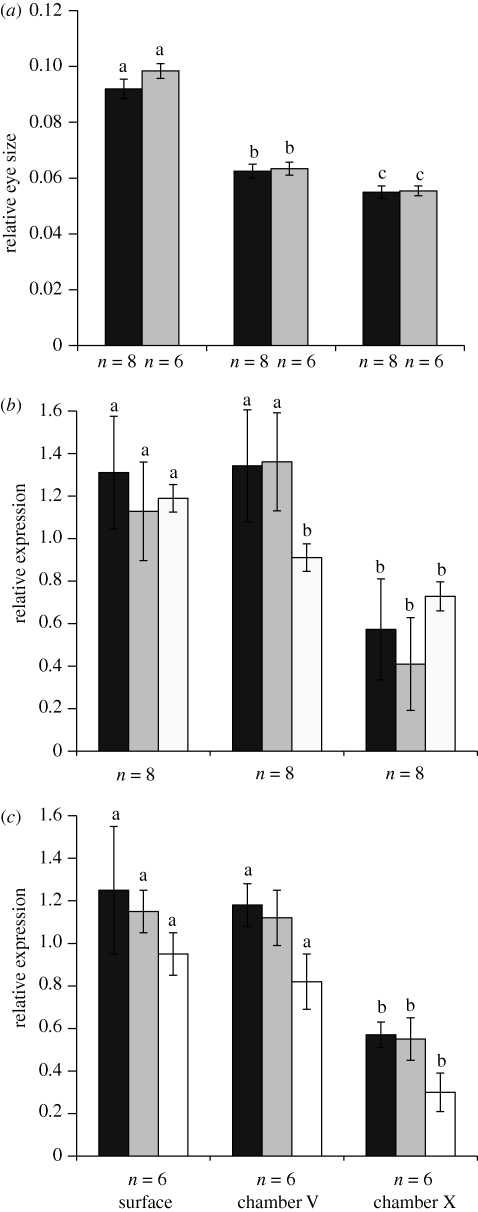
We found significant differences in eye size in wild-caught (F2,21 = 31.359, p < 0.001) and laboratory-reared fish (F2,15 = 19.10, p < 0.001), with surface fish having larger eyes than cave-dwelling fish (figure 1a). However, eye size did not significantly predict opsin gene expression (ANCOVA: F1,20 ≤ 0.173, p ≥ 0.682 for all three genes); hence we removed the covariate from the final population comparisons.
Figure 1.
(a) Relative eye size (eye diameter/standard length) for wild-caught (black bars) and laboratory-reared (light grey bars) fish. (b,c) Relative expression levels of SWS1 (black bars), Rh2 (light grey bars) and LWS (white bars) for (b) wild caught and (c) laboratory-reared populations. Within groups ((a) wild-caught versus laboratory-reared) and (b,c) genes, bars with different letters above are significantly different (p < 0.05; Fisher's LSD post hoc comparisons based on ANOVA).
Among populations of wild-caught P. mexicana, we found significant differences in the expression of SWS1 (F2,21 = 5.206, p = 0.015), Rh2 (F2,21 = 8.999, p = 0.002) and LWS (F2,21 = 4.608, p = 0.022) (figure 1b). As predicted, the highest expression levels were observed in the surface-dwelling population, with similarly high levels of SWS1 and Rh2 expression in the population living in the front cave chamber. LWS expression, however, was significantly lower in this population. In fish from the deep cave chamber, the expression of all opsin genes was reduced (figure 1b). Comparisons of gene expression patterns among laboratory-reared fish revealed a similar pattern as in wild-caught fish (SWS1: F2,15 = 3.93, p = 0.042; Rh2: F2,15 = 4.61, p = 0.028; LWS: F2,15 = 6.85, p = 0.008). Laboratory-reared fish from surface-dwelling and front-cave populations had significantly higher expression of all three opsin genes than did laboratory-reared fish derived from the deep cave population (figure 1c).
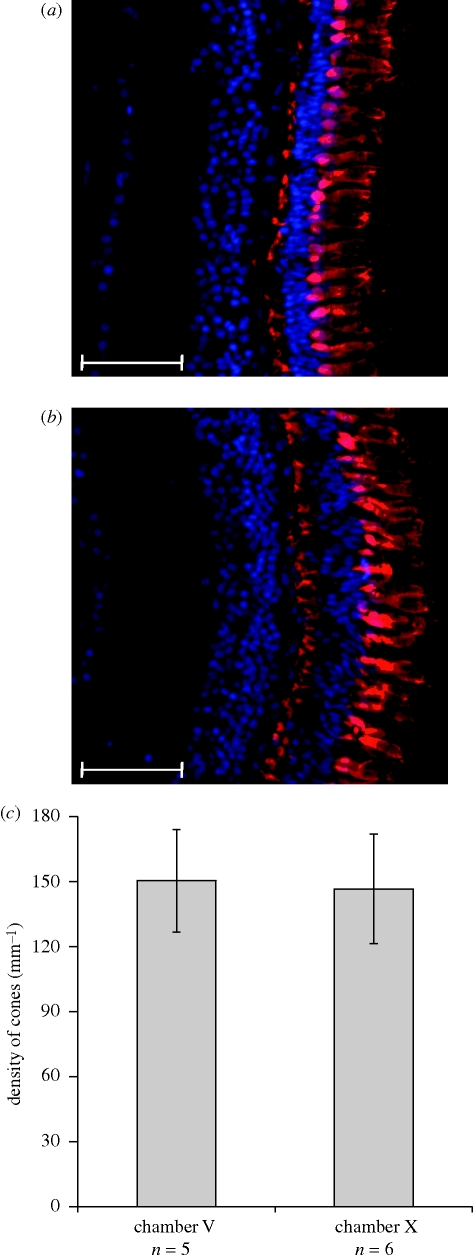
Laboratory-reared fish from chambers V and X did not differ in the densities of cones in the retina (t9 = 0.257, p = 0.803, figure 2). Furthermore, qualitative inspection of rod densities revealed no differences between the two populations (electronic supplementary material).
Figure 2.
(a,b) Representative labelling of red/green double cones (red dye) in P. mexicana from the cave, (a) chamber V and (b) chamber X. The blue-dye-labelled cell nuclei. (c) Mean (±s.d.) red/green double cone density in fish from the two cave chambers. Scale bar, (a,b) 40 µm.
4. Discussion
The regressive evolution of structures associated with vision in cave-dwelling organisms is the focus of much controversy because of the difficulties inherent in falsifying the null hypothesis of evolution by genetic drift (Romero & Green 2005; Wilkens 2007). Most research has focused on extreme differences between sighted surface animals and their completely blind counterparts, and multiple genes are involved in the complex developmental pathways leading to eye regression (Jeffery 2005; Protas et al. 2007). Troglodytic organisms, which retain some visual function, may prove critical in understanding the mechanisms underlying the initial regression of visual pathways. We suggest that cave-dwelling Atlantic mollies are one such system. In this study, we found an overall reduction in opsin gene expression in mollies from perpetually dark cave chambers, which could not be explained by differences in eye size. Also, while surface and front-cave mollies have similarly high levels of SWS1 and Rh2 expression, front-cave mollies showed reduced expression of LWS opsin. The reduction in LWS opsin expression in individuals from the front cave chamber might be owing to the rapid attenuation of long-wavelength light characteristic of low-light conditions (Bradbury & Vehrencamp 1998).
To test whether opsin gene expression is a phenotypically plastic response to local photic environment (Fuller et al. 2004), we quantified opsin gene expression in fish derived from these same three natural populations that were born and reared in the laboratory under identical lighting conditions. We found similar opsin gene expression patterns in laboratory-reared fish as in wild-caught fish, suggesting that the reduction in opsin expression in wild-caught cave-dwelling mollies is not entirely phenotypically plastic. Instead, these results suggest heritable differentiation among populations. Changes in opsin gene expression may consequently represent a first evolutionary step in the regression of vision in cave organisms.
Retinal immunohistochemistry indicated that the decrease in opsin gene expression does not merely reflect a decrease in photoreceptor number, as the two cave populations—albeit different in gene expression—do not differ in cone and rod densities. Consequently, the structure of the retina appears to remain unchanged while the level of opsin gene transcription per photoreceptor has decreased.
Whether the heritable reductions of eye size (Plath et al. 2007) and opsin gene expression (this study) in cave mollies are driven by natural selection or genetic drift is as yet unclear. In other systems, however, there is mounting evidence that the regression of visual systems is adaptive and caused by antagonistic pleiotropic effects, where traits beneficial to survival in darkness are enhanced at the expense of others (Jeffery 2005; Protas et al. 2007). Specifically, the development of eyes has recently been shown to be dependent on the same regulatory genes as the development of jaws and taste buds; i.e. selection on the latter two structures may directly affect eye development (Yamamoto et al. 2009). Incidentally, cave mollies—like Astyanax cavefish—have also diverged in taste bud density (Parzefall 2001) and jaw morphology (Tobler 2008). This raises the question as to whether common developmental trade-offs underlie the convergent evolution of troglodytic phenotypes.
Acknowledgements
We thank I. Schlupp for help in the field and providing fish from laboratory stocks, J. Tine for expertise in qPCR processing, and K. Winemiller for his continuous support. Funding was provided from the Swiss National Science Foundation (PBZHA-121016), the National Institutes of Health (IF32GM077143), and the US National Science Foundation (IOB-0636712 and SEGR-105086500).
All protocols were approved by the Texas A&M University Animal Care and Use Committee.
References
- Bradbury J. W., Vehrencamp S. L.1998Principles of animal communication Sunderland, MA: Sinauer [Google Scholar]
- Endler J. A., Basolo A.1998Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 13, 415–420 (doi:10.1016/S0169-5347(98)01471-2) [DOI] [PubMed] [Google Scholar]
- Fuller R., Carleton K. L., Fadool J. M., Spady T. C., Travis J.2004Population variation in opsin expression in the bluefin killifish, Lucania goodei: a real-time PCR study. J. Comp. Physiol. A 190, 147–154 (doi:10.1007/s00359-003-0478-z) [DOI] [PubMed] [Google Scholar]
- Jeffery W. R.2005Adaptive evolution of eye degeneration in the Mexican blind cavefish. J. Hered. 96, 185–196 (doi:10.1093/jhered/esi028) [DOI] [PubMed] [Google Scholar]
- Körner K. E., Schlupp I., Plath M., Loew E. R.2006Spectral sensitivity of mollies: comparing surface- and cave-dwelling Atlantic mollies, Poecilia mexicana. J. Fish Biol. 69, 54–65 (doi:10.1111/j.1095-8649.2006.01056.x) [Google Scholar]
- Langecker T. G.2000The effect of continuous darkness on cave ecology and cavernicolous evolution. In Ecosystems of the world 30: subterranean ecosystems (eds Wilkens H., Culver D. C., Humphreys W. F.), pp. 135–157 Amsterdam, The Netherlands: Elsevier Science [Google Scholar]
- Parry J. W. L., Carleton K. L., Spady T. C., Carboo A., Hunt D. M., Bowmaker J. K.2005Mix and match colour vision: tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids. Curr. Biol. 15, 1734–1739 (doi:10.1016/j.cub.2005.08.010) [DOI] [PubMed] [Google Scholar]
- Parzefall J.2001A review of morphological and behavioural changes in the cave molly, Poecilia mexicana, from Tabasco, Mexico. Environ. Biol. Fish. 62, 263–275 (doi:10.1023/A:1011899817764) [Google Scholar]
- Plath M., Hauswaldt S., Moll K., Tobler M., Garcia de Leon F. J., Schlupp I., Tiedemann R.2007Local adaptation and pronounced genetic differentiation in an extremophile fish, Poecilia mexicana, inhabiting a Mexican cave with toxic hydrogen sulfide. Mol. Ecol. 16, 967–976 (doi:10.1111/j.1365-294X.2006.03212.x) [DOI] [PubMed] [Google Scholar]
- Porter M., Crandall K.2003Lost along the way: the significance of evolution in reverse. Trends Ecol. Evol. 18, 541–547 (doi:10.1016/S0169-5347(03)00244-1) [Google Scholar]
- Protas M. E., Conrad M., Gross J., Tabin C., Borowsky R.2007Regressive evolution in the Mexican cave tetra, Astyanax mexicanus. Curr. Biol. 17, 452–454 (doi:10.1016/j.cub.2007.01.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero A., Green S. M.2005The end of regressive evolution: examining and interpreting the evidence from cave fishes. J. Fish Biol. 67, 3–32 (doi:10.1111/j.0022-1112.2005.00776.x) [Google Scholar]
- Tobler M.2008Divergence in trophic ecology characterizes colonization of extreme habitats. Biol. J. Linn. Soc. 95, 517–528 [Google Scholar]
- Tobler M., DeWitt T. J., Schlupp I., Garcia de Leon F. J., Herrmann R., Feulner P., Tiedemann R., Plath M.2008aToxic hydrogen sulfide and dark caves: phenotypic and genetic divergence across two abiotic environmental gradients in Poecilia mexicana. Evolution 62, 2643–2649 (doi:10.1111/j.1558-5646.2008.00466.x) [DOI] [PubMed] [Google Scholar]
- Tobler M., Schlupp I., Plath M.2008bDoes divergence in female mate choice affect male size distribution in two cave fish populations? Biol. Lett. 4, 452–454 (doi:10.1098/rsbl.2008.0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic T. S., Doro C. J., Hyde D. R.1999Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis. Neurosci. 16, 571–585 (doi:10.1017/S0952523899163168) [DOI] [PubMed] [Google Scholar]
- Wilkens H.2007Regressive evolution: ontogeny and genetics of cavefish eye rudimentation. Biol. J. Linn. Soc. 92, 287–296 (doi:10.1111/j.1095-8312.2007.00840.x) [Google Scholar]
- Yamamoto Y., Byerly M. S., Jackman W. R., Jeffery W. R.2009Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cave fish evolution. Dev. Biol. 330, 200–211 (doi:10.1016/j.ydbio.2009.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S.2000Molecular evolution of vertebrate visual pigments. Prog. Retin. Eye Res. 19, 385–419 (doi:10.1016/S1350-9462(00)00002-1) [DOI] [PubMed] [Google Scholar]