Abstract
According to theory, directional female choice for male sexual ornaments is expected to erode underlying genetic variation. Considerable attention, in this regard, has been given to understanding the ubiquity of heritable genetic variation in both female choice and male sexual traits. One intriguing possibility emerging from this work is that persistent genetic variation could be maintained, over time, by variation in female mate preferences. Here, we report the results of a four-year study showing significant year-to-year fluctuations in mate preferences in a small marine fish, the sand goby, Pomatoschistus minutus. Although the average size of mature fish varied across years, we were unable to find direct evidence linking this variation to differences in female preferences among years. Our results, nevertheless, underscore the importance of temporal fluctuations in female mate preferences, as these can have important consequences for understanding variation in sexual traits and the intensity of sexual selection.
Keywords: body size, lek paradox, mate choice, sand goby, sexual selection, temporal fluctuation
1. Introduction
Male sexual ornaments have typically been thought to evolve as a consequence of selection pressures driven by female choice (Andersson 1994). For this to happen, females need to prefer an extreme expression of the male trait, and the preferences themselves should be concordant among individuals within the population and remain fairly consistent across time (Mead & Arnold 2004). Paradoxically, this type of directional female choice is also expected to exhaust genetic variation in male sexual traits by fixation of the alleles that enhance reproductive success. As a result, the benefits of choice accruing to females should gradually disappear (Kirkpatrick & Ryan 1991; Kotiaho et al. 2008). Such an outcome, however, does not accord with empirical data, which suggests that there is often extensive heritable genetic variation in both female choice and male sexual traits (Pomiankowski & Møller 1995).
It has been suggested that variation in female preferences could weaken the strength of selection operating on sexual ornaments, slowing their exaggeration and allowing underlying genetic variation to persist (Qvarnström 2001). Such flexibility could be favoured, for example, if male attractiveness is not absolute, but instead depends on male genotypic quality in a particular ecological or social context (Qvarnström 2001). Indeed, there should be no a priori reasons why traits underlying female choice should be any less plastic or variable than male signal traits (Bakker et al. 1999; Qvarnström 2001). To date, however, very little is known about the temporal consistency of female preferences (Jennions & Petrie 1997; Qvarnström 2001; Candolin 2003), although changes in environmental or social conditions within a breeding season have recently been shown to affect female mating behaviour (Forsgren et al. 2004). Long-term oscillations in female preferences are also conceivable, and have even been predicted by some theoretical models (Houle & Kondrashov 2002). Furthermore, females could also pay more attention to cues that show the greatest variation at any given time, and then shift their emphasis when variation in those traits is reduced owing to directional selection (Qvarnström 2001; Candolin 2003; Chaine & Lyon 2008). Despite these considerations, longitudinal studies in sexual selection are still surprisingly uncommon, with few empirical studies examining variability of female preferences over successive breeding seasons.
Here, we present a four-year study of female preferences in the sand goby (Pomatoschistus minutus), a small, sexually dimorphic marine fish. During their single, prolonged breeding season, males of the species attract females to their sand-covered nests and take exclusive care of the developing brood. Recently, sand goby females have been found to use multiple cues in mate choice, exhibit individual variation in mate preferences and make context-dependent mating decisions (Lehtonen & Lindström 2008; Lehtonen & Wong in press). In addition, the strength of female mate choice in two closely related gobiid fish has been shown to change over the course of the breeding season (Borg et al. 2002; Forsgren et al. 2004). However, as far as we are aware, very few studies (on any species) have examined longer-term fluctuations in mate choice (for a recent exception in birds, see Chaine & Lyon 2008). We not only tested for such fluctuations but also measured body sizes of mature males and females each year, as these could potentially be important in predicting mate choice (Andersson 1994; Jennions & Petrie 1997).
2. Material and methods
The study was carried out at the Tvärminne Zoological Station, southern Finland, during the sand goby breeding season (May–July) over four consecutive years (2003–2006). Aquaria used for the study were housed under natural light conditions and supplied with a continuous through-flow of seawater. Fish were fed twice a day with frozen chironomid larvae or live Neomysis integer shrimps. All fish were weighed and measured (total length) immediately before experimentation.
We tested the association preferences of gravid females in tanks measuring 70 × 25 cm (length × width). Each ripe, ready-to-spawn focal female (n2003 = 25, n2004 = 63, n2005 = 21, n2006 = 41) was placed into a central compartment within the aquarium and presented with a choice between two males differing in total length by at least 1 mm. Males were placed into two separate compartments (20 × 25 cm) at opposite ends of the tank and provided with a nesting resource (halved clay flowerpot), following the design of Lehtonen and Lindström (2008). We spot-sampled each female's behaviour every 5 min over the course of two 100 min sessions, recording the number of times she associated with each of the two males (Lehtonen & Lindström 2008). Using these data, we defined the ‘preferred’ male as the one with whom the female had spent the most number of times in association (absolute preference). We also calculated the female's relative preference for this male, that is, the number of times the female associated with him divided by the average of the number of times the female spent associating with both males (relative ‘fitness’ sensu, Arnold & Wade 1984). Similarly, the size (total length) of the preferred male and the quality of his nest construction (estimation of the percentage of nest covered by sand) were determined relative to that of the other male by dividing the preferred male's value for each trait by the average value for both males.
Body size is a common target of female choice (Andersson 1994), and preferences can also be affected by the female's own size (Jennions & Petrie 1997). To assess the average size of males in the field, we introduced ceramic tiles (10 × 10 cm) to an area of shallow water within a sandy cove near the field station each June. Males that built their nests using the tiles were then collected with a dip-net (n2003 = 86, n2004 = 35, n2005 = 254, n2006 = 189). As a proxy for the size distribution of ready-to-spawn females in the field, we measured the total lengths of hand-trawled females from the same location as soon as they had ripened with eggs in the laboratory and were therefore ready to spawn (n2003 = 25, n2004 = 63, n2005 = 21, n2006 = 41). If adult body size is important in predicting patterns of mate choice (Andersson 1994; Jennions & Petrie 1997), female preferences should vary concomitantly with any year-to-year differences in adult body size.
The statistical analyses were conducted using Systat 12 (SPSS Inc.) software.
3. Results
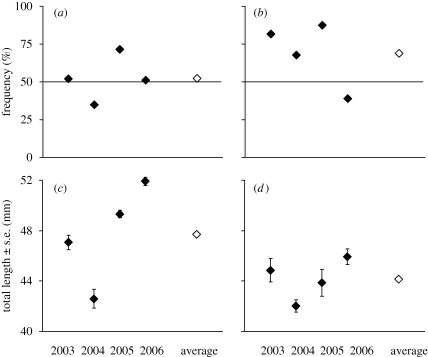
Absolute female preference for male size (G-test of independence, G = 9.44, d.f. = 3, p = 0.024) and nest cover (G-test of independence, G = 8.80, d.f. = 3, p = 0.032) varied significantly among years (figure 1a,b). Similarly, relative female preference scores varied annually, as did preferences for relative male length and nest cover (year × preference interactions) (table 1).
Figure 1.
(a,b) The frequency with which the male with the larger trait value was preferred (absolute preference) for (a) male size, (b) nest cover. (c,d) Yearly distributions of fish size for (c) males, (d) females. Open symbols indicate values averaged out across all four years.
Table 1.
General linear model analysis of among year variation in relative female preference (log-transformed) for relative male body size and relative nest cover percentage. (Year is a categorical variable (factor), whereas male total length and nest cover are covariates. Values for length and nest cover are expressed as the preferred male relative to the non-preferred male. SS, sum of squares; MS, mean squares.)
| source | SS | d.f. | MS | F | p |
|---|---|---|---|---|---|
| year | 0.314 | 3 | 0.105 | 3.564 | 0.016 |
| length | 0.083 | 1 | 0.083 | 2.836 | 0.094 |
| nest | 0.064 | 1 | 0.064 | 2.173 | 0.14 |
| year × length | 0.317 | 3 | 0.106 | 3.600 | 0.015 |
| year × nest | 0.307 | 3 | 0.102 | 3.484 | 0.018 |
| length × nest | 0.078 | 1 | 0.078 | 2.655 | 0.11 |
| year × length × nest | 0.309 | 3 | 0.103 | 3.515 | 0.017 |
| error | 3.932 | 134 | 0.029 |
We found significant year-to-year differences in average sizes of both mature males and females (males, one-way ANOVA, F3,560 = 47.1, p < 0.001; females, F3,146 = 7.86, p < 0.001) (figure 1c,d). However, fluctuations in adult body size did not directly predict differences in patterns of female preferences from year to year (figure 1). Similarly, the degree of variation in male or female length within each year did not correlate (or match in year-to-year sequence) with the corresponding variation in relative female preference for male size or nest cover (in all cases p > 0.10). Nevertheless, there was a negative overall correlation between female size and relative nest cover of the preferred male (combined correlation over the four-year period, rS = −0.180, d.f. = 147, p = 0.028), indicating that the smaller the female, the stronger her preference for a well-covered nest.
4. Discussion
Here, we have shown that female preferences in the sand goby vary significantly among years. Such long-term fluctuations are important for understanding persistence of genetic variation underlying male traits, with fluctuating preferences potentially altering the intensity or direction of sexual selection, thereby slowing the loss of variation (Qvarnström 2001). Year-to-year differences in preferences also underscore the importance of moving beyond a ‘snap shot’ view of sexual selection (Badyaev & Duckworth 2003). In this regard, ignoring the potential for temporal variation in female choice could lead to a misinformed view of how sexual selection pressures operate within a population (Jennions & Petrie 1997). Within taxa, the role of different signals in mate choice is not always unanimous (Parker & Ligon 2003). Our study highlights the possibility that such discrepancies could be owing to temporal variation in female preferences.
We found that the average size of males and females fluctuated strongly from year to year. However, even though the size of the female had a weak, but significant, influence on the strength of her preference for males with well-covered nests, we were unable to find any direct evidence linking variation in body size to differences in female preferences among years. Note, however, that our samples are based on only four years of data. Given fluctuations in female size, the link between female length and her nest cover preference could nevertheless help to explain why previous studies on sand gobies have yielded different results with regard to the role of nest cover in female mate choice (Lehtonen & Wong in press). The fact that average male size also varied among years implies that females may need to be sensitive to the current distribution of male sizes, rather than relying, for example, on a threshold choice criterion (Janetos 1980) that is fixed across the years. Such a strategy could, quite conceivably, contribute to the maintenance of variation within the population and warrants further investigation (Mead & Arnold 2004).
Body size aside, what other factors might contribute to temporal fluctuation of female preferences measured in controlled laboratory conditions? Female lifetime experience prior to experimentation may vary among years owing to environmental fluctuations (e.g. water temperature and food availability). Furthermore, male sexual signals, and hence female mating decisions, are sometimes influenced by non-stable environmental factors, as has been shown in three-spined sticklebacks (Gasterosteus aculeatus), in which socially enforced signals of male quality are compromised under turbid conditions (Wong et al. 2007). Alternatively, as females of some species exhibit individual differences in mate preferences (Brooks & Endler 2001), population-level preferences could fluctuate among years owing to corresponding fluctuations in the abundance of different female types. Regardless of the underlying causes, our study underscores the importance of temporal fluctuations in female mate preferences, as these could have important consequences for understanding variation in sexual traits and the intensity of sexual selection.
Acknowledgements
The study complies with all the relevant laws of Finland and was approved by Finnish authorities, permit no. HY 84-2003, Licensing Committee Koe-eläintoimikunta, University of Helsinki.
The authors thank the Tvärminne Zoological Station for hosting the research, M. Järvi-Laturi for samples and anonymous reviewers for helpful comments. Financial support was provided by the Academy of Finland, the Finnish Cultural Foundation (T.K.L.) and the Australian Research Council (B.B.M.W.).
References
- Andersson M.1994Sexual selection Princeton, NJ: Princeton University Press [Google Scholar]
- Arnold S. J., Wade M. J.1984On the measurement of natural and sexual selection: theory. Evolution 38, 709–719 (doi:10.2307/2408383) [DOI] [PubMed] [Google Scholar]
- Badyaev A. V., Duckworth R. A.2003Context-dependent sexual advertisement: plasticity in development of sexual ornamentation throughout the lifetime of a passerine bird. J. Evol. Biol. 16, 1065–1076 (doi:10.1046/j.1420-9101.2003.00628.x) [DOI] [PubMed] [Google Scholar]
- Bakker T. C. M., Künzler R., Mazzi D.1999Condition-related mate choice in sticklebacks. Nature 401, 234 (doi:10.1038/45727) [Google Scholar]
- Borg Å. A., Forsgren E., Magnhagen C.2002Plastic sex-roles in the common goby: the effect of nest availability. Oikos 98, 105–115 (doi:10.1034/j.1600-0706.2002.980111.x) [Google Scholar]
- Brooks R., Endler J. A.2001Female guppies agree to differ: phenotypic and genetic variation in mate-choice behaviour and the consequences for sexual selection. Evolution 55, 1644–1655 [DOI] [PubMed] [Google Scholar]
- Candolin U.2003The use of multiple cues in mate choice. Biol. Rev. 78, 575–595 (doi:10.1017/S1464793103006158) [DOI] [PubMed] [Google Scholar]
- Chaine A. S., Lyon B. E.2008Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science 319, 459–462 (doi:10.1126/science.1149167) [DOI] [PubMed] [Google Scholar]
- Forsgren E., Amundsen T., Borg Å. A., Bjelvenmark J.2004Unusually dynamic sex roles in a fish. Nature 429, 551–554 (doi:10.1038/nature02562) [DOI] [PubMed] [Google Scholar]
- Houle D., Kondrashov A. S.2002Coevolution of costly mate choice and condition-dependent display of good genes. Proc. R. Soc. Lond. B 269, 97–104 (doi:10.1098/rspb.2001.1823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetos A. C.1980Strategies of female mate choice: a theoretical analysis. Behav. Ecol. Sociobiol. 7, 107–112 (doi:10.1007/BF00299515) [Google Scholar]
- Jennions M. D., Petrie M.1997Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327 (doi:10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M., Ryan M. J.1991The paradox of the lek and the evolution of mating preferences. Nature 350, 33–38 (doi:10.1038/350033a0) [Google Scholar]
- Kotiaho J. S., LeBas N. R., Puurtinen M., Tomkins J. L.2008On the resolution of the lek paradox. Trends Ecol. Evol. 23, 1–3 (doi:10.1016/j.tree.2007.09.012) [DOI] [PubMed] [Google Scholar]
- Lehtonen T. K., Lindström K.2008Repeatability of mating preferences in the sand goby. Anim. Behav. 75, 55–61 (doi:10.1016/j.anbehav.2007.04.011) [Google Scholar]
- Lehtonen T. K., Wong B. B. M. Should females prefer males with elaborate nests? Behav. Ecol. In press. ( doi:10.1093/beheco/arp091) [Google Scholar]
- Mead L. S., Arnold S. J.2004Quantitative genetic models of sexual selection. Trends Ecol. Evol. 19, 264–271 (doi:10.1016/j.tree.2004.03.003) [DOI] [PubMed] [Google Scholar]
- Parker T. H., Ligon J. D.2003Female mating preferences in red junglefowl: a meta-analysis. Ethol. Ecol. Evol. 15, 63–72 [Google Scholar]
- Pomiankowski A., Møller A. P.1995A resolution of the lek paradox. Proc. R. Soc. Lond. B 260, 21–29 (doi:10.1098/rspb.1995.0054) [Google Scholar]
- Qvarnström A.2001Context-dependent genetic benefits from mate choice. Trends Ecol. Evol. 16, 5–7 (doi:10.1016/S0169-5347(00)02030-9) [DOI] [PubMed] [Google Scholar]
- Wong B. B. M., Candolin U., Lindström K.2007Environmental deterioration compromises socially enforced signals of male quality in three-spined sticklebacks. Am. Nat. 170, 184–189 (doi:10.1086/519398) [DOI] [PubMed] [Google Scholar]