Abstract
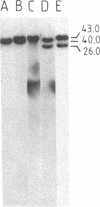
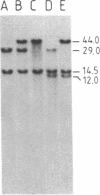
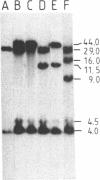
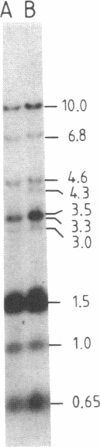
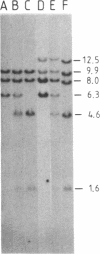
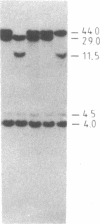
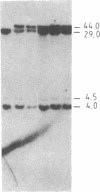
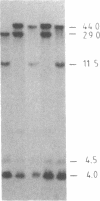
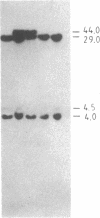
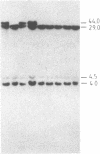
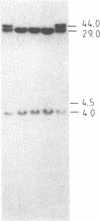
The "debrisoquine polymorphism" is a clinically important genetic defect of drug metabolism affecting 5-10% of individuals in Caucasian populations. It is inherited as an autosomal recessive trait. A full-length cDNA for human cytochrome P-450db1, the deficient enzyme (also designated P450IID1 for P450 family II subfamily D isozyme 1), has recently been cloned. Leukocyte DNA from "extensive metabolizers" (EMs) or "poor metabolizers" (PMs) of debrisoquine was examined by Southern analysis. Two polymorphic restriction fragments were associated with the PM phenotype when DNAs from 24 unrelated PM and 29 unrelated EM individuals were probed with P-450db1 cDNA after digestion with Xba I restriction endonuclease and Southern blotting: a polymorphic 44-kilobase (kb) fragment was found in 58% of PMs but only in 3.4% of EMs, and a polymorphic 11.5-kb fragment was present in 33% of PMs but in none of the EMs. Seventy-five percent of PMs had either the 44-kb or the 11.5-kb fragment or both. Segregation of these restriction fragment length polymorphisms in the families of six PM probands demonstrated that each of the two fragments is allelic with the 29-kb fragment present in all EM individuals and suggests that they identify two independent mutated allels of the P-450db1 gene (designated P450C2D1). At least a third mutated allele not detected by these restriction fragment length polymorphisms must be present in the population. The Xba I 44-kb fragment and 11.5-kb fragment were in linkage disequilibrium with restriction fragment length polymorphisms generated by four and five additional restriction endonucleases, respectively, which can be used to identify the same mutant alleles for the P-450db1 gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayesh R., Idle J. R., Ritchie J. C., Crothers M. J., Hetzel M. R. Metabolic oxidation phenotypes as markers for susceptibility to lung cancer. Nature. 1984 Nov 8;312(5990):169–170. doi: 10.1038/312169a0. [DOI] [PubMed] [Google Scholar]
- Dayer P., Kronbach T., Eichelbaum M., Meyer U. A. Enzymatic basis of the debrisoquine/sparteine-type genetic polymorphism of drug oxidation. Characterization of bufuralol 1'-hydroxylation in liver microsomes of in vivo phenotyped carriers of the genetic deficiency. Biochem Pharmacol. 1987 Dec 1;36(23):4145–4152. doi: 10.1016/0006-2952(87)90573-9. [DOI] [PubMed] [Google Scholar]
- Distlerath L. M., Reilly P. E., Martin M. V., Davis G. G., Wilkinson G. R., Guengerich F. P. Purification and characterization of the human liver cytochromes P-450 involved in debrisoquine 4-hydroxylation and phenacetin O-deethylation, two prototypes for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1985 Jul 25;260(15):9057–9067. [PubMed] [Google Scholar]
- Eichelbaum M., Baur M. P., Dengler H. J., Osikowska-Evers B. O., Tieves G., Zekorn C., Rittner C. Chromosomal assignment of human cytochrome P-450 (debrisoquine/sparteine type) to chromosome 22. Br J Clin Pharmacol. 1987 Apr;23(4):455–458. doi: 10.1111/j.1365-2125.1987.tb03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Spannbrucker N., Steincke B., Dengler H. J. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur J Clin Pharmacol. 1979 Sep;16(3):183–187. doi: 10.1007/BF00562059. [DOI] [PubMed] [Google Scholar]
- Evans D. A. Ethnic differences in reactions to drugs and xenobiotics. Therapy. Prog Clin Biol Res. 1986;214:491–526. [PubMed] [Google Scholar]
- Evans D. A., Harmer D., Downham D. Y., Whibley E. J., Idle J. R., Ritchie J., Smith R. L. The genetic control of sparteine and debrisoquine metabolism in man with new methods of analysing bimodal distributions. J Med Genet. 1983 Oct;20(5):321–329. doi: 10.1136/jmg.20.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A., Mahgoub A., Sloan T. P., Idle J. R., Smith R. L. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet. 1980 Apr;17(2):102–105. doi: 10.1136/jmg.17.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Skoda R. C., Kimura S., Umeno M., Zanger U. M., Nebert D. W., Gelboin H. V., Hardwick J. P., Meyer U. A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature. 1988 Feb 4;331(6155):442–446. doi: 10.1038/331442a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Vilbois F., Hardwick J. P., McBride O. W., Nebert D. W., Gelboin H. V., Meyer U. A. Human debrisoquine 4-hydroxylase (P450IID1): cDNA and deduced amino acid sequence and assignment of the CYP2D locus to chromosome 22. Genomics. 1988 Feb;2(2):174–179. doi: 10.1016/0888-7543(88)90100-0. [DOI] [PubMed] [Google Scholar]
- Gut J., Catin T., Dayer P., Kronbach T., Zanger U., Meyer U. A. Debrisoquine/sparteine-type polymorphism of drug oxidation. Purification and characterization of two functionally different human liver cytochrome P-450 isozymes involved in impaired hydroxylation of the prototype substrate bufuralol. J Biol Chem. 1986 Sep 5;261(25):11734–11743. [PubMed] [Google Scholar]
- Gut J., Gasser R., Dayer P., Kronbach T., Catin T., Meyer U. A. Debrisoquine-type polymorphism of drug oxidation: purification from human liver of a cytochrome P450 isozyme with high activity for bufuralol hydroxylation. FEBS Lett. 1984 Aug 6;173(2):287–290. doi: 10.1016/0014-5793(84)80792-9. [DOI] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Merry D., Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci U S A. 1986 Jan;83(1):130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. A., Gut J., Kronbach T., Skoda C., Meier U. T., Catin T., Dayer P. The molecular mechanisms of two common polymorphisms of drug oxidation--evidence for functional changes in cytochrome P-450 isozymes catalysing bufuralol and mephenytoin oxidation. Xenobiotica. 1986 May;16(5):449–464. doi: 10.3109/00498258609050251. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986 Aug;73(4):320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- Schmid B., Bircher J., Preisig R., Küpfer A. Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther. 1985 Dec;38(6):618–624. doi: 10.1038/clpt.1985.235. [DOI] [PubMed] [Google Scholar]