Abstract
Many animal species employ natural hypothermia in seasonal (hibernation) and daily (torpor) strategies to save energy. Facultative daily torpor is a typical response to fluctuations in food availability, but the relationship between environmental quality, foraging behaviour and torpor responses is poorly understood. We studied body temperature responses of outbred ICR (CD-1) mice exposed to different food reward schedules, simulating variation in habitat quality. Our main comparison was between female mice exposed to low foraging-cost environments and high-cost environments. As controls, we pair-fed a group of inactive animals (no-cost treatment) the same amount of pellets as high-cost animals. Mice faced with high foraging costs were more likely to employ torpor than mice exposed to low foraging costs, or no-cost controls (100% versus 40% and 33% of animals, respectively). While resting-phase temperature showed a non-significant decrease in high-cost animals, torpor was not associated with depressions in active-phase body temperature. These results demonstrate (i) that mice show daily torpor in response to poor foraging conditions; (ii) that torpor incidence is not attributable to food restriction alone; and (iii) that high levels of nocturnal activity do not preclude the use of daily torpor as an energy-saving strategy. The finding that daily torpor is not restricted to conditions of severe starvation puts torpor in mice in a more fundamental ecological context.
Keywords: hypothermia, environmental quality, foraging costs, energy balance
1. Introduction
Endothermic vertebrates have evolved a variety of physiological strategies to cope with energetic challenges (Geiser 2004). One such strategy causes a reduction in metabolic rate and tolerance of sub-euthermic body temperature in the state of total inactivity: torpor. Natural hypothermia occurs in the majority of mammalian families, suggesting that torpor may be possible for many, if not all, mammals (Heldmaier et al. 2004). Torpor physiology has recently attracted increased attention for its potential therapeutic applications (Carey et al. 2003), which include neuroprotection in cardiac-arrest patients, preservation of organs for transplantation, and the treatment of neurodegenerative diseases like Alzheimer's (Malatesta et al. 2007).
Mice are a model for mammalian physiology, but little is known about the biology of natural murine torpor. Although they do not hibernate, house mice (Mus musculus domesticus) exhibit hypothermic responses to overnight fasting (Hudson & Scott 1979), low ambient temperatures (Tomlinson et al. 2007) and pharmacological agents (i.e. adenosine monophosphate (Swoap et al. 2006), H2S (Blackstone et al. 2005) and 2-deoxy-d-glucose (Freinkel et al. 1972)). Whether these responses are functionally similar to natural torpor has remained unclear (Swoap et al. 2006), in part because manipulations such as fasting entail a non-trivial risk of death (Hudson & Scott 1979).
For small animals exhibiting daily torpor, food consumed during foraging is the most important source of energy (Geiser 2004). To understand the natural context of murine torpor, we studied body temperature regulation in mice exposed to poor environments. We elicited torpor in mice by exposing them to increased foraging costs per food reward (hereafter simply ‘foraging costs’) which simulated increased travel distances between food patches. High and low foraging costs were imposed by exercise-driven reward schedules. To control for the effect of food restriction, an additional group of non-foraging mice received the same amount food as high foraging-cost animals.
2. Material and methods
(a). Animals and housing
Virgin female mice (Mus musculus domesticus, Hsd:ICR (CD-1) outbred) were housed at 21 ± 1°C on an L:D cycle of 12:12 (lights on 0400 GMT+1). At four months of age, we implanted temperature transmitters (Series 3000 XM-FH, Mini Mitter, USA) i.p. under 1.5 per cent isoflurane anaesthesia. Animals were post-surgically injected s.c. with 0.5 mg kg−1 Temgesic for pain relief and allowed to recover for one week. Body mass (1–2 h before lights out), food intake and wheel-running activity (where applicable) were measured daily.
(b). Experimental manipulation
Detailed methods of the foraging cost manipulation are described by Schubert et al. (2008). Briefly, mice were trained to run in wheels for 45 mg food pellets (TestDiet 5TUM/PJAI, Sandown Chemicals, Hampton, UK) given by a dispenser (Med Associates ENV-203, Sandown Scientific, Hampton, UK) linked to a steering computer (Series 3 Programmable Controller, General Electric). Foraging costs were calculated individually for each animal after collecting baseline data. Low foraging costs were set to the ratio of spontaneous activity to ad libitum food intake, thus representing a balanced energy budget a priori. High foraging costs started at the baseline level, then increased by 10 per cent every 2 days until 200 per cent of baseline (20 days), and thereafter remained stable. On average, females in the low foraging-cost group needed to run 114 revolutions (50 m) for one 45 mg pellet of food, while mice in the high foraging-cost group needed to run 278 revolutions (122 m) per pellet. As food restriction controls, a no-cost group without running wheels was used. Each of these was pair-fed (once daily, at lights-out) a ration of pellets that matched the previous day's intake of an individual female from the high foraging-cost group.
We trained 24 mice for the experiment but omitted some animals from analysis due to technical problems (e.g. malfunctioning food dispensers or transmitter batteries). Final sample sizes per group were 5 (low cost), 4 (high cost) and 6 (no cost). We obtained temperature recordings during the baseline period and from experimental day 0 through ≥20 (depending on transmitter battery life). In addition, we measured euthermic (non-torpor) resting metabolic rate (RMR, kJ d−1) once during each experimental period at an ambient temperature of 21°C, placing mice in an open-flow respirometry system for 23 h; during measurements, mice were given a food ration matched to their previous day's intake (see Schubert et al. 2008).
(c). Data handling and analysis
Body temperature was recorded with the Dataquest LabPRO system (v. 3.10, receiver model RPC-1; Data Sciences International, Inc., St. Paul, MN, USA) taking a 2 s signal trace every minute. We processed data to remove noise and outliers from the signals, applying cut-off filters separately for the inactive (22.0–39.5°C) and active (34.0–39.5°C) circadian phase. We scored raw data by eye but applied a median smoothing filter over a range of 60 data points for visualization (utility by A.S.B.). We classified torpor as continuously declining temperature depressions ending below 30°C and arousal as re-warming to a stable euthermic temperature. We tested for differences between groups with generalized linear models (GLZ) in JMP 7.0.1 (2007, SAS Institute Inc., USA), performing post hoc tests with planned contrasts. Two-tailed p-values of ≤0.05 were considered statistically significant.
3. Results
(a). General responses
Our experimental manipulation had dramatic effects on both body mass and energy intake (see Schubert et al. 2008). Briefly, while high- and low-cost females ran about the same amount (approx. 9–11 km d−1), high-cost females (and their no-cost controls) consumed approximately 25 per cent less energy per day. This directly affected the body mass of high-cost females, which declined steadily over the course of the study with a slope of approximately 0.12 g d−1. Estimates of net energy balance from food intake and daily energy expenditure (doubly labelled water method) suggested that high-cost females were in a zero or negative balance, while the other groups remained in a positive balance. All females faced with high foraging costs ceased oestrous cyclicity (Schubert et al. 2008), and of the 10 females who eventually showed torpor, eight had ceased oestrous cyclicity before their first torpor day.
(b). Torpor frequency and duration
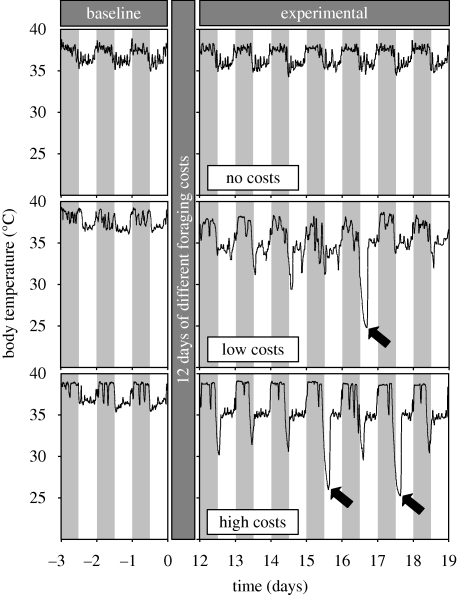
All mice maintained normal circadian body temperature rhythms in the baseline period. In the experimental period, many animals exhibited daily torpor bouts (figure 1). Although torpor was observed in some mice from each experimental group (table 1), treatment significantly predicted whether an animal ever showed torpor (binomial GLZ: χ22,15 = 6.36; p = 0.04; contrasts high–low, high–control, low–control p < 0.05). On average, torpor bouts lasted longer in high and low foraging-cost groups than the no-cost control group (table 1; linear GLZ: F2,5 = 6.23, p = 0.04, contrasts of high–control and low–control p < 0.05). Treatment neither predicted the total number of torpor bouts or minimum torpor temperature, nor did it affect average temperatures in the active or inactive circadian phase (all p-values > 0.1).
Figure 1.
Body temperature responses of female laboratory mice to variation in foraging conditions at an ambient temperature of 21 ± 1°C. Examples of one individual per treatment group are shown: no foraging costs given a food ration matched to the intake of high-cost animals (top panels), low foraging costs (middle panels) and high foraging costs (bottom panels). The figure shows part of the overall temperature record, where day 0 is the start of experimental manipulation. Shading shows the light–dark cycle, and black arrows highlight torpor bouts.
Table 1.
Characteristics of daily torpor in female ICR (CD-1) laboratory mice exposed to varying foraging conditions. Summary data per group are given as means and s.d. Zero or n/a indicates that an animal did not show torpor.
| mouse number | group | days scored (no.) | average active phase temperature (°C) | average resting phase temperature (°C) | torpor incidence (0/1)a | total torpor bouts (no.) | average bout duration (min) | average low torpor temperature (°C)b | record low torpor temperature (°C)c |
|---|---|---|---|---|---|---|---|---|---|
| 3 | L | 28 | 37.0 | 35.9 | 0 | 0 | n/a | n/a | n/a |
| 8 | L | 29 | 37.0 | 33.9 | 1 | 8 | 416.5 | 24.9 | 23.5 |
| 12 | L | 16 | 35.2 | 34.8 | 0 | 0 | n/a | n/a | n/a |
| 18 | L | 29 | 37.3 | 33.0 | 1 | 29 | 228.9 | 27.6 | 21.9 |
| 22 | L | 29 | 36.4 | 35.2 | 0 | 0 | n/a | n/a | n/a |
| 6 | H | 29 | 37.5 | 34.5 | 1 | 13 | 323.7 | 27.7 | 21.9 |
| 7 | H | 29 | 38.2 | 37.3 | 1 | 2 | 282.5 | 28.4 | 28.3 |
| 11 | H | 19 | 35.3 | 33.4 | 1 | 3 | 416.7 | 22.2 | 21.0 |
| 17 | H | 29 | 36.5 | 33.3 | 1 | 10 | 340.5 | 25.7 | 24.7 |
| 2 | N | 21 | 37.4 | 35.8 | 0 | 0 | n/a | n/a | n/a |
| 4 | N | 20 | 36.6 | 35.0 | 1 | 13 | 188.9 | 29.5 | 27.2 |
| 9 | N | 20 | 37.5 | 35.8 | 0 | 0 | n/a | n/a | n/a |
| 10 | N | 24 | 37.4 | 35.8 | 1 | 5 | 191.2 | 27.4 | 23.6 |
| 16 | N | 19 | 37.1 | 35.6 | 0 | 0 | n/a | n/a | n/a |
| 21 | N | 19 | 37.5 | 36.2 | 0 | 0 | n/a | n/a | n/a |
| low-cost group (L) | 25.9 (5.5) | 36.9 (0.9) | 35.0 (1.1) | 0.4 (0.5) | 7.4 (12.6) | 322.7 (132.7) | 26.2 (14.4) | 22.7 (12.4) | |
| high-cost group (H) | 26.1 (4.8) | 36.9 (1.3) | 34.6 (1.8) | 1.0 (0.0) | 7.0 (5.4) | 340.8 (56.1) | 26.0 (2.8) | 24.0 (3.3) | |
| no-cost group (N) | 20.5 (1.9) | 37.2 (0.4) | 35.7 (0.4) | 0.3 (0.5) | 3.0 (5.3) | 190.1 (98.1) | 28.4 (14.7) | 25.4 (13.2) | |
a0, no torpor; 1, torpor.
bIndividual average of the lowest temperature typically reached by the end of torpor bout.
cIndividual record of the single lowest temperature ever reached during a torpor bout.
(c). Correlates of torpor bouts
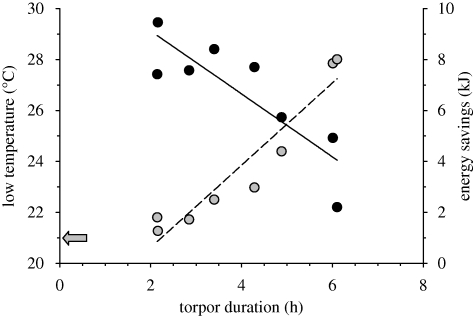
Neither body mass (g; y = −0.54x + 19.97, R2 = 0.29, F1,6 = 2.452, p > 0.10) nor energy intake (kJ; y = −0.01x + 4.57, R2 = 0.003, F1,6 = 0.02, p > 0.10) predicted torpor bout duration (h) when averaging data per individual for the subset of animals showing torpor. Assuming that metabolic depression precedes temperature declines, we estimated metabolic rate in torpor (MR2) from the equation: MR2 = (MR1×(Tb2−Ta))/(Tb1−Ta) (Heldmaier & Ruf 1992); MR1 was euthermic RMR, Tb1 was individual euthermic resting-phase temperature (mean ± s.d. = 35.9 ± 0.91°C), Tb2 was average low torpor temperatures and Ta was ambient temperature (21°C). The change in metabolic rate over time represented energy savings which ranged from 1–8 kJ d−1 (figure 2).
Figure 2.
Body temperature and estimated energy savings in relation to torpor duration. Data points are individual average low temperatures (black symbols and solid line; y = −1.24 × +31.60, R2 = 0.74, F1,6 = 16.67, p = 0.007) and energy savings (grey symbols and dashed line; y = 1.62 × −2.62, R2 = 0.90, F1,6 = 56.34, p = 0.0003). n = 8 because six animals did not show torpor. The grey arrow shows the ambient temperature of 21°C.
4. Discussion
Female mice exposed to high foraging costs, and to some extent any foraging costs, showed body temperature reductions well below the normal temperature of the inactive circadian phase. The depth and duration of these temperature depressions are consistent with the idea that mice show daily torpor as a response to poor environmental conditions. This is new evidence that torpor in mice is not restricted to conditions of starvation and inactivity, but also occurs in response to high foraging costs in poor quality habitat. As an ecologically relevant strategy to maintain energy balance, natural torpor in mice is likely to be accompanied by a suite of physiological adaptations such as those shown by obligate hibernators (Carey et al. 2003). Our results, therefore, provide an important context for comparing torpor physiology between species and for improving our functional understanding of hypometabolic states.
Although others have reported natural torpor in mice (Hudson & Scott 1979; Tomlinson et al. 2007), ours is the first to show that mice use torpor when food becomes more difficult to obtain. In a sense, mice in our experiment were fed ad libitum, since food was available to them on demand. Rather than increasing activity and expenditure, these animals reduced their total energy budget (Schubert et al. 2008). This somewhat counterintuitive response can be explained by reductions in metabolic rate to balance the reduced energy intake. Although the occurrence of torpor to maintain energy balance in poor foraging conditions has been suggested earlier (Perrigo & Bronson 1983; Vaanholt et al. 2007), ours is the first study to document this phenomenon.
Experimental animals did not appear to decrease their active phase temperature. In this respect, body temperature regulation in mice differs drastically from responses of food-deprived rats, which seem incapable of torpor and show gradual declines in both resting and active-phase body temperature when food restricted (Severinsen & Munch 1999). Although we chose a strict criterion (<30°C) to classify torpor, mice are likely to reduce temperature to varying extents on a daily basis. Future studies profiling temperature rhythms in detail will allow us to relate energy balance to torpor behaviour, but our data have already shown that reduced food intake is only one of the several factors contributing to trigger torpor in mice.
These findings cast murine torpor in a new light. We have little understanding of whether pharmacological interventions to lower metabolism also trigger natural physiological adjustments; it is therefore imperative to validate them against natural torpor. Our experimental approach is promising for studies of tissue- and cell-level processes occurring in torpid animals. Work to understand neuroprotection—one of the most exciting translational aspects of torpor physiology (Drew et al. 2001)—has already moved beyond species following a pattern of obligate hibernation to those that hibernate facultatively (Härtig et al. 2007). Studies of the physiology of foraging-induced torpor in mice will be an exciting next step in understanding the biology of hypometabolic states.
Acknowledgements
All procedures concerning animal care and treatment were in accordance with the regulations of the Ethical Committee for Use of Experimental Animals (DEC licence 4321B).
G. H. Visser, F. Stavasius and G. Overkamp helped with study design and data collection. R. A. Hut, E. A. v.d. Zee, S. Verhulst and two reviewers provided useful comments. K.A.S. and L.M.V. were supported by the University of Groningen, and S.D. by EC grant LSHM-CT 2006-018741 (EUCLOCK).
References
- Blackstone E., Morrison M., Roth M. B.2005H2S induces a suspended animation-like state in mice. Science 308, 518 (doi:10.1126/science.1108581) [DOI] [PubMed] [Google Scholar]
- Carey H. V., Andrews M. T., Martin S. L.2003Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153–1181 [DOI] [PubMed] [Google Scholar]
- Drew K. L., Rice M. E., Kuhn T. B., Smith M. A.2001Neuroprotective adaptations in hibernation: therapeutic implications for ischemia–reperfusion, traumatic brain injury and neurodegenerative diseases. Free Rad. Biol. Med. 31, 563–573 (doi:10.1016/S0891-5849(01)00628-1) [DOI] [PubMed] [Google Scholar]
- Freinkel N., Metzger B. E., Harris E., Robinson S., Mager M.1972The hypothermia of hypoglycemia. Studies with 2-deoxy-d-glucose in normal human subjects and mice. N. Engl. J. Med. 287, 841–845 [DOI] [PubMed] [Google Scholar]
- Geiser F.2004Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274 (doi:10.1146/annurev.physiol.66.032102.115105) [DOI] [PubMed] [Google Scholar]
- Härtig W., Stieler J., Boerema A. S., Wolf J., Schmidt U., Weißfuß J., Bullmann T., Strijkstra A. M., Arendt T.2007Hibernation model of tau phosphorylation in hamsters: selective vulnerability of cholinergic basal forebrain neurons—implications for Alzheimer's disease. Eur. J. Neurosci. 25, 69–80 [DOI] [PubMed] [Google Scholar]
- Heldmaier G., Ruf T.1992Body temperature and metabolic rate during natural hypothermia in endotherms. J. Comp. Physiol. B 162, 696–706 (doi:10.1007/BF00301619) [DOI] [PubMed] [Google Scholar]
- Heldmaier G., Ortmann S., Elvert R.2004Natural hypometabolism during hibernation and daily torpor in mammals. Resp. Physiol. Neurobiol. 141, 317–329 (doi:10.1016/j.resp.2004.03.014) [DOI] [PubMed] [Google Scholar]
- Hudson J. W., Scott I. M.1979Daily torpor in the laboratory mouse, Mus musculus var. albino. Physiol. Zool. 52, 205–218 [Google Scholar]
- Malatesta M., Biggiogera M., Zancanaro C.2007Hypometabolic induced state: a potential tool in biomedicine and space exploration. Rev. Environ. Sci. Biotechnol. 6, 47–60 (doi:10.1007/s11157-006-9101-4) [Google Scholar]
- Perrigo G., Bronson F. H.1983Foraging effort, food intake, fat deposition and puberty in female mice. Biol. Reprod. 29, 455–463 (doi:10.1095/biolreprod29.2.455) [DOI] [PubMed] [Google Scholar]
- Schubert K. A., Vaanholt L. M., Stavasius F., Demas G. E., Daan S., Visser G. H.2008Female mice respond differently to costly foraging versus food restriction. J. Exp. Biol. 211, 2214–2223 (doi:10.1242/jeb.017525) [DOI] [PubMed] [Google Scholar]
- Severinsen T., Munch I. C.1999Body core temperature during food restriction in rats. Acta Physiol. Scand. 165, 299–305 (doi:10.1046/j.1365-201x.1999.00488.x) [DOI] [PubMed] [Google Scholar]
- Swoap S. J., Rathvon M., Gutilla M.2006AMP does not induce torpor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R476–R473 [DOI] [PubMed] [Google Scholar]
- Tomlinson S., Withers P. C., Cooper C.2007Hypothermia versus torpor in response to cold stress in the native Australian mouse Pseudomys hermannsburgensis and the introduced house mouse Mus musculus. Comp. Biochem. Physiol. A 148, 645–650 [DOI] [PubMed] [Google Scholar]
- Vaanholt L. M., De Jong B., Garland T., Jr, Daan S., Visser G. H.2007Behavioural and physiological responses to increased foraging effort in male mice. J. Exp. Biol. 210, 2013–2024 (doi:10.1242/jeb.001974) [DOI] [PubMed] [Google Scholar]