Abstract
Theories of music evolution agree that human music has an affective influence on listeners. Tests of non-humans provided little evidence of preferences for human music. However, prosodic features of speech (‘motherese’) influence affective behaviour of non-verbal infants as well as domestic animals, suggesting that features of music can influence the behaviour of non-human species. We incorporated acoustical characteristics of tamarin affiliation vocalizations and tamarin threat vocalizations into corresponding pieces of music. We compared music composed for tamarins with that composed for humans. Tamarins were generally indifferent to playbacks of human music, but responded with increased arousal to tamarin threat vocalization based music, and with decreased activity and increased calm behaviour to tamarin affective vocalization based music. Affective components in human music may have evolutionary origins in the structure of calls of non-human animals. In addition, animal signals may have evolved to manage the behaviour of listeners by influencing their affective state.
Keywords: music evolution, vocal communication, affective responses, tamarins, species-specific music
1. Introduction
Has human music evolved from similar traits to other species (Brown 2000; McDermott & Hauser 2005; Fitch 2006)? ‘Song’ is described in birds, whales and the duets of gibbons, but the possible musicality of other species has rarely been studied. Non-human species generally rely solely on absolute pitch, with little or no ability to transpose to another key or octave (Fitch 2006). Studies of cotton-top tamarins and common marmosets found that both species preferred slow tempos. However, when any type of human music was tested against silence, monkeys preferred silence (McDermott & Hauser 2007).
Consistent structures are seen in acoustic signals that communicate affective state, with high-pitched, tonal sounds common to expressions of submission and fear, and low, loud, broadband sounds common to expressions of threats and aggression (Owings & Morton 1998). Prosodic features in speech of parents (‘motherese’) influence the affective state and behaviour of infants, and similar processes occur between owners and working animals to influence behaviour (McConnell 1991; Fernald 1992). Abrupt increases in amplitude for infants and short, upwardly rising staccato calls for animals lead to increased arousal. Long descending intonation contours produce calming. Convergence of signal structures used to communicate with both infants and non-human animals suggests these signals can induce behavioural change in others. Little is known about whether animal signals induce affective response in other animals.
Musical structure affects the behaviour and physiology of humans. Infants look longer at a speaker providing consonant compared with dissonant music (Trainor et al. 2002). Mothers asked to sing a non-lullaby in the presence or absence of an infant sang in a higher key and with slower notes to infants than when singing without infants (Trehub et al. 1993). In adults, upbeat classical music led to increased activity, reduced depression and increased norepinephrine levels, whereas softer, calmer music led to an increased level of well-being (Hirokawa & Ohira 2003). These results suggest that combined musical components of pitch, timbre and tempo can specifically alter affective, behavioural and physiological states in infant and adult humans as well as companion animals.
Why then are monkeys responsive to tempo but indifferent to human music (McDermott & Hauser 2007)? The tempos and pitch ranges of human music may not be relevant for another species. In the current study, we used a musical analysis of the tamarin vocal repertoire to identify common prosodic/melodic structures and tempos in tamarin calls that were related to specific behavioural contexts. We used these commonalities to compose music within the frequency range and tempos of tamarins with specific motivic features incorporating features of affiliation or of fear/threat-based vocalizations and played this music to tamarins. We predicted that music composed for tamarins would have greater behavioural effects than music composed for humans. Furthermore, we hypothesized that contrasting forms of music would have appropriately contrasting behavioural effects on tamarins. That is, music with long, tonal, pure-tone notes would be calming, whereas music that had broad frequency sweeps or noise, and rapid, staccato notes and abrupt amplitude changes would lead to increased activity and agitation.
2. Material and methods
(a). Subjects
We tested seven heterosexual pairs of adult cotton-top tamarins housed in the Psychology Department, University of Wisconsin, Madison, USA. One animal in each pair had been sterilized for colony management purposes and all pairs had lived together for at least a year. Pairs were housed in identical cages (160 × 236 × 93 cm, length × height × width) fitted with branches and ropes to simulate an arboreal environment. Food and water were available ad libitum.
(b). Music selection and composition
We prepared two sets of stimuli representing human and tamarin affiliation-based music and human and tamarin fear/threat-based music (totalling eight different stimuli) for playback to tamarins (see the electronic supplementary material).
Tamarin music was produced by voice or on an Andre Castagneri (1738) cello and recorded on a Sony ECM-M907 one point stereo electret condenser microphone with a frequency response of 100–15 000 Hz with Adobe Audition recording software. Vocal sounds were recorded and played back in real time, artificial harmonics on the cello were transposed up one octave in the playback (twice as fast as the original recording), and normal cello playing was transposed up three octaves in the playback (eight times faster than the original recording). (See the electronic supplementary material for each of the stimuli used.)
(c). Testing
We tested tamarins in two phases, three months apart, with each of the four stimulus types presented in each phase. All pieces were edited to approximately 30 s with variation allowing for resolution of chords. The amplitude of all pieces was equalized. We presented stimuli in counter-balanced order across the seven pairs so that one to two pairs were presented with each piece in each position. Each pair was tested with one stimulus, once a week.
Musical excerpts were recorded to the hard drive of a laptop computer and played through a speaker hidden from the pair being tested. An observer recorded behaviour for a 5 min baseline. Then the music stimulus was played and behavioural data were gathered for 5 min after termination of the music. The observer was naive to the hypotheses of the study and had previously been trained to a more than 85 per cent agreement on behavioural measures. Data were recorded using Noldus Observer 5.0 Software.
(d). Data analyses
We clustered data into five main categories for analysis. Head and body orientation to the speaker served as a measure of interest in the stimulus. Foraging (eating or drinking) and social behaviour (grooming, huddling, sex) served as measures of calm behaviour. Rate of movement from one perch to another was a measure of arousal. We combined several behaviours indicative of anxiety or arousal (piloerection, urination, scent marking, head shaking and stretching) into a single measure. Data from both phases for each stimulus type were averaged before analysis.
First, we examined responses in the baseline condition to determine if behavioural categories differed prior to stimulus presentation. Second, we compared responses to tamarin stimuli versus human stimuli, and tamarin fear/threat-based music to tamarin affiliation-based music, for both the playback and the post-playback periods. Third, we compared behavioural responses between baseline and post-stimulus conditions for each stimulus type. Data distributions did not differ from normality. We used paired sample two-tailed t-tests with a critical value of p < 0.05 and degrees of freedom based on the number of pairs. We report effect sizes (d) to indicate strength of results despite multiple comparisons of data and with small d.f. Effect sizes >0.80 are generally considered to be ‘strong’.
3. Results
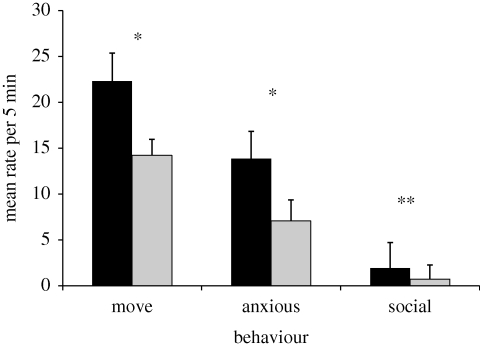
There were no differences in baseline behaviour owing to stimulus condition. During the 30 s playbacks there were no significant responses to tamarin music. In the post-stimulus condition there were no effects of human-based music. However, there were several differences between the tamarin fear/threat-based music and tamarin affiliation-based music. Monkeys moved more (fear/threat-based 22.3 ± 3.1, affiliation-based 14.2 ± 1.75, t6 = 2.70, p = 0.036, d = 1.02), showed more anxious behaviour (fear/threat-based 13.86 ± 2.78, affiliation-based 7.07 ± 1.56, t6 = 3.09, p = 0.021, d = 1.17) and more social behaviour following fear/threat-based music (fear/threat-based 1.923 ± 0.45, affiliation-based 0.71 ± 0.31, t6 = 6.58, p = 0.0006, d = 2.49) (figure 1).
Figure 1.
Responses to tamarin fear/threat-based music versus tamarin affiliation-based music in the 5 min following playback. Error bars show s.e.m. *p < 0.05, **p < 0.01. Black bars, tamarin fear/threat music; grey bars, tamarin affiliation music.
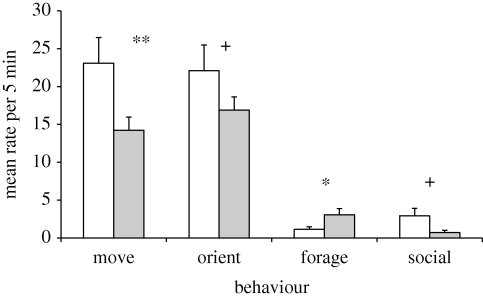
Compared with baseline, tamarins decreased movement following playback of the tamarin affiliation-based music (baseline 23.07 ± 3.4, post-stimulus 14.21 ± 1.75, t6 = 3.77, p = 0.009, d = 1.40) and showed trends toward decreased orientation (baseline 22.07 ± 1.93, post-stimulus 16.93 ± 2.3, t6 = 2.37, p = 0.056, d = 0.90) and decreased social behaviour (baseline 2.93 ± 0.97, post-stimulus 0.79 ± 0.31, t6 = 2.35, p = 0.057, d = 0.89). By contrast, foraging behaviour increased significantly (baseline 1.14 ± 0.33, post-stimulus 3.07 ± 0.80, t6 = 2.68, p = 0.036, d = 1.01) (figure 2). Following playback of tamarin fear/threat-based music, orientation increased (baseline 16.57 ± 2.91, post-stimulus 21.14 ± 2.98 t6 = −4.53, p = 0.004, d = 1.69).
Figure 2.
Responses to tamarin affiliation-based music after playback compared with baseline behaviour. Error bars show s.e.m. + 0.10 > p > 0.05, *p < 0.05, **p < 0.01. White bars, baseline; grey bars, after music.
Two significant baseline to post-stimulus comparisons followed playback of human-based music. Movement following playback of the human fear/threat-based music was significantly reduced (baseline 24.43 ± 1.78, post-stimulus 3.0 ± 0.54, t6 = 11.77, p = 0.00002, d = 4.45), which contrasts sharply with the increased movement following tamarin fear/threat-based music, and anxious behaviour decreased following playback of the human affiliation-based music (baseline 11.36 ± 1.26, post-stimulus 7.93 ± 1.11, t6 = 2.99, p = 0.024, d = 1.13).
4. Discussion
Tamarin calls in fear situations were short, frequently repeated and contained elements of dissonance compared with both confident threat and affiliative vocalizations. In contrast to human signals where decreasing frequencies have a calming effect on infants and working animals (McConnell 1991; Fernald 1992), the affiliation vocalizations of tamarins contained increasing frequencies throughout the call. Ascending two-note motives of affiliation calls had diminishing amplitude, whereas fear and threat calls had increasing frequencies with increasing amplitude. Tamarins have no vocalizations with slowly descending slides, whereas humans have few emotional vocalizations with slowly ascending slides. This marked species difference demonstrates that music intended for a given species may be more effective if it reflects the melodic contours of the vocalizations of that species.
Music composed for tamarins had a much greater effect on tamarin behaviour than music composed for humans. Although monkeys did not respond significantly during the actual playback, they responded primarily to tamarin music during the 5 min after stimulus presentations ended. Tamarin fear/threat-based music produced increased movement, anxious and social behaviour relative to tamarin affiliation-based music. Increased social behaviour following fear/threat-based music was not predicted, but huddling and grooming behaviour may provide security or contact comfort in the face of a threatening stimulus. In comparison with baseline behaviour, tamarin affiliation-based music led to behavioural calming with decreased movement, orientation and social behaviour, and increased foraging behaviour. Tamarin threat-based music showed an increase in orientation compared with baseline. The only exceptions to our predictions, that tamarins would respond only to tamarin-based music, were that human fear/threat-based music decreased movement and human affiliation-based music decreased anxious behaviour compared with the baseline. In all other measures, tamarins displayed significant responses only to music specifically composed for tamarins. We used two different versions of each type of music and presented each piece just once to each pair using conservative statistical measures. The effects cannot be explained simply by one possibly idiosyncratic composition. The robust responses, found in the 5 min after music playback ended, suggest lasting effects beyond the playback.
We did not test preferences, but the effect of tamarin-specific music may account for failures of monkeys to show preference for human music (McDermott & Hauser 2007). Those who have listened to the tamarin stimuli find both types to be unpleasant, further supporting species specificity of response to music. These results, together with those of McDermott & Hauser (2007), have important implications for husbandry of captive primates where broadcast music is often used for enrichment. Playback of human music to other species may have unintended consequences.
A simple playback of spontaneous vocalizations from tamarins may have produced similar behavioural effects, but responses to spontaneous call playbacks may result from affective conditioning (Owren & Rendall 1997). By composing music containing some structural features of tamarin calls but not directly imitating the calls, the structural principles (rather than conditioned responses) are likely to be the bases of behavioural responses. The results suggest that animal signals may have direct effects on listeners by inducing the same affective state as the caller. Calls may not simply provide information about the caller, but may effectively manage or manipulate the behaviour of listeners (Owings & Morton 1998).
Acknowledgements
The research protocol was approved by the University of Wisconsin College of Letters and Science Animal Care and Use Committee.
This work was supported by USPSH grant no. MH029775 and a Hilldale Professorship. E. Abbs tested the animals. We thank two anonymous reviewers for valuable critiques.
References
- Brown S.2000The ‘musiclanguage’ model of music evolution. In The origins of music (eds Wallin N. L., Merker B., Brown S.), pp. 271–300 Cambridge, MA: MIT Press [Google Scholar]
- Fernald A.1992Human maternal vocalizations to infants as biologically relevant signals: an evolutionary perspective. In The adapted mind (eds Barkow J., Cosmides L., Tooby J.), pp. 391–428 New York, NY: Oxford University Press [Google Scholar]
- Fitch W. T.2006The biology and evolution of music: a comparative perspective. Cognition 100, 173–215 (doi:10.1016/j.cognition.2005.11.009) [DOI] [PubMed] [Google Scholar]
- Hirokawa E., Ohira H.2003The effects of music listening after a stressful task on immune functions, neuroendocrine responses and emotional states of college students. J. Mus. Ther. 60, 189–211 [DOI] [PubMed] [Google Scholar]
- McConnell P. B.1991Lessons from animal trainers: the effects of acoustic structure on an animal's response. In Perspectives in ethology (eds Bateson P., Klopfer P.), pp. 165–187 New York, NY: Plenum Press [Google Scholar]
- McDermott J., Hauser M.2005The origins of music: innateness, uniqueness and evolution. Music Percept. 23, 29–59 (doi:10.1525/mp.2005.23.1.29) [Google Scholar]
- McDermott J., Hauser M. D.2007Nonhuman primates prefer slow tempos but dislike music overall. Cognition 104, 654–668 (doi:10.1016/j.cognition.2006.07.011) [DOI] [PubMed] [Google Scholar]
- Owings D. H., Morton E. S.1998Animal vocal communication: a new approach New York, NY: Cambridge University Press [Google Scholar]
- Owren M. J., Rendall D.1997An affect-conditioning model of nonhuman primate vocal signaling. In Perspectives in ethology, vol. 12 (eds Beecher M. D., Owings D. H., Thompson N. S.), pp. 329–346 New York, NY: Plenum Press [Google Scholar]
- Trainor L. J., Chang C. D., Cheung V. H. W.2002Preference for sensory consonance in 2- and 4-month old infants. Mus. Percept 20, 187–194 (doi:10.1525/mp.2002.20.2.187) [Google Scholar]
- Trehub S. E., Unyk A. M., Trainor L. J.1993Maternal singing in cross-cultural perspective. Inf. Behav. Develop. 16, 285–295 (doi:10.1016/0163-6383(93)80036-8) [Google Scholar]