Abstract
Sexual selection often promotes the evolution of elaborate colour signals in males, but the importance of sexually selected colour signals remains poorly studied in amphibians. We used reflectance spectrometry to document pronounced sexual dichromatism and dramatic colour change in Bufo luetkenii, a toad that breeds in large aggregations at the onset of the rainy season in Costa Rica. Our observations suggest that males fade rapidly from a vibrant lemon yellow to a dull brown once they have paired with a female. We demonstrate this by showing that males are much brighter than females and that unpaired males are more colourful than males in amplexus. We also show that coloration fades rapidly when males are briefly held captive. This is, to our knowledge, the first study to document such dynamic change in male coloration and sexual dichromatism in anurans.
Keywords: Bufo luetkenii, colour change, dichromatism, sexual selection, toad
1. Introduction
The evolution of sexual dimorphism is a hallmark of sexual selection. In some species, males compete for females through physical contests, leading to sexual dimorphism in body size. In other species, however, males compete through ritualized interactions often involving complex songs, elaborate behaviours or vibrant colours. Sexual dichromatism, or differences in coloration between males and females, usually results from sexual selection for conspicuous colours in males and opposing natural selection for cryptic colours in females (e.g. Darwin 1871; Wallace 1889; Doucet et al. 2007). Across species, the magnitude of sexual dichromatism has been linked to many traits associated with sexual selection, including mating system and sperm competition (e.g. Dunn et al. 2001; Gonzalez-Voyer et al. 2008).
In frogs and toads, male song is the most widely studied target of sexual selection (Gerhardt 1994). Indeed, bright coloration is typically associated with aposematism rather than sexual selection in this group (e.g. Summers & Clough 2000). A recent review revealed that only 32 of approximately 4400 species of anuran are sexually dichromatic (Hoffman & Blouin 2000), although this number is probably an underestimate owing to lack of research in this area. Despite this, recent research suggests that coloration may play a more important role in sexual selection among anurans than previously thought (e.g. Summers et al. 1999; Gomez et al. 2009).
Although toads are reputedly dull in coloration, there are some striking exceptions. In his original description of Bufo periglenes, the golden toad of Monteverde, Savage (1966) states:
In striking contrast to the usual Bufo situation, Bufo periglenes exhibits the most startling coloration and development of sexual dichromism recorded in the genus … I must confess that my initial response when I saw them was one of disbelief and suspicion that someone had dipped the examples in enamel paint … The new form is assuredly the most spectacularly colored Bufo known and is among the gaudiest of anurans.
Although this species is now believed to be extinct (Savage 2002), at least one of its congeners may exhibit similarly striking coloration and provide an opportunity for understanding the evolution of conspicuous colour in anurans.
Here we investigate dynamic colour change and sexual dichromatism in the Neotropical toad Bufo luetkenii. This common yet poorly studied species is distributed in Pacific lowlands and dry interior valleys from Chiapas, Mexico to central Costa Rica (Savage 2002). In northwestern Costa Rica, B. luetkenii breeds explosively at the onset of the rainy season in early May. Males congregate at temporary ponds and call from the shore to attract females (Savage 2002). Amplexus is axillary and can last more than an hour (S. M. Doucet & D. J. Mennill 2009, personal observation), with each female laying hundreds to thousands of eggs in two strings (Savage 2002). Males are described as having a yellow brown to yellow green dorsum with a discoloured yellowish green throat, whereas females and juveniles are variably patterned in dark and light browns (Savage 2002). In May 2007, we conducted opportunistic observations at a breeding aggregation that formed in the early morning following the first substantial rainfall of the year. Surprisingly, males exhibited a bright lemon-yellow dorsum and white underside at dawn, and their colour appeared to fade progressively once they began amplexing. By sunset, only the few non-amplexing males remained bright yellow, whereas amplexing males had faded to such an extent that they could have been mistaken for another species. To our knowledge, such rapid and dramatic changes in mating coloration have never been documented in anurans. Our objective in this study was to quantify this unique temporal change in male coloration and sexual dichromatism by comparing the coloration of males and females, by comparing the coloration of males before and after temporary captivity, and by comparing the coloration of unpaired males versus males in amplexus.
2. Material and methods
We studied B. luetkenii in Sector Santa Rosa, Area de Conservación Guanacaste, Costa Rica (10°40′ N, 85°30′ W). This Neotropical dry forest exhibits pronounced seasonality, with a dry season extending from December to May and a rainy season from May to November. On 6 May 2009, the morning following the first rain of the rainy season, we located a breeding aggregation of B. luetkenii in a temporary pond along the seasonally dry streambed of the Cuajiniquil river. We captured toads by hand and quantified coloration using a reflectance spectrometer.
We quantified coloration in three groups of males and one group of females. First, we captured and measured the reflectance of 10 males between 06.00 and 06.39 central standard time, placed them in small individual terrariums containing soil and water from the breeding pond, and measured them approximately 4 h later between 10.15 and 10.34. Second, we captured 10 amplexing pairs between 10.35 and 13.00 and measured the coloration of the male and female in each pair. We also captured and measured an additional 10 unpaired males during this time period. To ensure that we did not measure the same individual twice, we retained measured males in a large terrarium until 13.00, before releasing them at the pond's edge. We allowed mated pairs to re-initiate amplexus by keeping individual pairs in a large bucket for a few minutes before releasing them in a partly isolated section of the pond. We ensured that all toads were kept in the shade during their brief captivity.
We used a USB4000 spectrometer and PX-2 light source to objectively quantify coloration in B. luetkenii. We measured the centre of the dorsum, flank and throat, and collected three measurements per region, each of which comprised 15 readings taken in rapid succession and averaged by the spectrometer software. We used means per region in our analyses. We summarized colour variation using principal components analysis of hue, chroma and brightness to create a single colour score; for the dorsum and flank, low principal component scores corresponded to duller brown colours and high scores corresponded to brighter yellow colours (details in the electronic supplementary material). We used non-parametric statistics when data were not normally distributed.
3. Results
(a). Sexual dichromatism
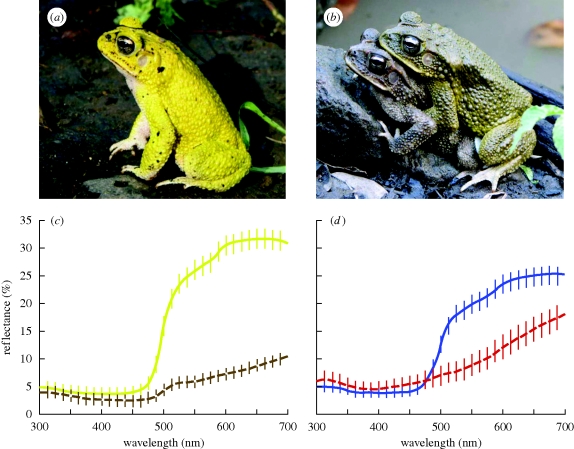
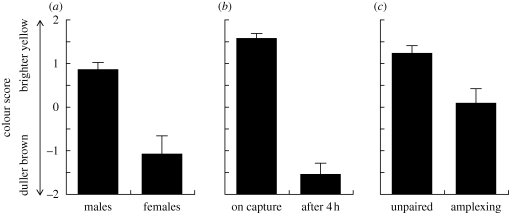
Male B. luetkenii exhibited more colourful skin than females (figure 1). On average, male toads had higher colour scores than females for the dorsum (figure 2; Mann–Whitney test: Z = 3.4, p = 0.0007, n = 30 males, 10 females) and flank (unpaired t-test: t38 = 3.2, p = 0.003), but not the throat (t38 = 0.9, p = 0.36).
Figure 1.
Bufo luetkenii exhibit dramatic sexual dimorphism and dynamic colour change. (a) A typical male B. luetkenii photographed at the edge of a mating aggregation in the early morning. (b) A typical male in amplexus with a female, photographed later on the same day. (c) Average reflectance spectra for the dorsal surface of 10 males measured early in the morning (yellow solid line) and the same 10 males measured 4 h later (dashed brown line). (d) Average reflectance spectra for the dorsal surface of 30 males (solid blue line) and 10 females (dashed red line). Vertical bars show s.e.
Figure 2.
Bufo luetkenii demonstrate dynamic colour variation. (a) Male toads were significantly more colourful than females. (b) Males were significantly less colourful after being detained in terraria for 4 h. (c) Unpaired males were significantly more colourful than males in amplexus. Vertical bars show s.e.
(b). Male colour change
Males changed dramatically from a bright lemon yellow to a dull brown when held in isolation for 4 h (figure 1). Compared with their initial colour, males held captive exhibited significantly lower colour scores on the dorsum (figure 2; Wilcoxon test: Z = 27.5, p = 0.002, n = 10 males) and flank (paired t-test: t9 = 6.2, p < 0.0001), but not the throat (t9 = 6.2, p = 0.08). This colour change occurred rapidly and may have arisen in response to stress since males appeared to revert to brighter coloration upon being released. Nevertheless, this change in colour was similar to that observed among amplexing males.
Unpaired males had higher colour scores than amplexing males for the dorsum (figure 2; Mann–Whitney test: Z = 3.2, p = 0.001, n = 20 unpaired males, 10 paired males) and flank (t-test: t28 = 3.5, p = 0.002), but not the throat (t28 = 0.2, p = 0.83).
4. Discussion
Bufo luetkenii demonstrate dramatic sexual dichromatism at the onset of breeding, and bright lemon-yellow coloration in males fades to a cryptic brown once they have secured a breeding partner. These findings suggest that sexual selection plays a role in regulating coloration in this species, although further research is needed to determine whether coloration does indeed influence mate choice by females or competition among males. Sexual dichromatism in B. luetkenii may match or exceed that described in the extinct golden toad (Savage 2002), a species believed to be the most spectacularly coloured Bufo in the world (Savage 1966).
Although sexual dichromatism in B. luetkenii is intriguing in its own right, the most surprising aspect of our study is the rapid colour change undergone by males. Aside from subtle lightening or darkening, colour change is rare in anurans, and the two species known to exhibit substantial colour change do so over a period of weeks to months (Hoffman & Blouin 2000). Such rapid colour change is reminiscent of that documented in chameleons (Cooper & Greenberg 1992), cephalopods (e.g. Hanlon 2007) and some species of fishes (e.g. Mäthger et al. 2003). Although colour change in these groups is traditionally associated with background matching for camouflage, intrasexual competition and mate choice may also play a role. Indeed, a recent comparative study suggests a hitherto unrecognized influence of sexual selection on colour changeability in chameleons (Stuart-Fox & Moussalli 2008). Bufo luetkenii may provide another system for investigating both the adaptive significance and the proximate basis of the intriguing but taxonomically restricted phenomenon of rapid colour change. In B. luetkenii, bright male coloration may function as part of a multiple ornament strategy that also includes a male song. Such multimodal displays may facilitate female evaluation of potential mates within the noise and confusion of a large breeding aggregation. That males quickly become duller in colour after successful pairing suggests that this conspicuous coloration is costly, perhaps owing to increased predation risk (e.g. Endler 1978). Further research is needed to indentify the function of bright yellow male coloration and the potential costs associated with this display.
Although the mechanism responsible for producing colour change in B. luetkenii remains to be identified, it probably involves at least two types of chromatophores (pigment cells): xanthophores that contain yellow pigments and melanophores that contain black melanin pigments. In other poikilothermic vertebrates, the dispersion of melanosomes in the dentritic cell processes of the melanophores results in a darkening of coloration (e.g. Camargo et al. 1999), and such a process may suffice to obscure and dampen the bright yellow coloration. It would be interesting to determine whether longer term changes in the yellow xanthophores also play a role and to investigate the proximate mechanism responsible for activating this dramatic change in colour.
Bufo luetkenii display a vivid colourful ornament in a group of animals typically recognized for vocal rather than visual displays, suggesting that visual communication may be more important than previously recognized in anurans. The dynamic colour change in males may reflect a unique adaptation for producing a bright, and presumably costly, sexual signal to coincide with an explosive annual mating event.
Acknowledgements
The experiment was approved by the University of Windsor's Animal Care Committee.
We thank the staff of the Guanacaste Conservation Area for logistical support; S. Douglas, J. Koloff, A. Osmun and K. Rieveley for field assistance and NSERC of Canada for financial support.
References
- Camargo C. R., Visconti M. A., Castrucci A. M. L.1999Physiological color change in the bullfrog Rana catesbeiana. J. Exp. Zool. 283, 160–169 (doi:10.1002/(SICI)1097-010X(19990201)283:2<160::AID-JEZ6>3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- Cooper W. E., Greenberg N.1992Reptilian coloration and behavior. In Biology of the Reptilia (eds Gans C., Crews D.), pp. 299–400 Chicago, IL: University of Chicago Press [Google Scholar]
- Darwin C. C.1871The descent of man, and selection in relation to sex. London, UK: Murray [Google Scholar]
- Doucet S. M., Mennill D. J., Hill G. E.2007The evolution of signal design in manakin plumage ornaments. Am. Nat. 169, S63–S80 [DOI] [PubMed] [Google Scholar]
- Dunn P. O., Whittingham L. A., Pitcher T. E.2001Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution 55, 161–175 [DOI] [PubMed] [Google Scholar]
- Endler J. A.1978A predator's view of animal color patterns. Evol. Biol. 11, 319–364 [Google Scholar]
- Gerhardt H. C.1994The evolution of vocalization in frogs and toads. Annu. Rev. Ecol. Syst. 25, 293–324 (doi:10.1146/annurev.es.25.110194.001453) [Google Scholar]
- Gomez D., Richardson C., Lengagne T., Plenet S., Joly P., Lena J. P., Théry M.2009The role of nocturnal vision in mate choice: females prefer conspicuous males in the European tree frog (Hyla arborea). Proc. R. Soc. B 276, 2351–2358 (doi:10.1098/rspb.2009.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Voyer A., Fitzpatrick J. L., Kolm N.2008Sexual selection determines parental care patterns in cichlid fishes. Evolution 62, 2015–2026 (doi:10.1111/j.1558-5646.2008.00426.x) [DOI] [PubMed] [Google Scholar]
- Hanlon R.2007Cephalopod dynamic camouflage. Curr. Biol. 17, R400–R404 (doi:10.1016/j.cub.2007.03.034) [DOI] [PubMed] [Google Scholar]
- Hoffman E. A., Blouin M. S.2000A review of colour and pattern polymorphisms in anurans. Biol. J. Linn. Soc. 70, 633–665 (doi:10.1111/j.1095-8312.2000.tb00221.x) [Google Scholar]
- Mäthger L. M., Land M. F., Siebeck U. E., Marshall N. J.2003Rapid colour changes in multilayer reflecting stripes in the paradise whiptail, Pentapodus paradiseus. J. Exp. Biol. 206, 3607–3613 (doi:10.1242/jeb.00599) [DOI] [PubMed] [Google Scholar]
- Savage J. M.1966An extraordinary new toad (Bufo) from Costa Rica. Rev. Biol. Trop. 14, 153–167 [PubMed] [Google Scholar]
- Savage J. M.2002The amphibians and reptiles of Costa Rica. Chicago, IL: University of Chicago Press [Google Scholar]
- Stuart-Fox D., Moussalli A.2008Selection for social signalling drives the evolution of chameleon colour change. PLoS Biol. 6, 22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K., Clough M. E.2000The evolution of coloration and toxicity in the poison frog family (Dendrobatidae). Proc. Natl Acad. Sci. USA 98, 6227–6232 (doi:10.1073/pnas.101134898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K., Symula R., Clough M., Bronin T.1999Visual mate choice in poison frogs. Proc. R. Soc. Lond. B 266, 2141–2145 (doi:10.1098/rspb.1999.0900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A. R.1889Darwinism: an exposition of the theory of natural selection with some of its applications. London, UK: Macmillan [Google Scholar]