Abstract
Ecological pressure paired with opportunism can lead to surprising innovations in animal behaviour. Here, we report predation of great tits (Parus major) on hibernating pipistrelle bats (Pipistrellus pipistrellus) at a Hungarian cave. Over two winters, we directly observed 18 predation events. The tits specifically and systematically searched for and killed bats for food. A substantial decrease in predation on bats after experimental provisioning of food to the tits further supports the hypothesis that bat-killing serves a foraging purpose in times of food scarcity. We finally conducted a playback experiment to test whether tits would eavesdrop on calls of awakening bats to find them in rock crevices. The tits could clearly hear the calls and were attracted to the loudspeaker. Records for tit predation on bats at this cave now span more than ten years and thus raise the question of whether cultural transmission plays a role for the spread of this foraging innovation.
Keywords: foraging innovation, novel food source, hibernation, torpor, prey detection, sensory ecology
1. Introduction
Ecological pressure paired with opportunism can lead to surprising innovations in animal behaviour, such as chimpanzee tool use (Goodall 1964), raiding of fox food caches by ravens (Careau et al. 2007), capture of emerging bats by raptors (Fenton et al. 1994) and problem solving in foraging guppies (Laland & Reader 1999). A recently discovered case is the predation of a large aerial hawking bat on night migrating songbirds (Popa-Lisseanu et al. 2007). Conversely, here we present evidence for opportunistic predation of a songbird, the great tit (Parus major), on bats. Four anecdotal reports have suggested that tits might prey on hibernating bats as an additional food source in winter. Ryberg (1947) described dead bats in front of hibernacula in Sweden with ‘injury, caused e.g. by titmice (possibly also bigger birds)’ (p. 31). Sachanowicz & Krasnodębski (1996) saw a great tit feeding on a dead bat at a cave in Poland. At the same place, Radzicki et al. (1999) found one dead and two live bats with injuries presumably from tit beaks. It was unclear in all cases whether the tits had captured and killed the bats or rather found them dead or dying on the ground. Estók (1996) reported a single event in which a great tit captured a live hibernating pipistrelle bat (Pipistrellus pipistrellus) in a cave in Hungary.
We returned to the same Hungarian cave and conducted observations over three winters. We investigated whether feeding on bats and bat carcasses is a chance event or whether foraging tits specifically search for and kill bats. We then conducted a preliminary provisioning experiment to test whether feeding on bats is a consequence of food scarcity. Upon disturbance and when waking up, torpid hibernating bats utter audible calls, which might help the tits localize bats in crevices. As a final step, we recorded these calls and played them back at the cave entrance to test whether they are audible to the tits and elicit any specific behavioural reaction.
2. Material and methods
(a). Field site
The study was conducted in the Istállós-kői-cave, Bükk Mountains, in northeastern Hungary (546 m elevation). The cave consists of a 57 m long and approximately 10 m high chamber and has a huge entrance (10 m by 7.5 m) which results in higher light levels and lower temperatures than in typical bat hibernacula. Nevertheless, the cold tolerant pipistrelle bat (Pipistrellus pipistrellus) and the noctule (Nyctalus noctula) regularly hibernate there (Estók 1996). Most bats retreat to narrow crevices high up in the wall and cave ceiling. Calls from bats audible to the human ear can be heard from many areas of the ceiling. In winter, loose flocks of great tits (P. major) with total numbers of 50 or more birds can be regularly observed in the vicinity of the cave. The tits were unmarked, but the number of individuals involved in observations and experiments was likely to be at least several dozen.
We conducted behavioural observations and preliminary food provisioning experiments in the winters 2004/2005 (13 days) and 2005/2006 (9 days). Visits took place in the morning and lasted from 0.5 to 5.5 h. Upon arrival at the cave, we inspected the cave floor carefully for bat carcasses or pieces of fur and skin. In February and March 2009, sound recordings of bat calls (7 days) and playback experiments (6 days) were performed.
(b). Provisioning experiment
Sunflower seeds and bacon were provided to the birds 30–50 m from the cave entrance. Once provisioning had started, we continuously provided food in quantities such that the birds had not completely depleted the food by the next visit. In the winters of 2004/2005 and 2005/2006, we searched for killed bats on the cave floor and observed the tits’ foraging behaviour for 10 no-provisioning days (1885 min observation) and 12 provisioning days (849 min).
(c). Call recording, analysis and playback experiment
We recorded calls of different individual awakening bats sitting in crevices of the cave wall from a distance of 0.5–1 m. In a first step, we used a Pettersson D240x bat detector (Pettersson Elektronik AB, Sweden; sampling rate 307 kHz, 8 bit depth) and then recorded the vocalizations 10× time-expanded onto a Microtrack II digital recorder (professional two-channel mobile digital recorder, M-Audio, USA; 16 bits). Analysis of these recordings revealed that the vocalizations contained only frequencies lower than 35 kHz. We therefore decided to directly use the Microtrack II (sampling rate, 96 kHz electret microphone) for sound recordings, because it is more sensitive to frequencies below 20 kHz, which dominated the bat vocalizations. Sound analysis was performed with the software Selena (Tübingen University; FFT 512, frequency resolution 188 Hz).
We assembled five playback files from calls of individual bats with 1 min duration and natural inter-call interval in Cool Edit Pro 2 (Syntrillium Software Corporation). Files were set to the same r.m.s. power for frequencies less than 10 kHz (great tit upper hearing limit at 8–10 kHz (Langemann et al. 1998)). Files were played back for 1 min from the MicroTrack II at 96 kHz D/A-conversion via a Mac Audio MPX 2000 amplifier and an Ekulit loudspeaker (LSM-50M/F, ±5 dB for 4–10 kHz, i.e. lower bat call limit to upper tit hearing limit). The speaker was hidden in rocks at ground level at four different locations 5–20 m from the cave entrance and operated remotely via a 20 m cable. The sound level of playback calls was about 40–46 dBA at 1 m (Voltcraft SL-400 sound level meter) and sounded realistic to the human ear.
3. Results
(a). Foraging and feeding behaviour
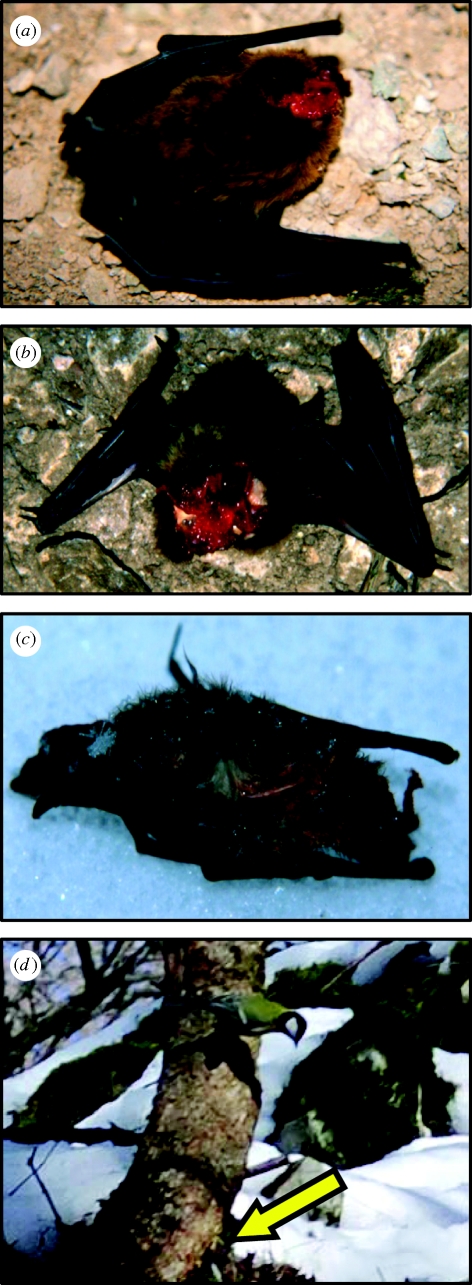
We observed a total of 16 predation events of great tits on pipistrelle bats in the winter 2004/2005 and two events in 2005/2006. The tits flew into the cave, flew slowly at close distance to cave walls and ceiling and repeatedly landed and sometimes vanished into crevices for few seconds (electronic supplementary material, video S1). We then saw them pecking the captured bats on rocks inside the cave (electronic supplementary material, videos S2 and S3) or on the cave floor. Typically 5–15 min would elapse from the time a tit entered the cave until we observed it with a captured bat. For transport, they took the bats into their beaks and sometimes carried them out of the cave to feed in nearby trees (electronic supplementary material, videos S4 and S5). The tits started eating the bats from either the head (figure 1a,b), back or abdomen. In addition to the direct observations, we found five pipistrelle carcasses in 2004/2005 and three in 2005/2006. All eight showed obvious traces of bird pecks, but no signs of chewing by a mammalian predator. Considerable parts of muscle mass had been pecked off these carcasses; on one, only skin and bones remained (figure 1c). The highest predation rates we observed were three predation events within 35 min on 8 February 2005 and five events within 1 h 40 min on 15 February 2005.
Figure 1.
(a) Freshly killed pipistrelle bat (P. pipistrellus), where a tit pecked off parts of snout and face. (b) Freshly killed pipistrelle, where a tit pecked off parts of head and ventral musculature. (c) Skin and skeleton of a dead pipistrelle found just below trees outside the cave in which tits had been observed feeding on bats. Inner organs, brain and flesh were missing; skin bore clear signs of bird beak pecks. (d) Great tit (P. major) looking at and approaching a hidden loudspeaker (arrow) that broadcast calls of an awakening pipistrelle bat.
(b). Provisioning experiment
We observed 17 events of a tit preying on a bat on 10 days without food provisioning, but only one predation event when the birds had access to experimentally provisioned, abundant food. Predation in non-provisioning times was significantly more common than expected by an even distribution of predation over observation time (see §2; binomial test, p = 0.0113). The number of bat carcasses with signs of bird feeding that we found upon arrival to the cave provided additional support to the hypothesis that the intensity of the predation on the bats was inversely correlated with availability of other food to the tits. On 10 visits to the cave without prior food provisioning, we found six carcasses, while on 12 visits with food provisioning we found only two, but this difference was not statistically significant (binomial test, p = 0.0927).
(c). Bat calls and playback experiment
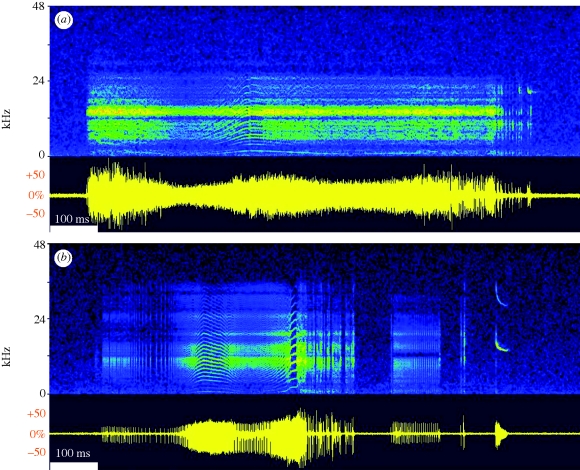
The vocalizations of awakening bats were noisy multi-harmonic sounds of about 0.8 s (examples in figure 2; summary statistics of call parameters in table 1). Often, they were followed by one or several tonal, mostly downward frequency modulated calls.
Figure 2.
Examples of vocalizations from awakening pipistrelle bats. Sonagram representation (FFT 512); oscillogram below, 70 dB dynamic range. (a) Typical example that closely corresponds to the average call parameters listed in table 1. (b) Example with especially prominent tonal components and long, trailing downward frequency modulated call. Scale bar, 100 ms.
Table 1.
Sound parameters of vocalizations from awakening pipistrelle bats (second order means ± s.d.).
| mean ± s.d. | n (sound files) | n (vocalizations) | |
|---|---|---|---|
| highest frequency | 29.8 ± 3.9 kHz | 8 | 51 |
| frequency with maximum amplitude | 15.1 ± 2.6 kHz | 8 | 51 |
| lowest frequency | 3.9 ± 1.6 kHz | 8 | 51 |
| duration | 0.78 ± 0.24 s | 10 | 61 |
| interval (onset to onset) | 2.25 ± 0.54 s | 9 | 60 |
| trailing tonal calls | 1.9 ± 0.6 | 7 | 50 |
We broadcast the vocalizations of awakening bats to one or several tits (maximum eight) that were present close to our loudspeaker for a total of 27 times. Forty-five out of 56 tits showed a clear response to the playback, 11 did not. A reaction consisted of orienting in the direction of the hidden speaker and approaching the speaker in flight or on foot (figure 1d). As the tits might observe and copy each other's behaviour, we did not regard the reaction of birds that were simultaneously present at the speaker as independent. Indeed, all tits present simultaneously always showed the same response; either all or none reacted to the playback. Overall, 18 playbacks elicited a response, while nine did not.
4. Discussion
The slow flights along and frequent landings on the cave walls and the direct observation of 18 predation events over 22 days with only 45 h of time at the cave indicates that the tits specifically and systematically searched for and killed bats. Extended pecking on the bats and substantial removal and ingestion of flesh, brain and other organs suggest that the birds were killing the bats for food and were not, for example, in competition for roosting sites in the cave. The substantial decrease of the observed killing of bats by tits after experimental provisioning of energy-rich food further supports that bat-killing served a foraging purpose. Tits were also observed to kill other songbirds in competition for nest holes in summer (Merilä & Wiggins 1995) and for food in winter (Caris 1958). Our data support a trend found for non-migrating birds, such as tits, to rely more on innovative feeding behaviours in winter than in other seasons (Sol et al. 2005).
Upon playbacks of calls from awakening bats, many tits approached and inspected the loudspeaker. This is evidence that they were able to hear and localize the bat calls, even though the frequency with maximum energy (table 1) was above their upper hearing limit (Langemann et al. 1998). Thus, tits could eavesdrop on awakening calls to find bats in crevices. Interestingly, similar calls of awakening bats in Canada were found to repel mammalian predators (Martin & Fenton 1978). It therefore is even conjecturable that bats vocalize in an attempt to defend themselves against predation (pursuit-deterrence; see Caro 1995). If calling indeed reveals the bats' location to opportunistic birds, the tits' foraging innovation may change the direction of selection on the use of this call.
The first observation of a tit preying on a bat at Istállós-kői-cave was in winter 1995/1996 (Estók 1996), 10 years before our present observations and thus clearly exceeding the typical lifespan for a wild tit (0.1% survival probability from egg to 8 years of age (Tinbergen & Daan 1990). The tits in our study were not marked; however, we are certain that several, if not many, individuals foraged for bats. This raises the question of whether cultural transmission plays a role for the spread of this foraging innovation, as was the case for the famous blue tits (Cyanistes caeruleus) in the British Isles that learned to open milk bottles (Fisher & Hinde 1949). If so, it raises another question to be addressed in future studies: does cultural transmission link the Hungarian bat-eating tits to the Polish ones (see §1) or did this behavioural innovation arise independently at the two sites, as a consequence of similar opportunities present?
Acknowledgements
We thank Annamária Gilyén who told us about a tit feeding on a bat at the cave in 1995, Leonie Baier for assembling figure 2 as well as Rachel Page, Henrik Brumm, John Quinn and an anonymous referee for commenting on the manuscript. This study was supported by the Max Planck Society.
References
- Careau V., Lecomte N., Giroux J. F., Berteaux D.2007Common ravens raid Arctic fox food caches. J. Ethol. 25, 79–82 (doi:10.1007/s10164-006-0193-7) [Google Scholar]
- Caris J. L.1958Great tit killing and carrying goldcrest. Br. Birds 51, 355 [Google Scholar]
- Caro T. M.1995Pursuit-deterrence revisited. Trends Ecol. Evol. 10, 500–503 (doi:10.1016/S0169-5347(00)89207-1) [DOI] [PubMed] [Google Scholar]
- Estók P.1996The bat-seizing of great tit (Parus major) at Istállóskői-cae (Bükk mountains) (in Hungarian). Denevérkutatás Hungarian Bat Research News 2, 40 [Google Scholar]
- Fenton M. B., Rautenbach I. L., Smith S. E., Swanepoel C. M., Grosell J., Vanjaarsveld J.1994Raptors and bats—threats and opportunities. Anim. Behav. 48, 9–18 (doi:10.1006/anbe.1994.1207) [Google Scholar]
- Fisher J., Hinde R. A.1949The opening of milk bottles by birds. Br. Birds 42, 347–357 [Google Scholar]
- Goodall J.1964Tool-using and aimed throwing in a community of free-living chimpanzees. Nature 201, 1264–1266 (doi:10.1038/2011264a0) [DOI] [PubMed] [Google Scholar]
- Laland K. N., Reader S. M.1999Foraging innovation in the guppy. Anim. Behav. 57, 331–340 (doi:10.1006/anbe.1998.0967) [DOI] [PubMed] [Google Scholar]
- Langemann U., Gauger B., Klump G. M.1998Auditory sensitivity in the great tit: perception of signals in the presence and absence of noise. Anim. Behav. 56, 763–769 (doi:10.1006/anbe.1998.0879) [DOI] [PubMed] [Google Scholar]
- Martin K. A., Fenton M. B.1978Possible defensive function for calls given by bats (Myotis lucifugus) arousing from torpor. Can. J. Zool. 56, 1430–1432 (doi:10.1139/z78-196) [Google Scholar]
- Merilä J., Wiggins D. A.1995Interspecific competition for nest holes causes adult mortality in the collared flycatcher. Condor 97, 445–450 (doi:10.2307/1369030) [Google Scholar]
- Popa-Lisseanu A. G., Delgado-Huertas A., Forero M. G., Rodriguez A., Arlettaz R., Ibanez C.2007Bats’ conquest of a formidable foraging niche: the myriads of nocturnally migrating songbirds. PLoS ONE 2, unpaginated (doi:10.1371/journal.pone.0000205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzicki G., Hejduk J., Banbura J.1999Tits (Parus major and Parus caeruleus) preying upon hibernating bats. Orn. Fenn. 76, 93–94 [Google Scholar]
- Ryberg O.1947Studies on bats and bat parasites Lund, Sweden: Berlingska Boktrykeriet [Google Scholar]
- Sachanowicz K., Krasnodębski I.1996Great tit Parus major feeding on dead bat (in Polish). Przegląd Przyrodniczy 7, 91 [Google Scholar]
- Sol D., Lefebvre L., Rodriguez-Teijeiro J. D.2005Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B. 272, 1433–1441 (doi:10.1098/rspb.2005.3099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen J. M., Daan S.1990Family-planning in the great tit (Parus major)—optimal clutch size as integration of parent and offspring fitness. Behaviour 114, 161–190 (doi:10.1163/156853990X00103) [Google Scholar]