Abstract
Many animals respond to predation risk by forming groups. Evolutionary explanations for group formation in previously ungrouped, but loosely associated prey have typically evoked the selfish herd hypothesis. However, despite over 600 studies across a diverse array of taxa, the critical assumptions of this hypothesis have remained collectively untested, owing to several confounding problems in real predator–prey systems. To solve this, we manipulated the domains of danger of Cape fur seal (Arctocephalus pusillus pusillus) decoys to provide evidence that a selfish reduction in a seals' domain of danger results in a proportional reduction in its predation risk from ambush shark attacks. This behaviour confers a survival advantage to individual seals within a group and explains the evolution of selfish herds in a prey species. These findings empirically elevate Hamilton's selfish herd hypothesis to more than a ‘theoretical curiosity’.
Keywords: selfish herd, predator–prey interaction, dilution effect, grouping, white shark, Cape fur seal
1. Introduction
Predation is one of the main evolutionary forces driving group formation, providing various benefits related to detecting, confusing, deterring and mobbing predators (reviews in Krause & Ruxton 2002; Caro 2005). When a predator can only target one or a few members of a group at a given time (Foster & Treherne 1981), an individual may be afforded a dilution of risk. However, risk is often not equally shared by group members and consequently individuals may behave to reduce their risk relative to others in the group. Hamilton's selfish herd hypothesis (1971) has arguably been the most popular model to explain how differential risk may cause loosely associated prey individuals to group up.
In Hamilton's simplest model, surface-living individuals are preyed upon by a bottom-dwelling predator. The predator attacks the nearest prey individual from the point at which it randomly appears on the surface. This creates ‘domains of danger’ for individual prey: an area within which an individual will be the ‘closest’ to a predator from its point of appearance at the surface. The larger this area, the greater the individual's predation risk relative to that of its neighbours. By moving towards neighbours, an individual can reduce this ‘domain of danger’, which hypothetically translates into lower predation risk (Hamilton 1971). If all individuals move in this way, Hamilton argued, compact groups may form.
The elegance of the selfish herd has endeared it as a framework within which to interpret the behaviour of prey in response to predators, and more than 600 studies on a diverse array of taxa (Morton et al. 1994) have provided support for it. While these studies have successfully quantified increased cohesiveness of groups when exposed to predators (Viscido 2003), shown that individuals prefer central to peripheral positions within a group (e.g. Krause 1993; Krause & Ruxton 2002; Caro 2005), and that centre individuals' risk are reduced relative to peripheral ones (e.g. Milinski 1977; Uetz 1993), this evidence is not unequivocal. The classification of centre versus edge individuals is open to bias (Stankowich 2003; Viscido 2003), there is ambiguity in the literature as to where animals are more at risk (Krause 1993; Krause & Ruxton 2002; Caro 2005) and results at group centres and edges may be strongly confounded by other benefits and costs of grouping (Fitzgibbon 1990; Krause 1993; Caro 2005). The prediction that an individual's spacing affords it differential predation risk has been largely neglected. To our knowledge, only one study has shown that predators (sparrow hawks) target more widely spaced prey (redshank), relative to non-attacked neighbours, while controlling for predator confusion, centre/edge positioning and, to some extent, vigilance (Quinn & Cresswell 2006).
The presence of confounding differential agents of risk within groups may be the most pertinent complication to selfish herd investigations: predators often avoid selecting more vigilant individuals (Quinn & Cresswell 2006), might find grouping individuals more difficult to target (Krause & Ruxton 2002; Caro 2005) or might preferentially target prey individuals of different body size (Uetz 1993; Romey & Wallace 2007), experience (Milinski 1977; Uetz 1993; Romey & Wallace 2007) or state of hunger (Milinski 1977; Romey & Wallace 2007).
The critical corroboration of the selfish herd hypothesis requires empirical validation of its central concept, namely that the size of the domain of danger is proportional to predation risk and that it alone embodies differential survival probability and is subject to selection pressure. We tested this prediction in a predator–prey system involving white sharks (Carcharodon carcharias) and Cape fur seals (Arctocephalus pusillus pusillus) at Seal Island in South Africa.
Seal Island is a small rocky outcrop situated in the northwest of False Bay, which is inhabited by approximately 77 000 Cape fur seals. Adult seals leave the island to travel to the foraging grounds approximately 24 km to the south of the island (Laroche et al. 2008) on trips lasting several days (David & Rand 1986). In the austral winter, seals are subject to high levels of predation by white sharks (Laroche et al. 2008). Predations are spatially and temporally predictable, typically occurring within 400 m of the south and west side of the island and within the first 2 h after sunrise (Laroche et al. 2008). Predation success is highly dependent on the element of surprise (Laroche et al. 2008), thus solitary hunting sharks (Le Boeuf 2004) typically attack seals at tremendous speed from directly below, resulting in their whole bodies breaching out of the water (Laroche et al. 2008).
This predator–prey relationship provides an excellent system for testing the selfish herd hypothesis for several reasons. First, there is a distinct spatial separation of foraging and predation zones: seals must traverse the ‘danger zone’ adjacent to the island, and groups which form prior to departure from the island subsequently break up, once out of the danger zone (Rand 1967; Laroche et al. 2008). Furthermore, predator–prey activity is spatio-temporally confined and predictable (Laroche et al. 2008), and the system, where the predator appears by surprise within a group is one that strongly resembles Hamilton's original model. Most propitiously, sharks detect their prey using surface moving silhouettes (Laroche et al. 2008), which allows for an opportunity to manipulate the system by constructing artificial seal silhouettes with variable domains of danger. Not only can exact distances between individual ‘seals’ be measured, but resulting domains of danger can be repeated and survival probability subsequently assigned to them. Furthermore, it allows a test of the selfish herd that controls for vigilance, the confusion effect, and phenotypical and behavioural variability within groups.
In this study, we test the prediction that animals with larger domains of danger will be at more risk than animals with smaller ones, and investigate if the size of an animal's domain of danger is proportional to its relative predation risk.
2. Material and methods
We used black (on ventral surfaces) Styrofoam boards to construct identical seal decoys which were fixed into positions on a raft, using lightweight reed poles secured to the dorsal surface of each decoy. By varying the distances between each decoy within a group, we could produce different and repeatable domains of danger. Decoy rafts were designed to mimic the size and spacing of natural seal groups in the study area. The mean (±s.d.) area of the domain of danger for real seal groups was 5.625 ± 2.064 m2 compared with 5.546 ± 1.453 m2 and 6.109 ± 0.867 m2 for artificial groups using five and four decoy seal groups, respectively.
Decoy rafts, comprising two different arrangements of four and five decoys, were towed at a distance of 30 m behind a 5 m semi-rigid boat at an average speed of 7 km h−1. Both seals and sharks at the island were habituated to the proximity of motor boats as tourist operators and researchers have engaged in shark- and seal-related activities for more than 10 years. We towed the decoy raft through five 1 km transects on 16 field days and recorded a total of 36 separate attacks. A power analysis was performed to determine when sufficient data to support or refute the hypothesis had been collected in accordance with conditions of the permit issued to perform this research. After each predation event we recorded which decoy (and associated domain of danger) was attacked. Decoys were designed to break free of the raft upon attack, reducing the chances of injury to the shark and enabling a conclusive assessment of which decoy was targeted. There was little difference in attack frequency on transects leaving from (n = 16) compared with travelling towards (n = 20) the island.
Domains of danger were determined using Voronoi tessellations (Viscido 2003) constructed by plotting decoy positions as single points on an x, y grid and using the Voronoi scatter plot function in Statistica to draw the lines of the tessellations that formed the boundary of each domain of danger. The size of each domain of danger was calculated by superimposing 0.25 m2 × 0.25 m2 cube grids onto the diagrams. In real systems, groups are realistically not edgeless, but bound by either a limited predatory attack or detection range (Pulliam 1973; James et al. 2004). We present results calculated when tessellations were bound with one predator body length (3 m) from edge individuals. From the observations we know that predators target individuals within groups and thus will not appear in a space more than one predator body length away from any given individual. We also calculated ‘limited domains of dangers’ (James et al. 2004) (a circular limitation), and bound tessellations with one and two prey body lengths (see Viscido & Wethey 2002; James et al. 2004, for alternative suggestions on how to calculate domains of danger).
3. Results and discussion
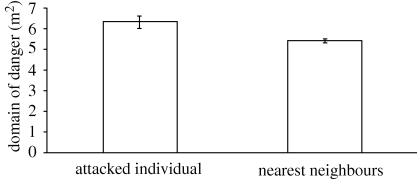
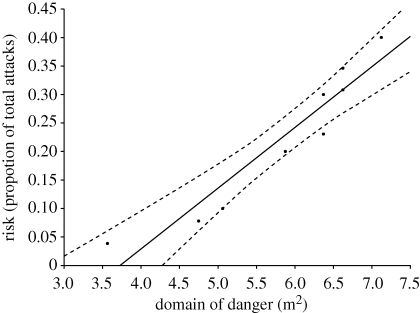
Our results provide strong support for Hamilton's hypothesis: the mean domain of danger for attacked decoys (mean ± s.e.m. = 6.365 ± 0.289 m2) was significantly larger (paired t-test: α = 0.05, n = 36, p = 0.000005) that of non-targeted decoys (mean ± s.e.m. = 5.514 ± 0.072 m2) (figure 1, table 1). Furthermore, we found a significant positive correlation (r2 = 0.9038, p = 0.00008, α = 0.05, n = 9, pooling attacks on five-decoy (n = 26) and four-decoy (n = 10) configurations) between the size of the domain of danger and relative predation risk; measured as the proportion of total trials in which an individual decoy was attacked (figure 2).
Figure 1.
Average domain of danger (m2) of targeted decoys and their nearest neighbours (n = 36). All other individuals in the group were considered neighbours in this analysis. Error bars represent the standard error of means at the 95 per cent confidence interval (paired t-test: α = 0.05, t = 5.379, p = 0.000005).
Table 1.
Alternative calculations of domains of danger (DOD, means±s.e.m.), as calculated by binding groups with prey body lengths (PL), two prey body lengths (2PL) and limited domains of danger (LDOD, n = 36). (Significance is depicted by an asterisk.)
| attacked individuals DOD | non-attacked individuals DOD | t-statistics | p-value | correlation of DOD with risk (r2 value) | |
|---|---|---|---|---|---|
| PL | 6.684 ± 0.591 | 6.254 ± 0.216 | 3.997 | 0.000315* | 0.844 |
| 2PL | 6.059 ± 0.497 | 5.601 ± 0.284 | 4.525 | 0.000067* | 0.859 |
| LDOD | 7.025 ± 0.141 | 6.897 ± 0.062 | 4.284 | 0.000136* | 0.9395 |
Figure 2.
The relationship between risk (proportion of total attacks) and domain of danger size (m2). We pooled results of domains of dangers from configurations of four (n = 10) and five (n = 26) individuals in this analysis and correlated it with the number of attacks experienced as a proportion of the total number of trials. Dashed lines represent the confidence interval at the 95 per cent level (p = 0.00008, n = 9, α = 0.05).
Broadly speaking, two limitations have impeded previous attempts to test the central tenets of selfish herds. First, quantifying individual animal spacing and relating this to risk (but see Quinn & Cresswell 2006) and second, disentangling selfish herd effects from other mechanisms that may affect predator targeting choice, i.e. vigilance (Fitzgibbon 1990; Krause 1993; Viscido & Wethey 2002; Quinn & Cresswell 2006), the confusion effect (Caro 2005) and phenotypical variation within groups (Romey & Wallace 2007).
Complexity is not absent from this predator–prey system: for one, surprise seems to be very important to the success of a predation (Laroche et al. 2008), suggesting that prey vigilance is important in this system and had to be controlled for. Furthermore. attack rates are not equal for different age classes (Laroche et al. 2008), suggesting a confounding effect of prey phenotype. However, our results are of particular significance because the predator attack strategy provided an opportunity to isolate the selfish herd effect from these other mechanisms, while, we believe, still retaining biological realism.
Overall, our results provide evidence that individual spacing within seal groups influences predation risk by white sharks and suggests that the selfish herd hypothesis may be a plausible theoretical framework for explaining the evolution of gregarious behaviour in similar systems. We provide empirical evidence that the domain of danger is a biologically real spatial construct of differential predation risk (and thus a real entity for natural selection to operate on) and thus offer some authentication of the selfish herd as more than a mere ‘theoretical curiosity’ (Viscido 2003).
Acknowledgements
This project was approved by the Science Faculty Animal Ethics Committee of the University of Cape Town (clearance number 2008/V15/JO) and under permit conditions of DEAT, marine and coastal management (V1/8/5/1).
Thanks to the extended members of the Zoology Department, UCT for assistance in data collection, DEAT for a research permit (V1/8/5/1); L. Atkins, T. Hoffman, M. Lewis, K. Potgieter, D. Ravasi, R. Simmons for comments on a draft; J. Puttick, M. Laird and R. Simmons for donation of equipment and the NRF for funding. Thanks to W. Cresswell and two anonymous reviewers for constructive comments on an earlier version of this manuscript.
References
- Caro T.2005Antipredator defenses in birds and mammals Chicago, IL: University of Chicago Press [Google Scholar]
- David J. H. M., Rand R. W.1986Attendance behaviour of South African fur seals. In Fur seals: maternal strategy on land and at sea (eds Kooyman G. L., Gentry R. L.), pp. 126–141 Princeton, NJ: Princeton University Press [Google Scholar]
- Fitzgibbon C.1990Mixed-species grouping in Thomson's and Grant's gazelles: the antipredator benefits. Anim. Behav. 39, 1116–1126 (doi:10.1016/S0003-3472(05)80784-5) [Google Scholar]
- Foster W. A., Treherne J. E.1981Evidence for the dilution effect in the selfish herd from fish predation on a marine insect. Nature 293, 466–467 (doi:10.1038/293466a0) [Google Scholar]
- Hamilton W. D.1971Geometry of the selfish herd. J. Theor. Biol. 31, 295–311 (doi:10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- James R., Bennett P. G., Krause J.2004Geometry for mutualistic and selfish herds: the limited domain of danger. J. Theor. Biol. 228, 107–113 (doi:10.1016/j.jtbi.2003.12.005) [DOI] [PubMed] [Google Scholar]
- Krause J.1993Differential fitness returns in relation to spatial position in groups. Biol. Rev. 69, 187–206 (doi:10.1111/j.1469-185X.1994.tb01505.x) [DOI] [PubMed] [Google Scholar]
- Krause J., Ruxton G. D.2002Living in groups Oxford, UK: Oxford University Press [Google Scholar]
- Laroche R. K., Kock A. A., Dill L. M., Oosthuizen W. H.2008Running the gauntlet: a predator–prey game between sharks and two age classes of seals. Anim. Behav. 76, 1901–1917 (doi:10.1016/j.anbehav.2008.06.025) [Google Scholar]
- Le Boeuf B.2004Hunting and migratory movements of white sharks in the eastern North Pacific. Mem. Natl Inst. Polar Res. 58, 89–100 [Google Scholar]
- Milinski M.1977Do all members of a swarm suffer the same predation? Z. Tierpsychol. 45, 373–378 [Google Scholar]
- Morton T. L., Haefner J. W., Nugala V., Decino R. D., Mendes L.1994The selfish herd revisited: do simple movement rules reduce relative predation risk? J. Theor. Biol. 167, 73–79 (doi:10.1006/jtbi.1994.1051) [Google Scholar]
- Pulliam H. R.1973On the advantages of flocking. J. Theor. Biol. 38, 419–422 (doi:10.1016/0022-5193(73)90184-7) [DOI] [PubMed] [Google Scholar]
- Quinn J. L., Cresswell W.2006Testing domains of danger in the selfish herd: sparrowhawks target widely spaced redshanks in flocks. Proc. R. Soc. B 273, 2521–2526 (doi:10.1098/rspb.2006.3612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand R. W.1967The Cape fur-seal (Arctocephalus pusillus): general behaviour on land and at sea. Invest. Rep. Div. Sea Fisheries South Africa 60, 1–39 [Google Scholar]
- Romey W. L., Wallace A. C.2007Sex and the selfish herd: sexual segregation within nonmating whirligig groups. Behav. Ecol. 18, 910–915 (doi:10.1093/beheco/arm057) [Google Scholar]
- Stankowich T.2003Marginal predation methodologies and the importance for predator preferences. Anim. Behav. 66, 589–599 (doi:10.1006/anbe.2003.2232) [Google Scholar]
- Uetz L. R. G.1993Ontogenetic shifts within the selfish herd: predation risk and foraging trade-offs change with age in colonial web-building spiders. Oecologia 95, 1–8 [DOI] [PubMed] [Google Scholar]
- Viscido S. V.2003The case for the selfish herd hypothesis. Comment. Theor. Biol. 8, 665–684 [Google Scholar]
- Viscido S. V., Wethey D. S.2002Quantitative analysis of fiddler crab flock movement: evidence for ‘selfish herd’ behavior. Anim. Behav. 63, 735–741 (doi:10.1006/anbe.2001.1935) [Google Scholar]