Abstract
Every autumn the entire eastern North American population of monarch butterflies (Danaus plexippus) undergoes a spectacular migration to overwintering sites in the mountains of central Mexico, where they form massive clusters and can number in the millions. Since their discovery, these sites have been extensively studied, and in many of these studies, monarchs were captured and sexes recorded. In a recent effort to compile the sex ratio data from these published records, a surprising trend was found, which appears to show a gradual decline in proportion of females over time. Sex ratio data from 14 collections of monarchs, all spanning 30 years and totaling 69 113 individuals, showed a significant negative correlation between proportion of females and year (r = −0.69, p = 0.007). Between 1976 and 1985, 53 per cent of overwintering monarchs were female, whereas in the last decade, 43 per cent were female. The relationship was significant with and without weighting the analyses by sampling effort. Moreover, analysis of a recent three-year dataset of sex ratios revealed no variation among nine separate colonies, so differences in sampling location did not influence the trend. Additional evidence from autumn migration collections appears to confirm that proportions of females are declining, and also suggests the sex ratio is shifting on breeding grounds. While breeding monarchs face a number of threats, one possibility is an increase in prevalence of the protozoan parasite, Ophryocystis elektroscirrha, which recent evidence shows affects females more so than males. Further study will be needed to determine the exact cause of this trend, but for now it should be monitored closely.
Keywords: monarch butterflies, Danaus plexippus, overwintering, sex ratios
1. Introduction
The migration and overwintering behaviour of monarch butterflies (Danaus plexippus) in eastern North America is one of the world's most amazing biological phenomena, as each autumn the entire population of butterflies east of the Rocky Mountains migrates to a select few mountaintop sites in central Mexico where they overwinter until the following March. In this location, which is designated as a sanctuary by the Mexican government, monarchs form tight clusters on the branches of oyamel fir trees and can number in the millions. Since this location was discovered in 1976 (Urquhart 1976), overwintering monarchs have been extensively studied in a variety of contexts, such as the impact of bird predation (e.g. Alonso-Mejia et al. 1998), the effect of freezing temperatures (e.g. Anderson & Brower 1996) and mating behaviour at the end of the winter (Van Hook 1993). In many cases, investigators have captured monarchs for their projects and reported the number of males versus females in their sample. Recently, in an attempt to determine the sex ratio of monarchs in the eastern population, these published accounts were compiled (listed in the elctronic supplementary material, table S1), and in the process an unexpected and alarming temporal trend was discovered, which is reported on here.
2. Material and methods
Information on sex ratios at the Mexico overwintering site was gathered from published sources (elctronic supplementary material, table S1), from recent collections by university researchers (S. Altizer 2008, unpublished data) and by staff of the World Wildlife Fund Mexico. Only reports where collections were made in an unbiased fashion were included. Reports of monarchs killed by bird predators were not included, because these tend to be highly male-biased (Brower & Calvert 1985; Alonso-Mejia et al. 1998), and collections made only in March were not included because colonies begin dispersing at this time (Van Hook 1993). Combined, the records (n = 14) reflect 69 113 monarch captures over 30 years. Because not all published records were from the same overwintering colonies, we examined data gathered by the WWF to explore possible differences in sex ratios among colonies. This dataset consisted of multiple collections from all colonies beginning in the 2004/2005 overwintering season, although sampling effort from the first season was uneven (within season and across colonies), so it was not included.
(a). Data analysis
With the WWF data we used analysis of variance to examine possible variation in per cent of females per sample (dependent) among colonies (categorical independent) and with year (three total) included as an independent variable. We also included a variable to examine possible within-season (i.e. overwintering season) temporal variation in sex ratio. For this, months were divided into ‘early’ or ‘late’ periods, so that there were eight time periods from late November to early March.
For the 30 year dataset, we used correlation analysis (comparing year versus percentage of females) to determine whether overwintering sex ratios have changed over time. Data were analysed with and without weighting by sampling effort. For weighted analyses, sex ratio records were assigned scores of 1–4 based on the level of sampling effort (electronic supplementary material, table S1), and this variable was used for weighting.
3. Results
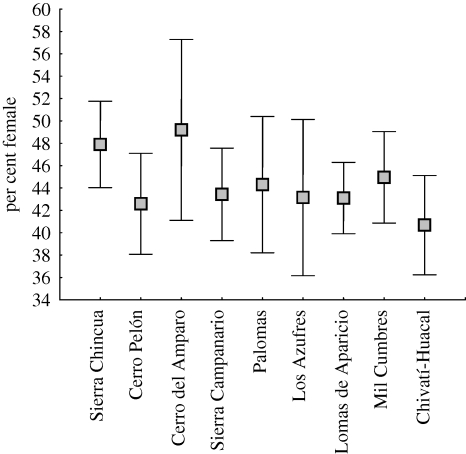
The WWF data showed that the percentage of females was similar among the nine overwintering colonies (F8,187 = 1.105, p = 0.362; figure 1). There was also no effect of year from the three years considered here (F2,187 = 2.138, p = 0.121). Sex ratios tended to vary within overwintering seasons (F7,187 = 3.20, p = 0.003), although there was no clear pattern; early-season samples (late November, early December) showed 46–48% females, then the ratio fluctuated between 42, 47 and 38 per cent (electronic supplementary material, table S2). By March, when colonies begin to break up, samples tended to be slightly female biased (53% female).
Figure 1.
Mean per cent of females across nine known overwintering colonies, based on data gathered between 2006 and 2008 by WWF personnel. Means are not significantly different (see text). Shaded squares, mean; vertical lines with upper and lower bar, mean ± 95 CI.
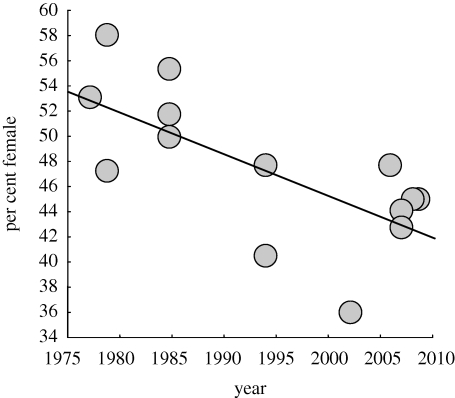
The correlation analyses of the 30 year data pointed to a gradually changing sex ratio over time at the Mexico overwintering colonies. The percentage of overwintering female monarchs was negatively correlated with year (r = −0.68, p = 0.007 (without weighting), r = −0.71, p < 0.001 (weighted by sampling effort); figure 2). Put another way, if one considers all of the samples between 1976 and 1985, the average percentage of females was 52.5 per cent. Meanwhile, the average of all samples from the current decade is 43.4 per cent, or a drop of approximately 10 per cent over 30 years. Because of the small sample size in the correlation analysis, and the possibility that single points could influence the significance of the relationship, additional (unweighted) correlations were performed with each data point removed once (14 tests in total). All tests resulted in significant (p < 0.05) negative correlations, with the lowest coefficient being −0.63.
Figure 2.
Sex ratio of overwintering monarch butterflies in Mexico over time. Points were from published and non-published sources that collectively examined 69 113 individuals over 30 years (electronic supplementary material, table S1). r = −0.69; p = 0.0065.
4. Discussion
The data compiled here indicate the sex ratios of monarch butterflies at Mexican overwintering colonies have been changing over the three decades since their discovery, from generally female biased (approx. 53% female) in the 1970s, to largely male-biased in recent years (approx. 43% female). This discovery was certainly unexpected and is not readily explainable. At first glance, one might think that the thinning of the sanctuary's forest over the past three decades from illegal logging (Brower et al. 2002) has gradually changed the microclimate, making it somehow less favourable for female survival in recent years. However, evidence to date does not indicate this species has any sex-related differences in cold tolerance to natural temperatures (i.e. per cent survival in relation to temperature) (Alonso-Mejia & Arellano-Guillermo 1992) or to experimentally induced freezing (Anderson & Brower 1993). Moreover, our own analysis (of the WWF data) provided no clear evidence of differential mortality throughout the overwintering period (i.e. percentage of females did not clearly decline from November to March).
A second possibility is that the clustering patterns have changed over time, making males somehow more likely to be sampled by researchers in recent collections. This also appears unlikely, considering what is known of overwintering behaviour in western North American monarchs. Frey & Leong (1993) showed that overwintering monarchs in California have no sex-related differences in tree height preference, which would lead to sex biases in captures at clusters. It follows then that the sexes would not behave differently (in terms of clustering position) at Mexican colonies.
The cause of the decline in females may not even be related to the overwintering sites at all. After uncovering the overwintering trend, we examined published records of autumn migrants and found evidence that the proportion of female monarchs has been declining in this phase as well. Long before the Mexico destination was known, Beall (1948) made a collection of 464 migrants in Ontario that was 55.6 per cent female. A collection of 266 migrants from Missouri in 1972 showed that 50.8 per cent were females (Brown & Chippendale 1974). Herman (1988) collected 1868 late-summer monarchs from 1976 to 1986 and 44 per cent were female. During 1998–2001, Borland et al. (2004) captured migrating monarchs at more than 20 locations in Minnesota and Texas and the percentage that were female averaged 40 per cent across all sites. Most recently, Brindza et al. (2008) captured 2224 migrants at two sites in Virginia from 2001 to 2006, of which 37 per cent were female. This decline in the proportion of female monarchs during the autumn migration provides further confirmation of the temporal trend we discovered at the overwintering sites and also indicates that the change in sex ratio is occurring at the breeding grounds, or at least in the late-summer migratory generation.
Breeding monarchs face a number of threats, such as anthropogenic habitat alterations, insecticides and other chemicals, and infections with parasites such as the protozoan Ophryocystis elektroscirrha (OE). Interestingly, there is recent evidence of differential impacts of infection on males and females; infected females experience greater infection intensities (de Roode et al. 2008) and larger parasite-induced reductions in body sizes than males (de Roode et al. 2007). Furthermore, field collections made in the past five years at Mexican wintering colonies have shown higher prevalence than those from 30 years ago (S. Altizer 2009, personal communication). It follows then that if OE prevalence is increasing, and if it negatively affects females more so than males, it is possible that population-level sex ratios at the breeding grounds could become skewed towards males. On the other hand, if OE is not the cause of this phenomenon, it may be that equal numbers of males and females are indeed being produced in the summer, but for some reason fewer female monarchs are now undertaking the autumn migratory journey.
Clearly, the temporal trend found here is a phenomenon that deserves additional research to uncover the causative factor, but it also highlights the many questions that remain to be answered about the biology of this unique insect migration and overwintering phenomenon, which is at risk of disappearing in the near future because of habitat loss at overwintering sites (Brower & Malcolm 1991) and along migration routes (Brower et al. 2006). For now, the changing sex ratio of monarchs in eastern North America remains a fascinating discovery and one which we will continue to monitor in the future.
Acknowledgements
Sonia Altizer provided data from collecting trips in 2007 and 2008 and contributed helpful comments to the manuscript. Support for A.K.D. came from the D.B. Warnell School of Forestry and Natural Resources at the University of Georgia. The WWF data in Mexico was collected with the support of the Alliance WWF-Telcel.
References
- Alonso-Mejia A., Arellano-Guillermo A.1992Influence of temperature, surface body moisture and height aboveground on survival of monarch butterflies overwintering in Mexico. Biotropica 24, 415–419 (doi:10.2307/2388612) [Google Scholar]
- Alonso-Mejia A., Montesinos-Patino E., Rendon-Salinas E., Brower L. P., Oyama K.1998Influence of forest canopy closure on rates of bird predation on overwintering monarch butterflies Danaus plexippus L. Biol. Conserv. 85, 151–159 (doi:10.1016/S0006-3207(97)00134-1) [Google Scholar]
- Anderson J. B., Brower L. P.1993Cold-hardiness in the annual cycle of the monarch butterfly. In Biology and conservation of the monarch butterfly (eds Malcolm S. B., Zalucki M. P.), pp. 155–164 Los Angeles, CA: Natural History Museum of Los Angeles County [Google Scholar]
- Anderson J. B., Brower L. P.1996Freeze-protection of overwintering monarch butterflies in Mexico: critical role of the forest as a blanket and an umbrella. Ecol. Entomol. 21, 107–116 (doi:10.1111/j.1365-2311.1996.tb01177.x) [Google Scholar]
- Beall G.1948The fat content of a butterfly, Danaus plexippus, as affected by migration. Ecology 29, 80–94 (doi:10.2307/1930346) [Google Scholar]
- Borland J., Johnson C. C., Crumpton T. W., III, Thomas M., Altizer S., Oberhauser K.2004Characteristics of fall migratory monarch butterflies, Danaus plexippus, in Minnesota and Texas. In The monarch butterfly, biology and conservation (eds Oberhauser K., Solensky M.), pp. 97–104 Ithaca, NY: Cornell University Press [Google Scholar]
- Brindza L., Brower L. P., Davis A. K., Van Hook T.2008Comparative success of monarch butterfly migration to overwintering sites in Mexico from inland and coastal sites in Virginia. J. Lepidopterists Soc. 62, 189–200 [Google Scholar]
- Brower L. P., Calvert W. H.1985Foraging dynamics of bird predators on overwintering monarch butterflies in Mexico. Evolution 39, 852–868 (doi:10.2307/2408685) [DOI] [PubMed] [Google Scholar]
- Brower L. P., Malcolm S. B.1991Animal migrations: endangered phenomena. Am. Zool. 31, 265–276 [Google Scholar]
- Brower L. P., Castilleja G., Peralta A., Lopez-Garcia J., Bojorquez-Tapia L., Diaz S., Melgarejo D., Missrie M.2002Quantitative changes in forest quality in a principal overwintering area of the monarch butterfly in Mexico, 1971–1999. Conserv. Biol. 16, 346–359 (doi:10.1046/j.1523-1739.2002.00572.x) [Google Scholar]
- Brower L. P., Fink L. S., Walford P.2006Fueling the fall migration of the monarch butterfly. Integr. Compar. Biol. 46, 1123–1142 (doi:10.1093/icb/icl029) [DOI] [PubMed] [Google Scholar]
- Brown J. J., Chippendale G. M.1974Migration of the monarch butterfly, Danaus plexippus: energy sources. J. Insect Physiol. 20, 1117–1130 (doi:10.1016/0022-1910(74)90218-2) [DOI] [PubMed] [Google Scholar]
- de Roode J. C., Gold L. R., Altizer S.2007Virulence determinants in a natural butterfly–parasite system. Parasitology 134, 657–668 (doi:10.1017/S0031182006002009) [DOI] [PubMed] [Google Scholar]
- de Roode J. C., Pedersen A. B., Hunter M. D., Altizer S.2008Host plant species affects virulence in monarch butterfly parasites. J. Anim. Ecol. 77, 120–126 (doi:10.1111/j.1365-2656.2007.01305.x) [DOI] [PubMed] [Google Scholar]
- Frey D., Leong K. L. H.1993Can microhabitat selection or differences in ‘catchability’ explain male-biased sex ratios in overwintering populations of monarch butterflies? Anim. Behav. 45, 1025–1027 (doi:10.1006/anbe.1993.1120) [Google Scholar]
- Herman W. S.1988Body weight and wing length changes in Minnesota populations of the monarch butterfly. J. Lepidopterists Soc. 42, 32–36 [Google Scholar]
- Urquhart F. A.1976Found at last: the monarch's winter home. Natl Geogr. 150, 161–173 [Google Scholar]
- Van Hook T.1993Non-random mating in monarch butterflies overwintering in Mexico. In Biology and conservation of the monarch butterfly (eds Malcolm S. B., Zalucki M. P.), pp. 49–60 Los Angeles, CA: Natural History Museum of Los Angeles County [Google Scholar]