Abstract
Sexual selection is a major force behind the rapid evolution of male genital morphology among species. Most within-species studies have focused on sexual selection on male genital traits owing to events during or after copulation that increase a male's share of paternity. Very little attention has been given to whether genitalia are visual signals that cause males to vary in their attractiveness to females and are therefore under pre-copulatory sexual selection. Here we show that, on average, female eastern mosquitofish Gambusia holbrooki spent more time in association with males who received only a slight reduction in the length of the intromittent organ (‘gonopodium’) than males that received a greater reduction. This preference was, however, only expressed when females chose between two large males; for small males, there was no effect of genital size on female association time.
Keywords: Gambusia, genital evolution, female choice, Poeciliidae, sexual selection
1. Introduction
Genitalia evolve rapidly and differ widely in morphology even among closely related species (Eberhard 1985). The consensus is that sexual selection is primarily responsible due to in copula or post-copulatory processes involving sperm competition, cryptic female choice and/or sexual conflict over insemination (Hosken & Stockley 2004). In support of this claim, genital divergence in polyandrous insect clades is more than double that of sister clades with monogamous females (Arnqvist 1998). In addition, studies of several insects show that intra-specific variation in male genital morphology influences fertilization success (e.g. House & Simmons 2003; Wenninger & Averill 2006). There is, therefore, compelling evidence that post-copulatory sexual selection drives the evolution of insect genitalia. By contrast, evidence from vertebrates is equivocal (mammals: Ramm 2007) or simply lacking.
One neglected possibility is that sometimes, male genitalia are sexual ornaments under pre-copulatory sexual selection driven by female mate choice based on visual assessment. Evidence for female mating preferences based on genital traits is, however, surprisingly rare. In humans, there seems to be stabilizing sexual selection on penis length (e.g. Dixson et al. 2007). Bertin & Fairbairn (2005) demonstrated the existence of pre-copulatory sexual selection for external genital size in water striders, but it is unclear if this is driven by sexual conflict over mating rates or active female choice (Arnqvist & Rowe 2005). Pre-copulatory female choice based on male genitalia is most likely to evolve in taxa with good visual systems and external genitals (e.g. mammals, spiders (pedipalps) and some fishes).
Poeciliid fishes (Family: Poeciliidae) are ideal for the study of genital evolution because of high genital diversity across species (Rosen & Bailey 1963). In addition, population variation in male genitals is associated with changes in the use of different mating tactics and predation risk (e.g. Kelly et al. 2000; Jennions & Kelly 2002; Langerhans et al. 2005). Male poeciliids inseminate females with their gonopodium, an intromittent organ modified from the anal fin. In some species, males actively display the gonopodium during courtship (e.g. Basolo 1995), suggesting that it might be a signal used by females (Langerhans in press). Closely related poeciliids often vary greatly in relative gonopodium length (Rosen & Tucker 1961). A female preference for males with longer gonopodia has only been shown in one species (Gambusia affinis) using video playbacks (Langerhans et al. 2005) and there is a weak correlative evidence for a similar preference in Poecilia reticulata (Brooks & Caithness 1995). To date, no study has actively manipulated the genital size of live fish. Here, we surgically alter gonopodium length in the eastern mosquitofish (Gambusia holbrooki) to test whether the gonopodium size affects female mate choice. Mating occurs exclusively through male coercion, so more time spent near a male should increase the likelihood of successful insemination (see the electronic supplementary material).
2. Material and methods
We collected fish in Canberra, Australia. The sexes were housed separately: 45 females per 120 l aquarium and 10 males per 6 l aquarium. Females were male-deprived for 4–6 weeks to increase sexual receptivity (Bisazza et al. 2001). Fish were kept at 28°C with a 12 : 12 h photoperiod and fed live Artemia and fishflakes ad libitum.
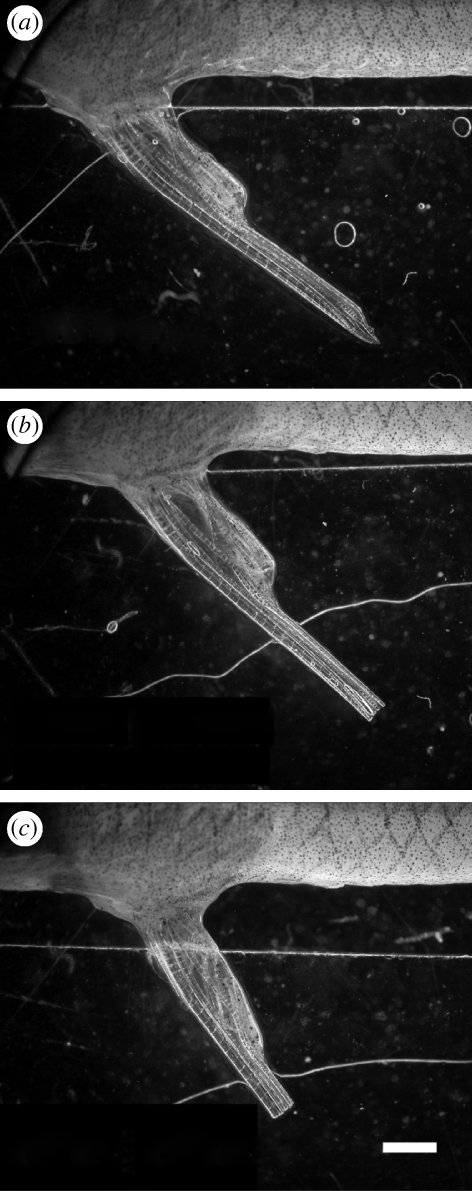
Male standard length (snout to posterior of last vertebrae) was measured (±0.5 mm) under a dissecting microscope to create two size classes: small (S) ≤20 mm and large (L) ≥24 mm representing the upper and lower population quartiles. Gonopodia were then experimentally shortened under anaesthetic. Half of the males in both body size classes had a major reduction treatment (s) and the others had a minor reduction (l) to account for any effects of surgery on female choice or male behaviour (see the electronic supplementary material). Post-manipulation, all males had blunt-ended gonopodia with terminal structures removed that differed only in length (figure 1). We created four distinct male types: Ll, Ls, Sl and Ss. Gonopodia of Ls males were 15 per cent shorter than those of Ll males (mean: 5.65 versus 6.65 mm, s.e. = 0.05) and gonopodia of Ss males were 17 per cent shorter than those of Sl males (mean: 4.52 versus 5.43 mm, s.e. = 0.04). After surgery, males were individually housed in 1 l aquaria. There was no mortality.
Figure 1.
Representative G. holbrooki showing the difference between wild-type (not used in trials) and treatment male gonopodia. (a) Wild-type; (b) minor reduction; (c) major reduction. Scale bar 1 mm.
(a). Experiment 1
Experiments were conducted in a test aquarium with five compartments (see the electronic supplementary material). A male from each class (Ll, Ls, Sl and Ss) was placed separately in one of the four end compartments. Each end pairing was size matched (±0.5 mm). A test female was then placed in a transparent cylinder in the central compartment. After 5 min, the cylinder was carefully removed. We made focal samples every 10 s for 10 min to measure female association preferences. Females were defined as associating with a male if they were <4 cm from the front of his compartment with their body oriented unambiguously towards him. After the trial, females were housed individually in 1 l aquaria for 7 days. We then repeated the trial but size-matched male pairs were swapped between ends, and within-pair positions randomized to control for any side bias. We used 60 test females (n = 120 trials). Owing to logistic constraints, male fish (n = 15 of each Ll, Ls, Sl and Ss) were used for several tests (4.0 ± 1.16 s.d.), but all male pairings were unique.
(b). Experiment 2
Given a female preference for large males with minor reduction in gonopodia length (see §3), a second two-male choice experiment was conducted to test female choice for gonopodium length in small males only (Sl versus Ss). The test aquarium was rearranged so that each male occupied a compartment equal in size to those in Experiment 1, but placed centrally at opposite ends. Trials occurred as outlined above, but with a continuous measure of association time. We used 15 females and 30 males. After the experiments, fish were euthanized and precise measurements of standard length taken.
Statistical analysis was by two-way analysis of variance (ANOVA; female identity: random; male type: fixed).
3. Results
(a). Experiment 1
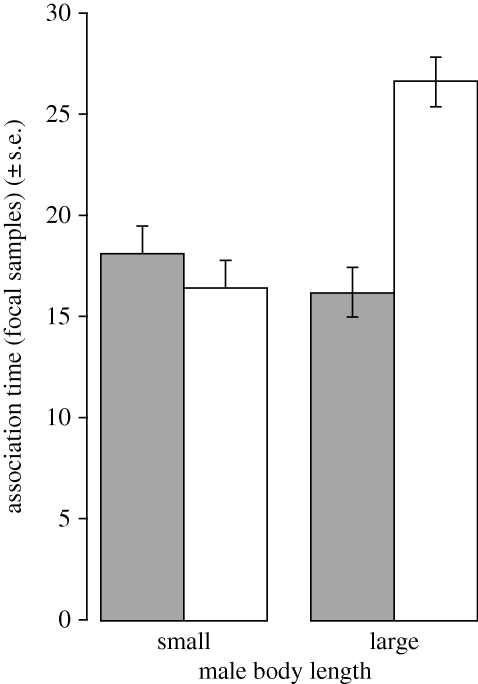
The mean time females spent associating with males varied significantly among the four male types. This was true whether the two trials were pooled (F3,177 = 10.52, p < 0.001) or considered separately (Trial 1: F3,177 = 10.22, p < 0.001; Trial 2: F3,177 = 2.91, p = 0.036). The difference in association time was primarily owing to females spending far more time in association with large males with minor reduction in gonopodia length (Ll) than any other class of males (figure 2; paired t-tests: Ll–Ss: t59 = 3.44, p = 0.001; Ll–Sl: t59 = 4.02, p < 0.001; Ll–Ls: t59 = 5.66, p < 0.001; all other pairs non-significant). The differences between Ll and the other three male types remained significant after Bonferroni correction.
Figure 2.
Pooled data for mean number of focal sample females associated with each male type. Grey bar, major reduction; white bar, minor reduction.
Female ‘choosiness’ (i.e. total time spent with males) was significantly repeatable between trials (ri = 0.29, p = 0.012), although the amount of time individual females spent with the Ll males was not (ri = −0.14, p = 0.849). Female body length correlated positively with choosiness (rs = 0.42, p = 0.004). There was no significant difference in body length among females categorized by the male type with whom they spent the most time (one-way ANOVA: F3,56 = 0.89, p = 0.45).
(b). Experiment 2
When females were given a choice between two small males, they did not preferentially associate with the male with the larger gonopodium (association time ± s.e.: Sl 66.2 ± 18.7 s, Ss 80.5 ± 16.5 s; F1,14 = 0.28, p = 0.61). Again, there was no relationship between female body length and the strength of their association preference (rs = 0.334, p = 0.223, n = 15). The correlation between female body length and choosiness was marginally non-significant (rs = 0.496, p = 0.06, n = 15).
4. Discussion
Our results show that male genital morphology influenced female mate choice in the eastern mosquitofish, G. holbrooki. Females spent significantly more time associating with large males with longer gonopodia. This is one of very few studies to investigate experimentally whether male genital size affects female mate choice based on visual cues. It corroborates reports of a female preference for longer male genitalia in the congeneric G. affinis (Langerhans et al. 2005). Together, these results suggest that pre-copulatory sexual selection might play a role in the rapid evolution of genitalia in Gambusia.
We found that, on average, females preferred to associate with larger males, which is consistent with previous studies of G. holbrooki (Bisazza et al. 2001), possibly owing to avoidance of harassment by smaller males (Dadda et al. 2005). However, our results were driven by the strong preference for large males with longer genitalia. Females spent the same amount of time associating with large males with short genitalia as with small males with large or small genitalia. Interestingly, a female preference for longer genitalia was absent when choosing between small males (Experiment 1), even when larger males were absent (Experiment 2). This suggests that the net benefit of producing a longer gonopodium might vary with male body size. One explanation for female choice is that G. holbrooki vary greatly in size at sexual maturation producing considerable overlap between juvenile and adult male body size. Some females might mistake smaller males with shortened genitalia for juveniles and also prefer to associate with a juvenile rather than a small adult male, resulting in no net preference for small males with a longer gonopodium. Another explanation is that female choice is for genetic gains, with correlation selection on insemination ability only favouring longer genitalia in larger bodied males who are less manoeuvrable.
We cannot confirm whether female mate choice generates stabilizing and/or directional selection with respect to the current average male genitalia length, because treatment males only had their genitalia shortened. At present, we lack a technique to experimentally lengthen the gonopodia of free-swimming males. If directional selection for longer genitalia is occurring, as in G. affinis (Langerhans et al. 2005), one might ask why males have not evolved still longer gonopodia? The most likely explanation is a counterbalancing effect of natural selection owing to reduced speed and/or increased predation risk: such trade-offs are well known for male ornaments (Andersson 1994, including poeciliids e.g. Basolo & Alcaraz 2003) and genitalia (House & Simmons 2003). Locomotor costs of non-retractable genitalia occur in G. affinis (Langerhans et al. 2005) and spiders (Ramos et al. 2004).
Finally, our study raises the intriguing question of why a female association preference exists in a mating system driven by coercive male mating (Bisazza et al. 2001). In poeciliids, pre-existing mating biases for male ‘swords’ (tail ornamentation) have been demonstrated in swordless species (Basolo 1990, 1995), and for large ‘sails’ (dorsal fin) in species lacking sails (MacLaren & Rowland 2006). It is possible that a similar sensory bias exists for long gonopodia in Gambusia spp. It would be informative to test formally whether female preferences for longer gonopodia occur in other poeciliids, particularly courting species, as these tend to have relatively short gonopodia (Rosen & Bailey 1963), as well as in species with colourful gonopodia (e.g. Poeciliopsis infans; Langerhans in press).
Acknowledgements
This study was approved under animal ethics permit FBTZ.26.08.
We thank James Davies for assistance, the Australian Research Council for funding (M.D.J.) and the ANU for scholarships (A.T.K. and B.M.).
References
- Andersson M.1994Sexual selection Princeton, NJ: Princeton University Press [Google Scholar]
- Arnqvist G.1998Comparative evidence for the evolution of genitalia by sexual selection. Nature 393, 784–786 (doi:10.1038/31689) [Google Scholar]
- Arnqvist G., Rowe L.2005Sexual conflict Princeton, NJ: Princeton University Press [Google Scholar]
- Basolo A. L.1990Female preference predates the evolution of the sword in swordtail fish. Science 250, 808–810 (doi:10.1126/science.250.4982.808) [DOI] [PubMed] [Google Scholar]
- Basolo A. L.1995Phylogenetic evidence for the role of a pre-existing bias in sexual selection. Proc. R. Soc. Lond. B 259, 307–311 (doi:10.1098/rspb.1995.0045) [DOI] [PubMed] [Google Scholar]
- Basolo A. L., Alcaraz G.2003The turn of the sword: length increases male swimming costs in swordtails. Proc. R. Soc. Lond. B 270, 1631–1636 (doi:10.1098/rspb.2003.2388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A., Fairbairn D. J.2005One tool, many uses: precopulatory sexual on genital morphology in Aquarius remiges. J. Evol. Biol. 18, 949–961 (doi:10.1111/j.1420-9101.2005.00913.x) [DOI] [PubMed] [Google Scholar]
- Bisazza A., Vaccari G., Pilastro A.2001Female mate choice in a mating system dominated by sexual coercion. Behav. Ecol. 12, 59–64 [Google Scholar]
- Brooks R., Caithness N.1995Female choice in a feral guppy population: are there multiple cues? Anim. Behav. 50, 301–307 (doi:10.1006/anbe.1995.0246) [Google Scholar]
- Dadda M., Pilastro A., Bisazzo A.2005Male sexual harassment and female schooling behaviour in the eastern mosquitofish. Anim. Behav. 70, 463–471 (doi:10.1016/j.anbehav.2004.12.010) [Google Scholar]
- Dixson B. J., Dixson A. F., Morgan B., Anderson M. J.2007Human physique and sexual attractiveness: sexual preferences of men and women in Bakossiland, Cameroon. Arch. Sex. Behav. 36, 369–375 (doi:10.1007/s10508-006-9093-8) [DOI] [PubMed] [Google Scholar]
- Eberhard W. G.1985Sexual selection and animal genitalia Cambridge, MA: Harvard University Press [Google Scholar]
- Hosken D. J., Stockley P.2004Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93 (doi:10.1016/j.tree.2003.11.012) [DOI] [PubMed] [Google Scholar]
- House C. M., Simmons L. W.2003Genital morphology and fertilization success in the dung beetle Onthophagus taurus: an example of sexually selected male genitalia. Proc. R. Soc. Lond. B 270, 447–455 (doi:10.1098/rspb.2002.2266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennions M. D., Kelly C. D.2002Geographical variation in male genitalia in Brachyrhaphis episcopi (Poeciliidae): is it sexually or naturally selected? Oikos 97, 79–86 (doi:10.1034/j.1600-0706.2002.970108.x) [Google Scholar]
- Kelly C. D., Godin J. G. J., Abdallah G.2000Geographical variation in the male intromittent organ of the Trinidadian guppy (Poecilia reticulata). Can. J. Zool. 78, 1674–1680 (doi:10.1139/cjz-78-9-1674) [Google Scholar]
- Langerhans R. B.In press.Genital evolution in poecillid fish. In Ecology and evolution of poecillid fishes (eds Evans J. R., Pilastro A., Schlupp I.). Chicago, IL: University of Chicago Press [Google Scholar]
- Langerhans R. B., Layman C. A., DeWitt T. J.2005Male genital size reflects a tradeoff between attracting mates and avoiding predators in two live-bearing fish species. Proc. Natl Acad. Sci. USA 102, 7618–7623 (doi:10.1073/pnas.0500935102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren R. D., Rowland W. J.2006Female preference for male lateral projection area in the shortfin molly, Poecilia mexicana: evidence for a pre-existing bias in sexual selection. Ethology 7, 678–690 (doi:10.1111/j.1439-0310.2006.01213.x) [Google Scholar]
- Ramm S. A.2007Sexual selection and genital evolution in mammals: a phylogenetic analysis of baculum length. Am. Nat. 169, 360–369 (doi:10.1086/510688) [DOI] [PubMed] [Google Scholar]
- Ramos M., Irschick D. J., Christenson T. E.2004Overcoming an evolutionary conflict: removal of a reproductive organ greatly increases locomotor performance. Proc. Natl Acad. Sci. USA 101, 4883–4887 (doi:10.1073/pnas.0400324101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D. E., Bailey R. M.1963The poeciliid fishes (Cyprinodontiformes), their structure, zoogeography, and systematics. Bull. Am. Mus. Nat. Hist. 126, 1–176 [Google Scholar]
- Rosen D. E., Tucker A.1961Evolution of secondary sexual characters and sexual behavior patterns in a family of viviparous fishes (Cyprinodontiformes: Poeciliidae). Copeia 2, 201–212 (doi:10.2307/1439999) [Google Scholar]
- Wenninger E. J., Averill A. L.2006Influence of body and genital morphology on relative male fertilization success in oriental beetle. Behav. Ecol. 17, 656–663 (doi:10.1093/beheco/ark013) [Google Scholar]